139 Fungal Infections
Medical advances continue to improve the prognosis of patients with cancer and other immunodeficiencies. In the past 50 years, the field of transplantation has greatly impacted the management of patients with cancer, renal, cardiac, and liver diseases. Moreover, advances in neonatology continue to increase the survival of premature infants. Undoubtedly these advances have benefited society greatly, but they have also fueled the emergence of systemic mycoses. Candida species first appeared as significant nosocomial pathogens approximately 30 years ago.1 For 2 decades, infections due to these pathogens increased dramatically. With the establishment of the National Healthcare Safety Network (NHSN) in 2005, several Centers for Disease Control and Prevention (CDC) surveillance systems, including the Nosocomial Infections Surveillance System (NNIS), were phased out. The NHSN provides broader surveillance data of healthcare-associated infections than the NNIS, thus the results of the two systems are not exactly comparable. Although the surveillance methods have changed, the trends have not. NHSN pathogen distribution data for 2006-2007 were comparable to that of the NNIS reports from 1986-1999.2
Fungal infections are generally more prevalent in ICUs than on the general medical wards.3 The importance of effective preventive measures against systemic mycosis is widely appreciated in critically ill oncology patients or hematopoietic stem cell transplant (HSCT) recipients. As our understanding of these infections in the general intensive care unit (ICU) setting continues to improve, so too does the ability to institute appropriate preventive measures. In the past decade, the development of agents possessing either a different mode or broader spectrum of activity, less toxicity, or a reduced propensity to interact with other drugs has increased the number of available systemic antifungal agents. Consequently, clinicians can now tailor antifungal therapy to specific patients. Moreover, our understanding of antifungal pharmacodynamics is developing, and methods to measure antifungal susceptibility are improving.
 Fungal Infections in the Critically Ill
Fungal Infections in the Critically Ill
Candida Infections in the ICU
Epidemiology
Candida albicans remains the fourth most common pathogen of healthcare-associated infections, and only coagulase-negative staphylococci, Staphylococcus aureus, and enterococci are more common.2 Candida spp. have consistently caused a substantial disease burden for at least the past decade. ICUs have a higher incidence of Candida bloodstream infections (BSIs) than medical and surgical wards.3 Although prior data had suggested the frequency of Candida BSIs among ICU patients in the United States had declined, estimates from national secondary databases and population-based studies suggest the disease burden may be shifting from the ICU to the general hospital population.1
C. albicans remains the most common invasive Candida spp. worldwide.4 However, decreasing trends in the isolation of this species over time have been observed in the ICU and non-ICU setting.4,5 An increased prevalence of C. albicans and Candida parapsilosis among neonatal ICU patients and an increasing prevalence of Candida glabrata infections among adults has been widely appreciated.1,4,5 C. albicans is responsible for approximately 45% of episodes of candidemia.6 The incidence of infection due to a particular Candida sp. varies considerably by the clinical service on which the patient is hospitalized. However, in general, C. albicans is the primary fungal pathogen in the ICU setting and is followed by C. glabrata, C. parapsilosis, Candida tropicalis, Candida krusei, and other Candida spp. (i.e., Candida guilliermondii, Candida lusitaniae, etc.).6 This rank order varies little across infection site, but it may vary with age.1,4–6 Surveillance data have noted that candidemia in neonatal ICUs is predominantly due to C. albicans and C. parapsilosis and rarely due to C. glabrata or other Candida spp.1,4–6 Surveillance studies have demonstrated that BSI due to C. albicans occurs less frequently with increasing age.1,4–6 In contrast, C. glabrata is rarely isolated among infants and children but is more frequently found with increasing patient age.1,4–6
C. albicans is part of the normal flora of the gastrointestinal tract. Infections including BSIs caused by most Candida spp., particularly C. albicans, arise endogenously from the gastrointestinal mucosa, skin, and urinary tract.7 Invasive Candida infections occur when alteration of endogenous flora leads to overgrowth of yeast which, in the presence compromised skin or gastrointestinal mucosa integrity, translocates from its commensal environment to the bloodstream.7 Candida spp., including C. albicans, may be transmitted exogenously in ICU settings.8,9 Exogenous transmission of non-albicans Candida spp. through indirect contact with the ICU environment occurs commonly.8 For example, C. parapsilosis is an exogenous pathogen known for its ability to form biofilms on catheters and inert devices. C. parapsilosis persists in the nosocomial environment.10 Moreover, it is spread throughout the hospital through hand carriage by healthcare workers.10 Therefore, colonization with this pathogen is not a prerequisite for infection.10
Mortality
Candida BSIs are often difficult to detect. Symptomatically, BSIs due to Candida spp. are indistinguishable from BSIs of bacterial etiology. Candida spp. are cleared from the blood very efficiently by several organs, particularly the liver, and blood cultures yield positive results in only 50% of patients with hematogenously disseminated candidiasis. However, the ability of automated blood culture systems to recover Candida spp. has continued to improve. For example, in a simulated candidemia study, Candida spp. were isolated in 74% (479/648) of blood culture bottles.11 However, isolation rates were highest in aerobic blood and mycology culture bottles (98% [211/216] and 97% [210/216], respectively) but lowest in anaerobic culture bottles (27% [58/216]).11 The ability to detect growth improved as inoculum size increased.11 Although the time to detect growth varied with Candida spp., most species were detected within 24 to 48 hours. Growth was detected faster in aerobic and mycology culture bottles than in anaerobic bottles. These data and other studies demonstrated the improved ability of current technology to detect simulated or clinical candidemia due to most common and uncommon Candida pathogens in aerobic cultures.11,12
Even with improved ability to recover Candida spp. from the blood, Candida BSIs carry a relatively poor prognosis. Candida spp. isolated from the blood have consistently been identified as an independent predictor of mortality.13–15 The overall attributable mortality of nosocomial BSIs among critically ill patients is 35%.16 This mortality rate for nosocomial BSIs in the ICU setting is comparable to the mortality rate associated with BSIs due to Candida spp. Historically, the estimated crude mortality rate associated with Candida BSIs hospital-wide and in the ICU setting has ranged from 35% to 69%, while the estimated attributable mortality has been 38%.14,17
Recent estimates suggest that the attributable mortality due to candidemia and other forms of invasive candidiasis ranges from 10% to approximately 50%.1 Moreover, data demonstrate that despite the advent of potent and safer anti-Candida antifungal therapy, the risk mortality associated with candidemia has essentially remained unchanged for at least 2 decades.19,20 Inadequate treatment may be a reason why mortality has not improved despite the availability of potent and safe antifungal therapy. Inadequate therapy resulting from delays in administration, treatment with an agent to which the organism is resistant, inadequate dosing or treatment duration, or failure to recognize and treat candidemia all contribute to the mortality associated with Candida BSI.21–27 In particular, it is increasingly clear that delaying initiation of adequate antifungal therapy even 12 to 48 hours is independently associated with mortality in candidemia patients.22,23,26,28,29
Candidemia produces significant morbidity and adds as much as a month to the length of hospital stay.1,7 Given the severity of illness associated with this infection, the added length of stay utilizes significant healthcare resources. Considering the incidence of candidemia in the United States alone, it is not surprising that the estimated annual healthcare costs associated with this infection easily exceed $1 billion.20
Risk Factors
Among critically ill patients, risk factors for Candida infections are well described.30,31 Broad-spectrum antimicrobial use, colonization, indwelling vascular catheters, and hemodialysis have been consistently identified as independent risk factors for Candida BSIs.14 In most ICU settings, many of these risk factors are commonly present and unavoidable. The ICU itself provides an ideal environment for transmission of Candida spp. among patients, thus it is not surprising that prolonged ICU stay has been identified as an independent risk factor.32 A study using validated risk factors in a simulated ICU population demonstrated that in the presence of multiple risk factors, the probability of infection increases exponentially.32 For example, in a hypothetical critical care unit, if a patient had prior exposure to 4 antibiotic classes, the calculated risk of candidemia for that patient would range from 5% to 35%, depending on the overall baseline candidemia rate in the ICU, varying between 1% and 5%. However, if that same hypothetical patient subsequently had Candida spp. cultured from another (non-bloodstream) anatomic site, the calculated risk would increase substantially to 40% to 80%.32 Given how common many of the risk factors (such as indwelling catheters, antibiotics, immunosuppressants, and TPN) are in the ICU, these data illustrate the need to accurately predict or identify patients who truly are at risk so that therapy can be instituted as early as possible.
