CHAPTER 277 Fungal and Tubercular Infections of the Spine
Epidemiology
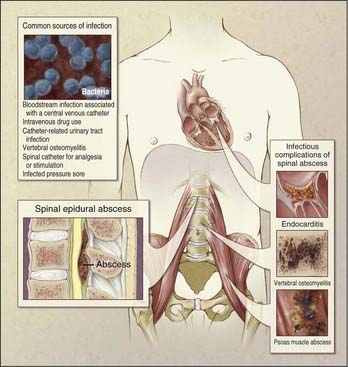
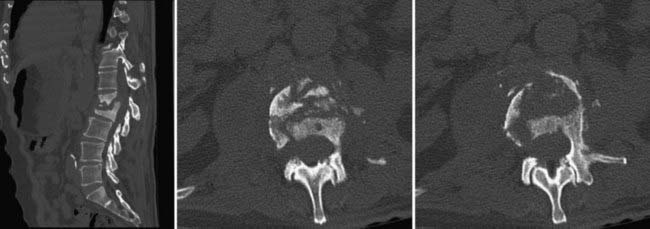
The incidence of fungal infections has increased greatly in recent years.1 This increase is thought to be due in part to the greater use of immunosuppressive drugs, more prevalent use of broad-spectrum antibiotics, and more widespread use of indwelling catheters, as well as the prevalence of acquired immunodeficiency syndrome (AIDS).2 The incidence of fungal and other nonbacterial infections is also increasing, not only because of the increasing number of immunocompromised patients but also because of the increasing use of intravenous therapy, central parenteral nutrition, surgery, and hemodialysis.3–5 Fungal infections of the spine are relatively uncommon but arise most commonly from hematogenous spread from another source, often the lung (Fig. 277-1).6–8 Additional routes of infection include direct spread from other organs or from contamination at the time of surgery.9,10 Unfortunately, in most industrialized countries the diagnosis of fungal infection is often delayed because of a variety of factors. This delay has been shown to lead to worse patient outcomes.11 Tubercular infections are simply so uncommon outside the Third World that they are often missed or misdiagnosed on initial evaluation. The importance of appropriate history taking, including a detailed travel history when such an infection is suspected, cannot be overemphasized.
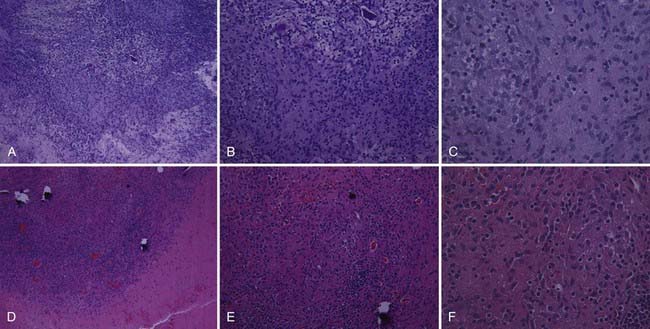
Candida (Fig. 277-2) is most commonly found in normal skin flora and within the gastrointestinal tract but can also exist in the sputum, female genital tract, and urine of patients with indwelling catheters.2 Candida and Aspergillus are the most common types of disseminated fungal infection in susceptible patients, and vertebral osteomyelitis is the most common form of bone infection.3,12–14 Ten species of Candida are pathogenic in humans; 62% of cases of candidal vertebral osteomyelitis are caused by Candia albicans, 19% by Candida tropicalis, and 14% by Candida glabrata.14 Because of the increased use of azole antifungals, the overall incidence of Candida infection of the spine is increasing,15 and Candida is reported to be responsible for approximately 0.7% to 2.7% of spine infections.16–18 The most common (83%14) initial complaint is back pain for more than 1 month. The lower thoracic and lumbar spine is most commonly involved.14
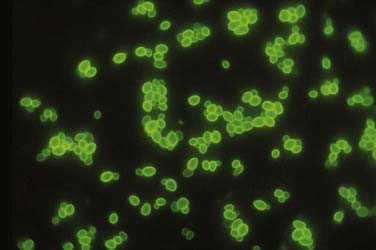
FIGURE 277-2 Appearance of oval budding yeast cells of Candida albicans with fluorescent antibody stain.
(Courtesy of Maxine Jalbert, Dr. Leo Kaufman, Centers for Disease Control and Prevention.)
Aspergillus is a ubiquitous fungus that creates small spores typically found in water, soil, decaying vegetation, hay, and grains,19 thereby allowing deposition into air conduction systems and subsequently human alveoli (Fig. 277-3).2 Although bone infection from disseminated Aspergillus is rare (<1%), the vertebral body is the most common site of such infection.20 The average delay in diagnosis is on the order of months.21 The most common species isolated in human infection is Aspergillus fumigatus.22 Even though Aspergillus most commonly infects the lung, those at risk for development of the disseminated form include patients with AIDS or chronic granulomatous disease and those who take antibiotics long term or abuse intravenous drugs.2 Spinal involvement in disseminated Aspergillus infection is uncommon. When it does infect the spine, the lumbar region is affected in 63% of cases.23 Aspergillus osteomyelitis typically arises as a result of hematogenous spread, extension from an adjacent organ, or direct inoculation at the time of surgery.24–27 Aspergillus vertebral osteomyelitis is similar to other fungal infections of the spine in that it has a male preponderance, bimodal age distribution, predilection for the lumbar spine, and back pain as the most common initial symptom.2
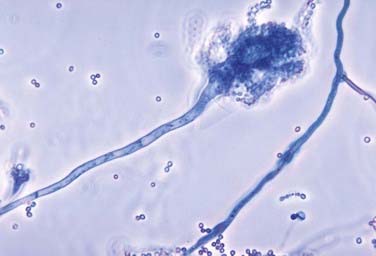
FIGURE 277-3 Photomicrograph depicting the appearance of a conidiophore of the fungus Aspergillus fumigatus.