The risk factors for non–C. albicans and C. albicans BSIs are similar, and the probability of a patient having either infection cannot be differentiated based on clinical characteristics alone.30,31 Several studies have developed prediction rules to stratify patients at increased risk for developing invasive infections with either C. albicans or non-albicans Candida spp. in hopes of providing guidance for clinical decision making to prevent candidemia in the ICU. These prediction rules are based upon retrospective studies and assess the combination of ICU length of stay, prior Candida colonization, and other host risks.33–36 While these systems demonstrate risk stratification is possible, they are somewhat complicated to apply, and some have questioned the practicality of certain components of individual prediction rules.31,37 Using the database from a large prospective multicenter Spanish study in which fungal colonization was assessed weekly along with other potential risk factors, León and colleagues developed the “Candida Score” based upon four independent risk factors: multifocal Candida spp. colonization, surgery upon ICU admission, severe sepsis, and total parenteral nutrition (TPN). The score, obtained by adding the statistical weight of each risk factor, has a cutoff value of 2.5, providing a sensitivity of 81% and specificity of 74% for identifying patients with current or future candidal infection. Patients with a score greater than 2.5 were more than 7 times as likely to have proven infection as patients with a Candida Score up to 2.5.35 A prospective multicenter observational study demonstrated that a Candida Score ≥3 discriminated between colonization and invasive candidiasis in non-neutropenic ICU patients colonized with Candida spp., with a minimum length of ICU stay of 7 days.37 These data lend credence to the idea of using the Candida Score for guiding the start of empirical antifungal therapy in the ICU. However, even though the Candida Score is promising, the clinical utility of such prediction rules in establishing the benefit of targeted antifungal prophylaxis remains to be established in prospective studies.38
Opportunistic Fungal Infections in Immunocompromised Critically Ill Patients
Invasive Aspergillosis in Critically Ill Patients with Hematologic Malignancies
In contrast to Candida spp., the burden of infection due to Aspergillus spp. is small.1 National hospital discharge data from the 1990s through 2003 reveal that there are approximately 10,000 aspergillosis-related hospitalizations annually in the United States.1 Nonetheless, Aspergillus spp. cause infection in critically ill populations immunocompromised by burns, cytotoxic chemotherapy, prolonged corticosteroid therapy, malignancy, leukemia, SOT or HSCT, and other congenital or acquired immunodeficiencies. Aspergillus spp. are ubiquitous environmental molds. While several hundred species of Aspergillus have been described, relatively few are known to cause disease in humans. Most Aspergillus infections are acquired exogenously via inhalation. In the absence of an effective immune response, airborne conidia invade sinus or lung vasculature. Although the lung is the most common site of invasive aspergillosis, Aspergillus spp. also demonstrate tropism for cutaneous, central nervous system (CNS), and cardiac vasculature.
The incidence of invasive aspergillosis in immunocompromised patients varies among specific populations.39 Among patients with hematologic malignancies, those with acute myelogenous leukemia have the highest incidence of invasive aspergillosis. For more than a decade the incidence of invasive aspergillosis in this population remained stable (5%-6%).40 However, advances in diagnosis (i.e., galactomannan assay, high-resolution computed tomography [CT] scan) have improved the ability to confirm cases that would previously been labeled as “suspected” invasive aspergillosis, and thus the incidence of this infection in patients with leukemia has risen significantly (12.7%).40 Like patients with leukemia, patients undergoing HSCT are at high risk for invasive aspergillosis. The incidence of invasive aspergillosis varies depending on transplant type but not type of conditioning regimen (myeloablative versus non-myeloablative).39 The incidence is higher among allogeneic HSCT recipients than among autologous HSCT recipients.39 In the HSCT population, whether the incidence of invasive aspergillosis is truly increasing or decreasing is difficult to ascertain, because the rate of autopsy continues to decline.41 The incidence of invasive aspergillosis among SOT is highest among lung transplant recipients and lowest among renal transplant recipients.39 Patients receiving HSCT or SOT can develop invasive aspergillosis shortly (within 40 days) after transplantation, but typically it occurs late post HSCT (>40-100 days) or SOT (>90 days).42–45
In patients with acute leukemia or in HSCT recipients, prolonged neutropenia after cytotoxic chemotherapy or HSCT is the primary risk for early invasive aspergillosis. Risk factors associated with invasive aspergillosis in HSCT and SOT recipients vary with time after the transplant. However, in general, risks early in the transplant process are related to transplant related factors (underlying disease, neutropenia, type of transplant), biological factors (hyperglycemia, iron overload), and extrinsic factors (excluding spores from the environment, air filtration). In contrast, risks for invasive aspergillosis occurring later in the transplant process include transplant complications (acute GVHD (grade ≥ 3) and high-dose corticosteroid therapy.43
Lesions associated with invasive pulmonary aspergillosis evolve over a period of weeks. CT findings, especially the “halo sign,” are strongly suggestive of invasive aspergillosis and infection from other angioinvasive fungi in immunocompromised patients. Moreover, this finding is associated with significantly improved response and survival if antifungal therapy is initiated shortly upon detection of this sign of infection.46 The combination of radiologic and clinical data may help in the differential diagnosis of fungal disease.
Recent diagnostic efforts have focused on detecting non–culture-based serum markers (e.g., galactomannan test, 1,3-β-D-glucan, polymerase chain reaction [PCR]). Galactomannan is a cell wall constituent of Aspergillus spp. that can be detected in the serum during invasive infection. The test is specific for invasive aspergillosis and is commercially available as a sandwich enzyme immunoassay (ELISA) that detects circulating galactomannan. The values from this test have been shown to strongly correlate with the clinical outcome of patients with invasive aspergillosis.47–49 Because 1,3-β-D-glucan is a cell-wall component of many fungal pathogens, it can be detected by colorimetric detection assays. Although the test is highly sensitive, the presence of 1,3-β-D-glucan in the serum is not specific for any fungi. Using both of these non–culture-based serum markers may improve the ability to diagnose invasive aspergillosis in high-risk populations and could lead to earlier diagnosis or improved monitoring of the success of antifungal therapy.50,51 The combination of radiologic, serologic, and clinical data may ultimately improve the diagnosis of invasive aspergillosis and speed up initiation of appropriate antifungal therapy.
Miscellaneous Pathogens in Critically Ill Patients with Hematologic Malignancies
Candida and Aspergillus spp. are the primary fungal pathogens in critically ill patients with hematologic malignancies. However, other pathogens such as Fusarium spp., Pseudallescheria spp., and the zygomycetes are increasing in frequency.7 Each of these less common organisms has characteristic clinical characteristics or tissue tropism. In addition, they are often less susceptible than Aspergillus spp. to systemic antifungal agents. Consequently, infections due to these pathogens are associated with high mortality. Of these, the zygomycetes (which cause mucormycosis) are the most common among critically ill patients, particularly in a surgical ICU. These angioinvasive pathogens are acquired through inhalation and produce a necrotic infection. Rhinocerebral and paranasal infections are common manifestations of zygomycetes. Common risks are diabetic ketoacidosis, immunosuppression, organ transplantation, skin damage, and a prolonged ICU stay. Data suggest that exposure to voriconazole prophylaxis to prevent invasive aspergillosis in certain immunosuppressed populations (i.e., HSCT recipients) may be a risk factor for zygomycosis.52
Cryptococcosis, Histoplasmosis, Blastomycosis, and Coccidioidomycosis in Critically Ill Patients
Cryptococcus neoformans, Histoplasma capsulatum var. capsulatum, Blastomyces dermatitidis, and Coccidioides immitis are not common pathogens in the ICU setting. These organisms can cause infection in patients with intact immune function. However, with the exception of B. dermatitidis, severe infections due to these pathogens are more common among critically ill immunocompromised populations, particularly those with AIDS and SOT recipients. Cryptococcosis is the third most common invasive fungal infection among SOT recipients.7
C. neoformans is a ubiquitous encapsulated yeast isolated from diverse environmental sources (i.e., soil, trees and plant material, and droppings from pigeons). This pathogen is primarily acquired by inhalation. In the lung, the organism elicits a cell-mediated response involving neutrophils, monocytes, and macrophages. The cryptococcal polysaccharide capsule, an important virulence factor, facilitates laboratory identification and recognition by host cell-mediated immune response and possesses immunosuppressive properties. The advent of AIDS significantly altered the incidence of cryptococcosis. Before the AIDS epidemic, cryptococcosis was an uncommon disease in the United States, but since then, the majority of cases have been associated with HIV infection. The prevalence of cryptococcosis in HIV in the United States has declined with the widespread use of fluconazole and highly active antiretroviral therapy to treat HIV infection. Cryptococcosis still produces significant acute mortality, but overall long-term outcomes have improved dramatically in the past 2 decades.53 Mortality among HIV-infected patients and SOT recipients is similar and is estimated to be approximately 15% to 20%.53–55
Histoplasmosis (caused by H. capsulatum var. capsulatum), blastomycosis (B. dermatitidis), and coccidioidomycosis (C. immitis) are the major endemic mycoses found in North America. Infections by these pathogens are reported primarily in distinct geographic areas, but owing to population mobility, they can be reported throughout the United States. H. capsulatum is endemically distributed primarily in the Mississippi and Ohio River valleys, B. dermatitidis is found primarily in the south central United States, the Mississippi and Ohio River valleys, and in certain regions of Illinois and Wisconsin. C. immitis is found primarily in the arid southwest regions of the United States. Infection with all these pathogens is acquired via inhalation. Overall, hospitalization is required in an estimated 4.6 and 28.7 cases per million children and adults, respectively.56 Nationwide, endemic mycoses require substantial healthcare resources to manage and produce significant crude mortality rates in children and adults (5% and 7%, respectively).56 The severity of histoplasmosis depends on host immune function and the extent of exposure, particularly in the immunocompetent host. Hematogenous dissemination from the lungs occurs in all infected patients, but in immunocompetent hosts, it is controlled by the reticular endothelial system. However, among elderly hosts or those with cell-mediated immune disorders (e.g., HIV infection), progressive disseminated infection readily occurs. After inhalation, B. dermatitidis can disseminate from the lungs to other organs as the yeast form. The primary pneumonia is often undetected and resolves without sequelae. Endogenous reactivation in the lungs, skin, or bones is often the first sign of infection.
C. immitis requires the inhalation of only a few arthroconidia to produce primary coccidioidomycosis. Like the other endemic mycoses, in the majority of patients, primary coccidioidomycosis typically manifests as an asymptomatic pulmonary disease. However, it can also manifest as an acute respiratory illness, chronic progressive pneumonia, pulmonary nodules and cavities, extrapulmonary nonmeningeal disease, and meningitis.57
Clinical manifestations of blastomycosis can mimic many other diseases, such as TB and cancer, but typically occurs as an asymptomatic infection, acute or chronic pneumonia, or disseminated (extrapulmonary) disease.58 Extrapulmonary blastomycosis typically afflicts the skin, bones, and genitourinary system.58 Cutaneous lesions are the most common skin manifestations of this disease.58 Extrapulmonary (disseminated) coccidioidomycosis afflicts 1% to 5% of all patients infected with C. immitis, and is deadly if not treated properly. Even with appropriate treatment chronic infection is common.57
 Systemic Antifungal Agents
Systemic Antifungal Agents
Amphotericin B Formulations
Amphotericin B Deoxycholate
Amphotericin B deoxycholate (AmB-d), a polyene antifungal agent, disrupts biological membranes, thereby increasing their permeability. AmB-d also stimulates the release of cytokines, which causes arteriolar vasoconstriction in the renal vasculature.59
Pharmacology and Pharmacokinetics
The majority (70%) of an administered AmB-d dose is recovered from the urine and feces over a 7-day period; approximately 30% of the administered dose remains in the body a week after dosing.60
Overview of Toxicity
AmB-d infusion-related reactions, including hypotension, fever, rigors, and chills, occur in approximately 70% of patients.61 These reactions occur early in therapy and often subside with time. Pretreatment regimens consisting of diphenhydramine, acetaminophen, meperidine, and hydrocortisone may be used to prevent infusion-related reactions. The efficacy of these regimens is unclear, so their routine use is discouraged until the reactions occur, after which pretreatment regimens should be employed with subsequent dosing.61 Although common and noxious, infusion-related reactions rarely cause early termination of AmB-d therapy or interfere with the use of other medications.
AmB-d also produces dose-related toxicities, including nephrotoxicity, azotemia, renal tubular acidosis, electrolyte imbalance, cardiac arrhythmias, and anemia.59 AmB-d–induced nephrotoxicity is the most common dose-related toxicity.62 In the ICU this toxicity often limits the use of AmB-d or interferes with the ability to use other medicines. Saline hydration before dosing can reduce the incidence of AmB-d–induced nephrotoxicity, but in the ICU setting, the utility of saline hydration may be limited by fluid restriction employed to manage the fluid status of critically ill patients.
Lipid Amphotericin B Formulations
Amphotericin B lipid complex (ABLC), amphotericin B colloidal dispersion (ABCD), and liposomal amphotericin B (LAmB) are lipid AmB formulations that in many centers have supplanted the use of AmB-d. They all retain the activity of AmB-d but have significantly less associated nephrotoxicity than the parent drug.62
Pharmacokinetic Comparisons of Lipid Amphotericin B Formulations
The lipid AmB formulations differ in physicochemical properties and composition. These differences produce subtle differences in their pharmacokinetic behavior that may ultimately prove to be clinically significant. The disposition and activity of these formulations in human tissue is poorly characterized. However, animal data indicate that high serum concentrations may influence the delivery of lipid AmB formulations to certain infection sites such as the CNS and lungs.63
Toxicity Comparisons of Lipid Amphotericin B Formulations
Compared with AmB-d, the lipid formulations have significantly less associated nephrotoxicity.62 The formulations differ in the incidence of infusion-related reactions and other adverse events associated with AmB-d infusion.64,65 These reactions typically do not result in early termination of therapy.64,66 Observational safety comparisons between ABLC and LAmB suggest the two formulations have a similar nephrotoxicity profile, but prospective comparative data suggest LAmB may be somewhat less nephrotoxic than ABLC.62,67 There are few data comparing the safety of lipid AmB formulations to the triazole antifungal agents in critically ill patients. Given the safety of triazoles, it is unlikely the lipid AmB formulations will prove to be any safer.
Azole Antifungal Agents
Fluconazole, Itraconazole, Voriconazole, Posaconazole
Pharmacology and Pharmacokinetics
Several studies have examined fluconazole pharmacokinetics in critically ill patients.68–70 In surgical ICU patients, fluconazole clearance correlates with creatinine clearance (CrCl), and its volume of distribution correlates with body weight.69 In addition, fluconazole volume of distribution is greater in this population than in healthy volunteers.69 The fluconazole half-life is markedly prolonged in surgical ICU patients.69 In patients with severe renal dysfunction (CrCl <30 mL/min), some recommend dosage reductions of 50%,69 but such reductions should be made cautiously and take into account the infecting pathogen in patients receiving fluconazole via enteral feeding tubes.69 Data suggest that the systemic availability of fluconazole is relatively unaffected by administration via enteral feeding tubes. However, serum concentrations obtained with standard doses administered via an enteral feeding tube may not be adequate to treat C. glabrata infections.68 Moreover, in critically ill abdominal trauma patients with and without abdominal wall closure, IV fluconazole may be warranted because the bioavailability of enterally dosed fluconazole in these patients is highly variable.70
Under fasting conditions in healthy adults, itraconazole is rapidly absorbed from the oral solution, and compared to the capsule there is less interpatient and intrapatient variability in serum concentrations.71 After IV administration, renal elimination of itraconazole is negligible, but HP-βCD is renally eliminated (80%-90%). IV itraconazole was contraindicated in cases of significant renal impairment (CrCl ≤ 30 mL/min) because of concerns over the renal accumulation of HP-βCD.
Voriconazole is a derivative of fluconazole with limited aqueous solubility and improved antifungal activity. It is available in IV and oral formulations. IV voriconazole contains sulfobutyl ether β-cyclodextrin (SBECD) as a solubilizing agent. There are few data on how critically ill patients handle voriconazole. In healthy volunteers, voriconazole exhibits good oral availability and wide tissue distribution, with hepatic metabolism and renal excretion of metabolites.72 In patients with moderate to severe renal function, SBECD accumulates, and it is recommended that oral dosing be used in patients with a CrCl less than 50 mL/min.73 Oral dosing in critically ill patients is often not possible, therefore how SBECD is handled in critically patients on dialysis has been examined. A small study observed accumulation of SBECD in three patients during hemodialysis. No toxicity due to accumulation of SBECD was observed, and the accumulated dose values were lower but comparable with those used in previous toxicity studies with animals.73 Nonetheless, if possible, use of IV voriconazole in patients on hemodialysis should be avoided. Data demonstrate that voriconazole achieves adequate CSF concentrations.72
Posaconazole is available as oral suspension and exhibits linear pharmacokinetics with dosages between 50 and 800 mg/d. However, absorption is saturated at doses exceeding 800 mg/d.74 Posaconazole absorption is influenced by gastric pH and is optimal under acidic conditions.75 There are no data describing the disposition of posaconazole in critically ill ICU patients. However, posaconazole absorption and exposure are maximized by dividing the total daily dose 4 times daily rather than administering it as a single dose.75,76 Posaconazole absorption and exposure are also enhanced by administration with or shortly after a meal. In the ICU it is often impractical to give posaconazole with or shortly after a meal, but absorption and exposure are also enhanced by administering the drug with a liquid nutritional supplement.75,77,78 Although posaconazole binds extensively (>95%) to plasma proteins, its large estimated volume of distribution suggests that it distributes widely throughout the body, but there are few data describing its penetration into the CSF.79 Posaconazole is primarily eliminated in feces and urine as unchanged drug.80
Overview of Toxicity
Fluconazole is perhaps the safest azole, and doses four to five times in excess of the recommended daily dose have been well tolerated. Adverse effects with itraconazole occur frequently and often may necessitate discontinuation of therapy.81 Although adverse effects associated with itraconazole are common, they are rarely life threatening, and symptoms typically abate when the drug is stopped or the dose is reduced.81 In addition to the adverse effects seen with other azoles, voriconazole produces transient visual disturbances in approximately 30% of patients, which rarely lead to discontinuation of therapy.82 These visual disturbances are acute and include changes in color discrimination, blurred vision, photophobia, and the appearance of bright spots.82 To date, the common adverse effects associated with posaconazole use have been similar to those observed with the other agents in the class (i.e., gastrointestinal, transient transaminase abnormalities).
Azole Drug Interactions
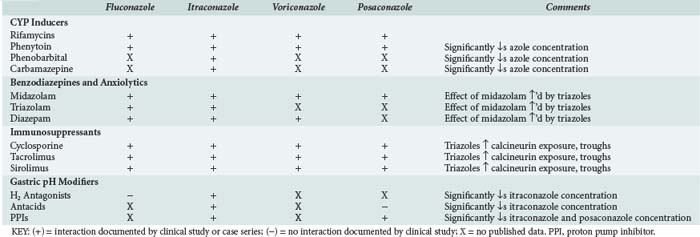
Drug interactions occur primarily in the intestine, liver, and kidneys by a variety of mechanisms. In the intestine they can occur as a result of changes in pH, complex formation with ions, or interference with transport and enzymatic processes involved in gut wall (i.e., presystemic) drug metabolism. In the liver, drug interactions can occur because of interference with drug-metabolizing enzymes. Drug interactions in the kidney can occur through interference with glomerular filtration, through active tubular excretion, or by other mechanisms. The azoles are one of the few drug classes that can cause or be involved in drug interactions at all of these anatomic sites by one or more of the above mechanisms. Drug interactions involving the azoles have been extensively reviewed.83 Several of the drug-drug interactions involving the azoles occur class wide. Therefore, when using the azoles, the clinician must be aware of the many drug-drug interactions, both real and potential, associated with this class.
Interactions involving the azoles result because of their physicochemical properties. All azoles are somewhat lipophilic and thus undergo CYP-mediated metabolism. The azoles all inhibit one or more CYP enzymes. Of the four azoles reviewed here, only itraconazole appears to interact significantly with P-glycoprotein (P-gp), which is a transport protein involved in drug distribution.83 Fluconazole is not affected by agents that increase gastric pH, but its potential to cause CYP-mediated interactions is more than that suggested by in vitro studies. CYP-mediated interactions involving fluconazole are often dose dependent and can involve drugs metabolized by CYP3A4 (e.g., midazolam, rifampin, phenytoin) and CYP2C9 (e.g., warfarin).83 Because of its linear and predictable pharmacokinetic properties, these interactions may sometimes be avoided or managed by using the lowest effective fluconazole dose.
Itraconazole is subject to pH-based interactions and interactions involving CYP3A4 and P-gp. Drugs that can interact with itraconazole include agents that increase gastric pH (e.g., protonics) and lipophilic CYP3A4 (e.g., HMG-CoA reductase inhibitors, benzodiazepines, immunosuppressive agents), and/or P-gp substrates (e.g., digoxin) with poor oral availability.83 Voriconazole is not affected by agents that increase gastric pH. However, CYP-mediated interactions involving voriconazole can involve drugs metabolized by CYP3A4 (e.g., midazolam, rifampin, phenytoin), CYP2C9 (e.g., warfarin), or CYP2C19 (e.g., omeprazole).83 Approximately 17% of a posaconazole dose undergoes biotransformation.84 Unlike other azoles, posaconazole is only minimally (2%) metabolized by CYP; instead its metabolites are glucuronide conjugates formed via uridine diphosphate glucuronosyltransferase (UGT) pathways.84,85 Although posaconazole is minimally metabolized by CYP, it inhibits hepatic CYP3A4.86 Like the other azoles, the most clinically significant interactions associated with posaconazole involve benzodiazepines (oral midazolam), calcineurin inhibitors (cyclosporine, tacrolimus), other immunosuppressive agents (sirolimus), and phenytoin.83 With more widespread use of posaconazole, the list of medications it interacts with will likely grow. Drug interactions involving the azoles that are relevant to the ICU setting are summarized in Table 139-1.
Echinocandin Antifungal Agents
Caspofungin, Micafungin, Anidulafungin
Pharmacology and Pharmacokinetics
Caspofungin binds extensively to plasma proteins (primarily albumin). Caspofungin distribution is multiphasic; initially it distributes to plasma and extracellular fluid before being actively transported slowly into the liver and other tissues via organic anion transport proteins.83 The prolonged elimination half-life (8-13 hours) of caspofungin is due in part to this slow multiphasic distribution.83 Caspofungin is slowly metabolized in the liver via N-acetylation and peptide hydrolysis to inactive metabolites, which are then excreted in bile and feces.87 Compared with healthy subjects, caspofungin average serum concentrations 24 hours after administration vary greatly and are elevated in surgical ICU patients.88 Body weight and hypoalbuminemia were found to be prognostic factors responsible for these increased caspofungin concentrations.88 The clinical significance of such findings is unclear. Dosage adjustment is not required in patients with impaired renal function, but the dose should be reduced by 50% in patients with significant hepatic impairment.89 Micafungin distribution and metabolism are not fully understood. Following IV administration, micafungin binds extensively to albumin, but the significance of this interaction on drug activity is unclear.90 Micafungin is hepatically metabolized to several metabolites, and it is predominately eliminated as parent drug and metabolite(s) in feces.90
Anidulafungin distribution and metabolism are not fully understood. Of all the other echinocandins, anidulafungin binds the least to plasma proteins; has a larger volume of distribution and achieves lower peak (Cmax) serum concentrations.91 Anidulafungin is not hepatically metabolized, but rather in the plasma it undergoes slow nonenzymatic chemical degradation to an inactive peptide breakdown product, which likely undergoes further enzymatic degradation and is excreted in feces and bile.91 The majority of an anidulafungin dose is excreted in feces or urine as unchanged drug.91
Toxicity and Drug Interactions
In general, caspofungin is well tolerated but is associated with nonspecific (i.e., fever, headache, nausea, phlebitis, rash, elevated hepatic enzymes) adverse effects which are generally mild and rarely cause early discontinuation of therapy. Similarly, caspofungin has low potential to interact with other drugs. Clinically insignificant interactions with the cyclosporine, tacrolimus, have been reported, but their clinical significance is unclear.83
 In Vitro Susceptibility Testing of Systemic Antifungal Agents
In Vitro Susceptibility Testing of Systemic Antifungal Agents
In vitro susceptibility testing of Candida spp. is now widely accepted. Standardized broth microdilution and disk diffusion methods developed by the Clinical and Laboratory Standards Institute (CLSI) for in vitro susceptibility testing of Candida spp. are reproducible and accurate. Interpretative breakpoints for Candida spp. exist for fluconazole, itraconazole, voriconazole, 5-FC, and the echinocandins but do not exist for amphotericin B formulations or posaconazole. Although interpretive breakpoints for AmB in the treatment of Candida spp. have not been established, minimum inhibitory concentrations (MICs) for most isolates of Candida are ≤1 µg/mL. In addition, resistance to AmB formulations among the most commonly isolated species is unusual. In contrast to Candida spp., in vitro susceptibility testing of C. neoformans is not routinely performed, because primary resistance to first-line antifungal drugs (5-FC, AmB, fluconazole) is not currently a significant clinical problem, and the susceptibility testing methods and interpretive breakpoints for Cryptococcus spp. against any antifungal are not validated.92 Validated broth microdilution methods for in vitro susceptibility testing methods of Aspergillus spp. for the azoles and AmB have been developed, but interpretive breakpoints for these agents have not been established.93 Validated agar-based disk diffusion methods and commercial kits (Etest) are available and may be reliable methods for determining susceptibilities for Aspergillus spp.93 Although broth microdilution methods for susceptibility testing for Aspergillus spp. for the echinocandins exist, the MIC is not the ideal measure of drug activity for this class of agents.93
 Treatment of Fungal Infections in the Critically Ill
Treatment of Fungal Infections in the Critically Ill
Candidiasis in the ICU
The paradigms of preventive antimycotic therapy are prophylaxis and “preemptive therapy” (sometimes referred to as empirical therapy). Prophylaxis is generally initiated in a population in anticipation of certain risk factors, regardless of whether they ever manifest. There are few data to justify the use of this paradigm in the ICU setting, where concerns regarding selection of resistant fungal pathogens with indiscriminate antifungal use persist.94 Moreover, the risk for invasive candidiasis is not the same for all ICU patients, and some risk factors evolve during an ICU stay. Therefore, universal institution of antifungal prophylaxis in the general ICU population is generally discouraged in favor of a more targeted approach selectively directed toward those patients at the highest risk.94,95
Preemptive therapy is the administration of antifungal treatment before the occurrence of a septic syndrome in patients with several risk factors for infection and evidence of significant Candida colonization.94 Historically, AmB-d was the sole option for prevention or treatment of candidiasis in the ICU setting. However, the risk of nephrotoxicity and the advent of safe and effective alternatives such as the echinocandins have diminished its use in the ICU.
Prophylaxis
Most studies of prophylactic antifungal use in the ICU setting have evaluated fluconazole. A placebo-controlled study for the prevention of intraabdominal Candida infections in a selected group of high-risk abdominal surgical patients showed that daily fluconazole (400 mg) significantly reduced the incidence of invasive candidiasis.96 This study included patients who had recurrent gastrointestinal perforations or anastomotic leakages; therefore, they were at very high risk of developing intraabdominal candidiasis. The patients in this study had moderate acuity (APACHE II score 13), but prophylactic fluconazole prevented Candida colonization and dissemination of Candida spp. Similar to experiences with HSCT recipients, this study illustrates that when the prophylactic paradigm is selectively applied it may benefit specific patient populations. This has also been shown in the HSCT population.96 Similar results were obtained in critically ill surgical patients staying in ICU longer than 3 days.68,97 However, these results should be interpreted cautiously. This was a single-center study, and true to the paradigm, patient selection was somewhat subjective and based on an anticipated ICU stay of 3 or more days and the clinician’s experience. Therefore, the results may not be widely generalizable. Others have also prospectively studied prophylactic fluconazole and shown an advantage for low-dose IV fluconazole (100 mg/d) in reducing Candida colonization and candidemia, with no effect on either invasive candidiasis or overall mortality.98 In this double-blind randomized placebo-controlled study, all patients received selective digestive decontamination. The incidence of Candida infections, particularly candidemia, was significantly less in the fluconazole-treated patients.
Using these three studies and others that included ketoconazole or nonabsorbable antifungal agents, three meta-analyses have attempted to provide further insight into the role of antifungal prophylaxis in critically ill patients, but with disparate results. One analysis concluded that prophylactic fluconazole administration to prevent mycoses in surgical ICU patients successfully decreased the rate of fungal infections, but it did not improve survival.99 Conversely, a second analysis demonstrated that antifungal prophylaxis indeed reduced the risk of candidemia and resulted in a reduction of overall mortality and attributable mortality (31% and 79%, respectively).100 The third and perhaps most rigorous meta-analysis demonstrated that antifungal prophylaxis in non-neutropenic critically ill patients reduces proven invasive fungal infections by approximately half and total mortality by approximately one-quarter.95 Although the analyses had slightly differing results, all concluded that if antifungal prophylaxis is employed, it should done so selectively and targeted toward those patients at high risk of developing infection.95,99,100 Thus, what the prophylactic studies have highlighted is the need to identify high-risk patients for preemptive therapy.
Preemptive Therapy
There are few randomized prospective data addressing preemptive therapy. Nonetheless, in the absence of mechanisms to identify patients who would most benefit by preemptive antifungal therapies, this strategy shares similar drawbacks to the prophylactic strategy. However, a growing body of data clearly demonstrate the importance of early institution of antifungal therapy in the adult ICU.* There are a number of predictive rules of varying complexity described in the literature. All of the studies have produced different predictive algorithms; few have been prospectively validated.37 While the methods are improving, published methods have yet to be widely applied in ICU patients as part of routine practice Moreover, there are few data describing the outcomes associated with preemptive therapy instituted based upon a predictive rule. One small study assessed the use of a scoring system to identify high-risk patients and demonstrated that fluconazole significantly decreased the incidence of invasive candidiasis in patients with a corrected colonization index (CCI) of ≥0.5.102 Another prospective study to assess whether preemptive antifungal therapy in high-risk ICU patients (CCI ≥ 0.4) would reduce invasive candidiasis demonstrated a significant decrease in the incidence of surgical ICU–acquired invasive candidiasis with preemptive therapy compared to historical controls.103 However, to generate the CCI, required weekly surveillance cultures at multiple anatomic sites in all ICU patients is necessary. This method is not practical for most ICUs, and it is doubtful that the CCI could be used with similar success without routine surveillance cultures.31
A prospective randomized double-blind study demonstrated that caspofungin is at least as effective as AmB-d for the treatment of invasive candidiasis.104 However, that study included ICU and non-ICU patients and assessed primary treatment of invasive candidiasis, not preemptive therapy. Lipid AmB formulations have lowered the risk of nephrotoxicity associated with AmB, but there are no data regarding their use in the ICU setting. The results of studies assessing these formulations as empirical or salvage therapy in immunocompromised hosts should not be extrapolated to the general ICU setting.
A cost analysis has illustrated the potential benefit of preemptive therapy. According to this analysis of empirical therapy, caspofungin is the most effective strategy for ICU patients, but its high cost made it less cost-effective than empirical fluconazole.105 The analysis also demonstrated that empirical AmB and the lipid AmB formulations were the least effective strategies, largely because of drug toxicities.105 The authors concluded that empirical fluconazole should reduce mortality at an acceptable cost.105 Similar to other decision model analyses, this study also recognized that in low-risk ICU patients, even empirical strategies are not justified.
The recommended antifungal therapy for candidiasis in the ICU setting is summarized in Table 139-2.106
TABLE 139-2 Summary of Recommended Antifungal Therapy for Aspergillosis and Candidiasis in the ICU Setting
| Infection | Recommended Treatment | Alternative Treatment |
|---|---|---|
| Aspergillosis | ||
| Invasive pulmonary aspergillosis therapy | VCZ, 6 mg/kg IV q 12 h for 1 day, followed by 4 mg/kg q 12 h; oral dose is 200 mg q 12 h | L-AmB, 3-5 mg/kg/d IV; ABLC, 5 mg/kg/d IV; caspofungin, 70 mg IV on day 1, and 50 mg/d IV thereafter |
| Empirical and preemptive antifungal therapy | L-AmB, 3 mg/kg/d IV; or Caspofungin, 70 mg IV on day 1, and 50 mg/d IV thereafter; or ITZ, 200 mg daily IV, or 200 mg BID; or VCZ, 6 mg/kg IV q 12 h for 1 day, followed by 3 mg/kg IV q 12 h; oral dosage is 200 mg q 12 h |
|
| Prophylaxis invasive aspergillosis | PCZ, 200 mg q 8 h in patients with GVHD and neutropenic patients with AML or MDS | ITZ, 200 mg q 12 h IV for 2 days, then 200 mg q 24 h IV; or ITZ, 200 mg PO q 12 h; micafungin (50 mg/d) |
| Invasive Candidiasis (Candidemia) | ||
| Treatment (non-neutropenic) | FCZ, 800 mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily; or an echinocandin* (for moderate-severe infection in patients with azole exposure) | LF-AmB, 3-5 mg/kg/d; or AmB-d, 0.5-1 mg/kg/d; or VCZ, 400 mg (6 mg/kg) BID for 2 doses, then 200 mg (3 mg/kg) BID |
| Treatment (neutropenic) | An echinocandin* or LF-AmB, 3-5 mg/kg/d (VCZ can be used when additional mold coverage is desired; removal of intravascular catheter is advised but is debatable.) | FCZ, 800 mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily; or VCZ, 400 mg (6 mg/kg) BID for 2 doses, then 200 mg (3 mg/kg ) BID |
| Suspected candidiasis treated with empirical antifungal therapy (non-neutropenic patients) | Treat as above for candidemia. An echinocandin* or fluconazole is preferred (for patients with moderate/severe infection or recent azole exposure, an echinocandin* is preferred). | LF-AmB, 3-5 mg/kg/d; or AmB-d, 0.5-1 mg/kg/d |
| Suspected candidiasis treated with empiric antifungal therapy (neutropenic patients) | LF-AmB, 3-5 mg/kg/d; or Caspofungin,* 70-mg loading dose, then 50 mg/d; or VCZ, 400 mg (6 mg/kg) BID for 2 doses, then 200 mg (3 mg/kg) BID |
FCZ, 800 mg (12 mg/kg) loading dose, then 400 mg (6 mg/kg) daily; or ITZ, 200 mg (3 mg/kg) BID |
| Prophylaxis | ||
| Neutropenic (HSCT) | FCZ, 400 mg/d while patients are at high risk | |
ABLC, amphotericin B lipid complex; AmB-d, amphotericin B deoxycholate; AML, acute myeloid leukemia; BID, twice daily; FCZ, fluconazole; GVHD, graft-versus-host disease; HSCT, hematopoietic stem cell transplant; ITZ, itraconazole; L-AmB, liposomal amphotericin B; LF-AmB, any marketed lipid amphotericin B formulation; MDS, myelodysplastic syndromes; PCZ, posaconazole; VCZ, voriconazole.
* Monitor for persistence; in vitro susceptibilities reveal caspofungin MICs for Candida parapsilosis higher than other Candida spp., and results of clinical trial demonstrated caspofungin to be effective in treatment of C. parapsilosis fungemia, but persistent cultures are common.
Adapted from Mora-Duarte J, Betts R, Rotstein C et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med 2002;347:2020-2029; Pappas PG, Kaufman CA, Andes D et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009;48:503-35; and Walsh TJ, Anaissie EJ, Denning DW et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008;46:327-60.
Invasive Aspergillosis and Other Opportunistic Mycoses in Bone Marrow Transplantation
Fever and neutropenia are common among critically ill immunocompromised individuals with hematologic malignancies. Although fever can be due to many causes, these patients, and particularly HSCT recipients, are at risk of developing systemic mycosis due to Candida or Aspergillus spp. Owing to the difficulty in diagnosing infections due to these pathogens, antifungal prophylaxis is standard in HSCT patients. Fluconazole has been shown to decrease the incidence of invasive infections with Candida spp. and is widely used in the prophylactic paradigm.96 As stated previously, invasive aspergillosis occurs relatively late after transplantation. Therefore, persistently febrile HSCT recipients should be treated empirically with antifungal agents with activity against molds, particularly Aspergillus spp.
For many years “high-dose” AmB-d was employed as standard empirical therapy of invasive aspergillosis, but within the last decade, based upon data from a randomized trial that compared voriconazole to AmB and suggested superiority with the azole, voriconazole has been considered the gold-standard therapy of documented and suspected aspergillosis.107 Although voriconazole is considered an initial option for prophylactic therapy, the choice of therapy may vary based upon the individual’s organ function. Voriconazole may not be ideal in cases where liver disease is present or if the patient is being treated with concomitant medicines that interact with this azole. Similarly, the presence of reduced renal function may preclude the use of lipid AmB formulations. The other azoles are not appropriate as preemptive therapy in HSCT. Fluconazole lacks activity against molds. Itraconazole has activity against Aspergillus spp., but as discussed previously, the capsule dosage form is not suitable for many critically ill patients and produces erratic blood levels. The oral solution of itraconazole is not well tolerated and is commonly associated with diarrhea. If available, IV itraconazole solution suffers the same drawback as lipid AmB formulations in patients with diminished renal function. Lastly, posaconazole is only available as an oral liquid, and it requires food and multiple daily dosing to optimize serum concentrations. These characteristics preclude its use in patients who experience vomiting, diarrhea, decreased appetite, and mucositis related to their cytotoxic chemotherapy.
With their lack of toxicity and low propensity for drug-drug interactions, the echinocandins are promising agents for empirical therapy of invasive aspergillosis in critically ill patients. However, their lack of cidal activity, and the lack of prospective data assessing their use in this population leads many to consider them only as a secondary option. Recommended antifungal therapy for the treatment of aspergillosis in the ICU setting is summarized in Table 139-2.108
Cryptococcosis, Histoplasmosis, and Blastomycosis
Although cryptococcosis, histoplasmosis, blastomycosis, and coccidioidomycosis are not considered nosocomial mycoses, patients with severe infections may require intensive care. The treatment of cryptococcosis, particularly that in the CNS, evolved from a series of classic clinical trials. Current guidelines base their recommendations on the best data available to address unresolved questions surrounding treatment of this infection. Recommended antifungal therapy for treatment of cryptococcosis in the ICU setting is summarized in Table 139-3.92
TABLE 139-3 Summary of Recommended Antifungal Therapy for Cryptococcosis and Endemic Mycoses in the ICU Setting
| Infection | Recommended Treatment(s) | Alternative Treatment |
|---|---|---|
| Cryptococcosis | ||
| CNS infection (HIV infected) | Induction: AmB, 0.7-1 mg/kg + 5-FC, 100 mg/kg/d for 2 wk; or L-AmB, 3-4 mg/kg/d; or ABLC, 5 mg/kg/d + 5-FC (100 mg/kg/d) for 2 wk |
AmB-d + FCZ FCZ + 5-FC |
| Consolidation: FCZ, 400 mg/d for 8 wk | ||
| CNS infection (transplant recipient) | Induction therapy: L-AmB, 3-4 mg/kg/d; or ABLC, 5 mg/kg/d + 5-FC, 100 mg/kg/d for 2 wk |
L-AmB, 6 mg/kg/d; or ABLC, 5 mg/kg/d for 4-6 wk |
| Consolidation therapy: FCZ, 400-800 mg/d for 8 wk | ||
| Maintenance therapy: FCZ, 200-400 mg/d for 6 mo-1 y | ||
| CNS infection (non-HIV, non-transplant recipient) | Induction: AmB-d, 0.7-1 mg/kg/d + 5-FC, 100 mg/kg/d for ≥ 4 wk; or AmB-d, 0.7-1 mg/kg/d for ≥ 6 wk; or L-AmB, 3-4 mg/kg/d; or ABLC, 5 mg/kg/d + 5-FC, if possible ≥ 4 wk; or AmB-d, 0.7 mg/kg/d + 5-FC, 100 mg/kg/d for 2 wk |
|
| Consolidation therapy: FCZ, 400-800 mg/d for 8 wk | ||
| Maintenance therapy: FCZ, 200 mg/d for 6 mo-1 y | ||
| Histoplasmosis | ||
| Acute pulmonary (moderately severe-severe) | LF-AmB, 3-5 mg/kg/d; or AmB, 0.7-1 mg/kg/d for 1-2 wk, then ITZ, 200 mg BID to finish 12 wk* |
|
| Progressive disseminated histoplasmosis (moderately severe to severe) | L-AmB, 3 mg/kg daily; or ABLC, 5 mg/kg daily; or AmB-d, 0.7-1 mg/kg/d for 1-2 wk; followed by ITZ, 200 mg BID for at least 1 y |
|
| Blastomycosis | ||
| Pulmonary (moderately severe to severe) | L-AmB, 3-5 mg/kg/d; or AmB-d, 0.7-1 mg/kg/d for 1-2 wk; followed by ITZ, 200 mg BID for 6-12 mo |
|
| Extrapulmonary (Disseminated) | ||
| CNS | L-AmB, 5 mg/kg/d for 4-6 wk is preferred; followed by an oral azole for at least 1 y | |
| Non-CNS (moderately severe to severe) | L-AmB, 3-5 mg/kg/d; or AmB-d, 0.7-1 mg/kg/d for 1-2 wk; followed by ITZ, 200 mg BID for 12 mo |
|
ABLC, amphotericin B lipid complex; AmB-d, amphotericin B deoxycholate; BID, twice daily; CNS, central nervous system; 5-FC, 5-fluorocytosine; FCZ, fluconazole; HIV, human immunodeficiency virus; ITZ, itraconazole; L-AmB, liposomal amphotericin B; LF-AmB, any marketed lipid amphotericin B formulation.
* Consider corticosteroids 60 mg × 2 wk.
Adapted from Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2010;50:291-322; Wheat LJ, Freifield AG, Kleiman MB et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45:807-25; and Chapman SW, Dismukes WE, Proia LA et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis 2008;46:1801-12.
Management of Increased ICP in CNS Cryptococcosis
Elevations in ICP occur in more than half of patients with cryptococcal meningitis and contribute significantly to the morbidity and mortality associated with this infection.92 There are much less data on treatment of HIV-negative patients with acute elevated ICP with regard to recommendations of pressure control. Therefore, ICP management may be underutilized in the management of non-HIV-infected patients with CNS cryptococcosis. Persistent elevations in ICP should be managed by sequential lumbar punctures.92 If necessary, more invasive procedures, including insertion of a lumbar drain or placement of a ventriculoperitoneal shunt, should be performed.92 The frequency with which sequential lumbar punctures are performed depends on the initial opening pressure and symptoms. For patients with elevated baseline opening pressure, lumbar puncture should be done to reduce the pressure 50% and performed daily to maintain the ICP in the normal range.92
Serum and CSF antigen titers are important in establishing the presumptive diagnosis and assessing the prognosis of CNS infection. The test measures cryptococcal polysaccharide capsule antigens but does not differentiate viable from nonviable organism. Therefore, once therapy is started, treatment decisions should not be based on antigen test results.92 A reduction in antigen titers during therapy is desired, but treatment decisions should be based on culture results.
Treatment of Histoplasmosis in Critically Ill Patients
The efficacy of AmB-d for therapy for disseminated histoplasmosis among immunocompetent patients is 70% to 90%. Therefore, AmB-d is recommended initially in severely ill patients. In a small study, all patients responded to itraconazole, 200 to 400 mg daily.109 Once an adequate response is noted to AmB-d, therapy can be switched to itraconazole.109 Few data exist concerning the efficacy of the lipid AmB formulations as therapy for disseminated histoplasmosis in immunocompetent patients. Recommended antifungal therapy for treatment of histoplasmosis in the ICU setting is summarized in Table 139-3.109
Treatment of Disseminated (Extrapulmonary) Blastomycosis in the Critically Ill
Disseminated blastomycosis and diffuse pulmonary infection are both associated with significant mortality. Treatment of these infections produces cure rates ranging from 85% to 90%, and the effective agents cause little associated toxicity.110 The optimal duration of therapy for the treatment of blastomycosis with existing antifungal agents is unknown and has been empirically derived from noncomparative studies and clinical experience. In cases of life-threatening infections or extrapulmonary disease and in patients who are severely immunocompromised or have already failed therapy with an azole, the risk of relapse is high.110 Therefore, the duration of therapy is lengthy to prevent relapse. Patients can be switched to safer azole therapy when significant improvement is observed.110 Pharmacologic treatment of blastomycosis in the ICU setting is summarized in Table 139-3.110
Key Points
Wey SB, Mori M, Pfaller MA, et al. Risk factors for hospital-acquired candidemia: a matched case-control study. Arch Intern Med. 1989;149:2349-2353.
Pittet D, Li Ning, Woolson RF, Wenzel RP. Microbiological factors influencing the outcome of nosocomial bloodstream infections: A 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068-1078.
Pelz RK, Hendix CW, Swoboda SM, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg. 2001;233:542-548.
Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25-31.
Golan Y, Wolf MP, Pauker SG, et al. Empirical anti-Candida therapy among selected patients in the intensive care unit: a cost-effectiveness analysis. Ann Intern Med. 2005;143:857-869.
1 Pfaller MA, Diekema DJ. Epidemiology of invasive Candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133-163.
2 Hidron AI, Edwards JR, Patel J, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the national healthcare safety network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996-1011.
3 Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309-317.
4 Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2005: an 8.5 year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2007;45:1735-1745.
5 Trick WE, Fridkin SK, Edwards JR, et al. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin Infect Dis. 2002;35:627-630.
6 Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695-1703.
7 Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1-53.
8 Ásmundsdóttir LR, Erlendsdóttir H, Haraldsson G, et al. Molecular epidemiology of candidemia: evidence of clusters of smoldering nosocomial infections. Clin Infect Dis. 2008;47:e17-e24.
9 Bliss JM, Basavegowda KP, Watson WJ, et al. Vertical and horizontal transmission of Candida albicans in very low birth weight infants using DNA fingerprinting techniques. Pediatr Infect Dis J. 2008;27:231-235.
10 Trofa D, Gacser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606-625.
11 Horvath LL, George BJ, Hospenthal DR. Detection of fifteen species of Candida in an automated blood culture system. J Clin Microbiol. 2007;45:3062-3064.
12 Bourbeau PP, Foltzer M. Routine incubation of BacT/ALERT FA and FN blood culture bottles for more than 3 days may not be necessary. J Clin Microbiol. 2005;43:2506-2509.
13 Miller PJ, Wenzel RP. Etiologic organisms as independent predictors of death and morbidity associated with bloodstream infections. J Infect Dis. 1987;156:471-477.
14 Wey SB, Mori M, Pfaller MA, et al. Risk factors for hospital-acquired candidemia: a matched case-control study. Arch Intern Med. 1989;149:2349-2353.
15 Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118:146-155.
16 Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infections in critically ill patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1994;271:1598-1601.
17 Pittet D, Li Ning, Woolson RF, et al. Microbiological factors influencing the outcome of nosocomial bloodstream infections: a 6-year validated, population-based model. Clin Infect Dis. 1997;24:1068-1078. 18
18 Spellberg BJ, Filler SG, Edwards JEJr. Current treatment strategies for disseminated candidiasis. Clin Infect Dis. 2006;42:244-251.
19 Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol. 2006;45:321-346.
20 Morgan J, Meltzer MI, Plikaytis BD, et al. Excess mortality, hospital stay, and cost due to candidemia: a case–control study using data from population–based candidemia surveillance. Infect Control Hosp Epidemiol. 2005;26:540-547.
21 Morrell M, Fraser VJ, Kollef MJ. Delaying empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for mortality. Antimicrob Agents Chemother. 2005;49:3640-3645.
22 Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25-31.
23 Armstrong-James D. Invasive Candida species infection: the importance of adequate empirical antifungal therapy. J Antimicrob Chemother. 2007;60:459-460.
24 Garey KW, Pai MP, Suda KJ, et al. Inadequacy of fluconazole dosing in patients with candidemia based on Infectious Diseases Society of America (IDSA) guidelines. Pharmacoepidemiol Drug Safety. 2007;16:919-927.
25 Parkins MD, Sabuda DM, Elsayed S, et al. Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infection. J Antimicrob Chemother. 2007;60:613-618.
26 Klevay MJ, Ernst EJ, Hollanbaugh JL, et al. Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diagn Microbiol Infect Dis. 2008;60:273-277.
27 Almirante B, Rodriguez D, Cuenca-Estrella M, et al. Epidemiology, risk factors, and prognosis of Candida parapsilosis bloodstream infections: case-control population-based surveillance study of patients in Barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2006;44:1681-1685.
28 Garroust-Orgeas M, Timsit JF, Tafflet M, et al. Excess risk of death from intensive care unit–acquired nosocomial bloodstream infections: a reappraised. Clin Infect Dis. 2006;42:1118-1126.
29 Chow JK, Golan Y, Ruthazer R, et al. Factors associated with candidemia caused by non-albicans Candida species versus Candida albicans in the intensive care unit. Clin Infect Dis. 2008;46:1206-1213.
30 Chow JK, Golan Y, Ruthazer R, et al. Risk factors for albicans and non-albicans candidemia in the intensive care unit. Crit Care Med. 2008;36:1993-1998.
31 Wenzel RP, Gennings C. Bloodstream infections due to Candida species in the intensive care unit: identifying especially high-risk patients to determine prevention strategies. Clin Infect Dis. 2005;41(Suppl.6):S389-S393.
32 Dupont H, Bourichon A, Paugam-Burtz C, et al. Can yeast isolation in peritoneal fluid be predicted in intensive care unit patients with peritonitis? Crit Care Med. 2003;31:752-757.
33 Paphitou NI, Ostrosky-Zeichner L, Rex JH. Rules for identifying patients at increased risk for candidal infections in the surgical intensive care unit: an approach to developing practical criteria for systematic use in antifungal prophylaxis trials. Med Mycol. 2005;43:235-243.
34 León C, Ruiz-Santana S, Saavedra P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in non-neutropenic critically ill patients with Candida colonization. Crit Care Med. 2006;34:730-737.
35 Ostrosky-Zeichner L, Sable C, Sobel J, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007;26:271-276.
36 León C, Ruiz-Santana S, Saavedra P, et al. Usefulness of the “Candida score” for discriminating between Candida colonization and invasive Candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624-1633.
37 Méan M, Marchetti O, Calandra T. Bench-to-bedside review: Candida infections in the intensive care unit. Crit Care. 2008;12:204-212.
38 Morgan J, Wannemuehler KA, Marr KA, et al. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med Mycol. 2005;43(Suppl.1):S49-S58.
39 Pagano L, Caira M, Picardi M, et al. Invasive aspergillosis in patients with acute leukemia: update on Morbidity and Mortality-SEIFEM-C Report. Clin Infect Dis. 2007;44:1524-1525.
40 Chamilos G, Luna M, Lewis RE, et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989-2003). Haematologica. 2006;91:986-989.
41 Gavalda J, Len O, San Juan R, et al. Risk factors for invasive aspergillosis in solid-organ transplant recipients: A case-control study. Clin Infect Dis. 2005;41:52-59.
42 Garcia-Vidal C, Upton A, Kirby KA, et al. Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis. 2008;47:1041-1050.
43 Neofytos D, Horn D, Anaissie E, et al. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter prospective antifungal therapy (PATH) alliance registry. Clin Infect Dis. 2009;48:265-273.
44 Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 7, 2010. [epub ahead of print]
45 Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373-379.
46 Woods G, Miceli MH, Grazziutti ML, et al. Serum Aspergillus galactomannan antigen values strongly correlate with outcome of invasive aspergillosis: a study of 56 patients with hematologic cancer. Cancer. 2007;110:830-834.
47 Miceli MH, Grazziutti ML, Woods G, et al. Strong correlation between serum Aspergillus galactomannan index and outcome of aspergillosis in patients with hematological cancer: clinical and research implications. Clin Infect Dis. 2008;46:1412-1422.
48 Maertens J, Buvé K, Theunissen K, et al. Galactomannan serves as a surrogate endpoint for outcome of pulmonary invasive aspergillosis in neutropenic hematology patients. Cancer. 2009;115:355-362.
49 Pazos C, Pontón J, Del Palacio A. Contribution of (1->3)-beta-D-glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. J Clin Microbiol. 2005;43:299-305.
50 Segal BH, Herbrecht R, Stevens DA, et al. Defining responses to therapy and study outcomes in clinical trials of invasive fungal diseases: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin Infect Dis. 2008;47:674-683.
51 Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350-1360.
52 Lortholary O, Poizat G, Zellar V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183-2191.
53 Singh N, Alexander BD, Lortholary O, et al. Cryptococcus neoformans in organ transplant recipients: impact of calcineurin-inhibitor agents on mortality. J Infect Dis. 2007;195:756-764.
54 Singh N, Dromer F, Perfect JR, et al. Cryptococcosis in solid organ transplant recipients: current state of the science. Clin Infect Dis. 2008;47:1321-1327.
55 Chu JH, Fuedtner C, Heydon K, et al. Hospitalizations for endemic mycoses: a population-based national study. Clin Infect Dis. 2006;42:822-825.
56 Parish JM, Blair JE. Coccidioidomycosis. Mayo Clin Proc. 2008;83:343-349.
57 Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.
58 Gallis HA, Drew RH, Pickard WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308-329.
59 Bekersky I, Fielding RM, Dressler DE, et al. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother. 2002;46:828-833.
60 Goodwin SD, Cleary JD, Walawander CA, et al. Pretreatment regimens for adverse events related to infusion of amphotericin B. Clin Infect Dis. 1995;20:755-761.
61 Saliba F, Dupont B. Renal impairment and amphotericin B formulations in patients with invasive fungal infections. Med Mycol. 2008;46:97-112.
62 Adler-Moore JP, Proffitt RT. Amphotericin B lipid preparations: what are the differences? Clin Microbiol Infect. 2008;14(Suppl. 4):25-36.
63 Paterson DL, David K, Mrsic M, et al. Pre-medication practices and incidence of infusion-related reactions in patients receiving AMPHOTEC: data from the Patient Registry of Amphotericin B Cholesteryl Sulfate Complex for Injection Clinical Tolerability (PRoACT) registry. J Antimicrob Chemother. 2008;62:1392-1400.
64 O’Connor N, Borley A. Prospective audit of the effectiveness of hydrocortisone premedication on drug delivery reactions following amphotericin B lipid complex. Curr Med Res Opin. 2009;25:749-754.
65 Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340:764-771.
66 Hachem RY, Boktour MR, Hanna HA, et al. Amphotericin B lipid complex versus liposomal amphotericin B monotherapy for Invasive aspergillosis in patients with hematologic malignancy. Cancer. 2008;112:1282-1287.
67 Pelz RK, Lipsett PA, Swoboda MS, et al. Enteral fluconazole is well absorbed in critically ill surgical patients. Surgery. 2002;131:534-540.
68 Rajagopalan P, Pelz RK, Lipsett PA, et al. Enteral fluconazole population pharmacokinetics in patients in the surgical intensive care unit. Pharmacotherapy. 2003;23:592-602.
69 Barquist ES, Gomez-Fein E, Block EFJ, et al. Bioavailability of oral fluconazole in critically ill abdominal trauma patients with and without abdominal wall closure: a randomized crossover clinical trial. J Trauma. 2007;63:159-163.
70 Barone JA, Moskovitz BL, Guarnieri J, et al. Enhanced bioavailability of itraconazole in hydroxypropyl-β-cyclodextrin solution versus capsules in healthy volunteers. Antimicrob Agents Chemother. 1998;42:1862-1865.
71 Scott LJ, Simpson D. Voriconazole: A review of its use in the management of invasive fungal infections. Drugs. 2007;67:269-298.
72 Von Mach MA, Burhenne J, Weilemann LS. Accumulation of the solvent vehicle sulphobutylether beta cyclodextrin sodium in critically ill patients treated with intravenous voriconazole under renal replacement therapy. BMC Clin Pharmacol. 2006;6:6.
73 Courtney R, Pai S, Laughlin M, et al. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob Agents Chemother. 2003;47:2788-2795.
74 Krishna G, Moton A, Ma L, et al. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob Agents Chemother. 2009;53:958-966.
75 Ezzet F, Wexler D, Courtney R, et al. Oral bioavailability of posaconazole in fasted healthy subjects: comparison between three regimens and basis for clinical dosage recommendations. Clin Pharmacokinet. 2005;44:211-220.
76 Courtney R, Wexler D, Radwanski E, et al. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br J Clin Pharmacol. 2003;57:218-222.
77 Sansone-Parsons A, Krishna G, Calzetta A, et al. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob Agents Chemother. 2006;50:1881-1883.
78 Rüping MJGT, Albermann N, Ebinger F, et al. Posaconazole concentrations in the central nervous system. J Antimicrob Chemother. 2008;62:1468-1470.
79 Nagappan V, Deresinski S. Posaconazole: a broad-spectrum triazole antifungal agent. Clinical Infect Dis. 2007;45:1610-1617.
80 Lestner JM, Roberts SA, Moore CB, et al. Toxicodynamics of itraconazole: implications for therapeutic drug monitoring. Clin Infect Dis. 2009;49:928-930.
81 Tan K, Brayshaw N, Tomaszewski K, et al. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse effects or liver function test abnormalities. J Clin Pharmacol. 2006;46:235-243.
82 Gubbins PO, Heldenbrand SD. Clinically relevant drug interactions of current antifungal agents. Mycoses. 2010;53:95-113.
83 Krieter P, Flannery B, Musick T, et al. Disposition of posaconazole following single-dose oral administration in healthy subjects. Antimicrob Agents Chemother. 2004;48:3543-3551.
84 Ghosal A, Hapangama N, Yuan Y, et al. Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of posaconazole (Noxafil). Drug Metab Dispos. 2004;32:267-271.
85 Wexler D, Courtney R, Richards W, et al. Effect of posaconazole on cytochrome P450 enzymes: a randomized, open-label, two-way crossover study. Eur J Pharm Sci. 2004;21:645-653.
86 Balani SK, Xu X, Arison B, et al. Metabolites of caspofungin acetate, a potent antifungal agent, in human plasma and urine. Drug Metab Disp. 2000;28:1274-1278.
87 Nguyen TH, Hoppe-Tichy T, Geiss HK. Factors influencing caspofungin plasma concentrations in patients of a surgical intensive care unit. J Antimicrob Chemother. 2007;60:100-106.
88 Hope WW, Shoham S, Walsh TJ. The pharmacology and clinical use of caspofungin. Expert Opin Drug Metab Toxicol. 2007;3:263-274.
89 Wiederhold NP, Lewis JS. The echinocandin micafungin: a review of the pharmacology, spectrum of activity, clinical efficacy and safety. Expert Opin Pharmacother. 2007;8:1155-1166.
90 Estes KE, Penzak SR, Calis KA, et al. Pharmacology and antifungal properties of anidulafungin, a new echinocandin. Pharmacotherapy. 2009;29:17-30.
91 Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:291-322.
92 Lass-Flörl C, Perkhofer S, Mayr A. In vitro susceptibility testing in fungi: a global perspective on a variety of methods. Mycoses. 2010;53:1-11.
93 Viscoli C. Antifungal prophylaxis and pre-emptive therapy. Drugs. 2009;69(Suppl 1):75-78.
94 Playford EG, Webster AC, Sorrell TC, et al. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother. 2006;57:628-638.
95 Eggimann P, Francioli P, Bille J, et al. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit Care Med. 1999;27:1066-1072.
96 Goodman JL, Winston DJ, Greenfield RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992;326:845-851.
97 Pelz RK, Hendix CW, Swoboda SM, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg. 2001;233:542-548.
98 Garbino J, Lew DP, Romand JA, et al. Prevention of severe Candida infections in non-neutropenic, high risk, critically ill patients: a randomized, double-blind, placebo controlled-trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002;28:1708-1717.
99 Shorr AF, Chung K, Jackson WL, et al. Fluconazole prophylaxis in critically ill surgical patients: A meta-analysis. Crit Care Med. 2005;33:1928-1935.
100 Cruciani M, de Lalla F, Mengoli C. Prophylaxis of Candida infections in adult trauma and surgical intensive care patients: a systematic review and meta-analysis. Intensive Care Med. 2005;31:1479-1487.
101 Lam SW, Eschenauer GA, Carver PL. Evolving role of early antifungals in the adult intensive care unit. Crit Care Med. 2009;37:1580-1593.
102 Pittet D, Monod M, Suter PM, et al. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994;220:751-758.
103 Piarroux R, Grenouillet F, Balvay P, et al. Assessment of preemptive treatment to prevent severe candidiasis in critically ill surgical patients. Crit Care Med. 2004;32:2443-2449.
104 Mora-Duarte J, Betts R, Rotstein C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002;347:2020-2029.
105 Golan Y, Wolf MP, Pauker SG, et al. Empirical anti-Candida therapy among selected patients in the intensive care unit: a cost-effectiveness analysis. Ann Intern Med. 2005;143:857-869.
106 Pappas PG, Kaufman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:503-535.
107 Marr KA. Fungal infections in hematopoietic stem cell transplant recipients. Med Mycol. 2008;46:293-302.
108 Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327-360.
109 Wheat LJ, Freifield AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:807-825.
110 Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801-1812.