(Courtesy of Centers for Disease Control and Prevention/Dr. Libero Ajello, Public Health Image Library.)
Cryptococcus infection is rare in immunocompetent hosts; however, 10% to 40% of human immunodeficiency virus (HIV)-negative patients with cryptococcosis have no underlying disease or deficiency.28,29 Although isolated cryptococcal osteomyelitis is rare, 5% to 10% of patients with disseminated central nervous system (CNS) cryptococcosis will have bone lesions.30 Cryptococcus neoformans (Fig. 277-4) is found in pigeon feces and in the soil2; it is the fourth most common infection in patients with HIV and is found in 1% to 5% of transplant patients.31 Typically, the pathogen enters the human host by way of inhalation and can remain dormant. Cryptococcus can then spread to the CNS and bone hematogenously and often resembles a cold abscess, similar to tuberculosis (TB).32 The lumbar spine is most often affected, followed by the cervical region.6 Other sites of osseous involvement include the femur, tibia, rib, and humerus.33 Unlike many other disseminated fungal infections, sinus tract and abscess formation are rare with Cryptococcus.33
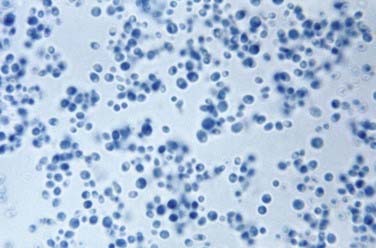
FIGURE 277-4 Photomicrograph depicting the yeast form of a Cryptococcus fungus.
(Courtesy of Centers for Disease Control and Prevention/Dr. Lucille K. Georg, Public Health Image Library.)
Blastomyces dermatitidis (Fig. 277-5) is found in soil and is believed to infect humans by way of inhalation of conidia.34 The pathogen is endemic to the southeastern and south central states, especially those bordering the Mississippi and Ohio River basins, and to the midwestern states bordering the Great Lakes.35 Like other fungal infections, Blastomyces spreads via the bloodstream and can affect any organ months to years after pulmonary infection.7 Even though the skin is the most common site of extrapulmonary infection, osseous involvement is seen in 14% to 60% of patients with disseminated infection. The spine is the most common bone site, followed by the skull, ribs, tibia, and bones of the wrist and foot.36,37 The lower thoracic and lumbar segments are affected most frequently, with the anterior aspect of the vertebral body being involved first. Infection can spread both via the intervertebral disk and along the anterior longitudinal ligament and affect other vertebral bodies; it can also lead to psoas or other paravertebral abscesses.2,33
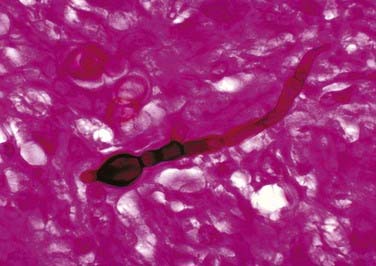
FIGURE 277-5 Micrograph showing the histopathologic changes that reveal the presence of the fungal agent Blastomyces dermatitidis.
(Courtesy of Centers for Disease Control and Prevention/Dr. Libero Ajello, Public Health Image Library.)
Coccidioides immitis (Fig. 277-6) is endemic to parts of the southwestern United States, including the San Joaquin Valley, as well as Central America and parts of South America.38,39 Humans most commonly become infected by inhalation of the spores and less commonly through abrasions of the skin.40 The primary focus of infection is typically in the lung, but in 0.5% of patients the disease is disseminated. Osseous involvement develops in 10% to 20% of those with disseminated disease, and spine involvement occurs in 10% to 60% of these individuals.8,24,33 Those at risk for the development of disseminated disease include Filipinos, African Americans, pregnant women in their third trimester, and younger and elderly individuals.40
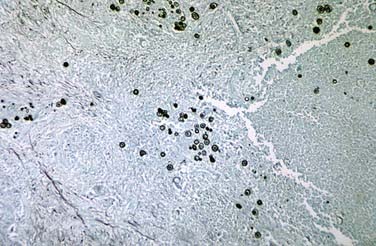
FIGURE 277-6 Methenamine silver–stained photomicrograph revealing spherules of Coccidioides immitis.
(Courtesy of Centers for Disease Control and Prevention/Dr. Martin D. Hicklin, Public Health Image Library.)
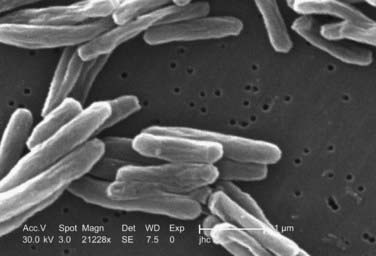
M. tuberculosis (Fig. 277-7) infects nearly one third of the world’s population. According to the World Health Organization (WHO), there were nearly 1.6 million deaths in 2005 alone as a result of TB, the most for any infectious disease. A person with active TB will infect between 10 and 15 other people every year, and according to WHO estimates, someone is newly infected with TB every second. Given these statistics, the burden of TB cannot be underestimated, and it causes significant morbidity when associated with disseminated disease to the spine.
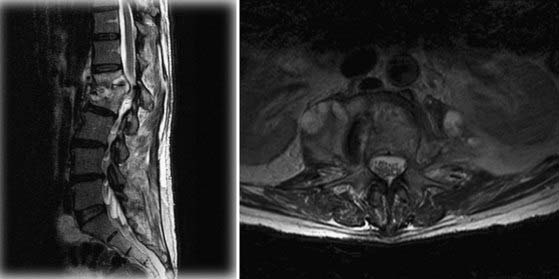
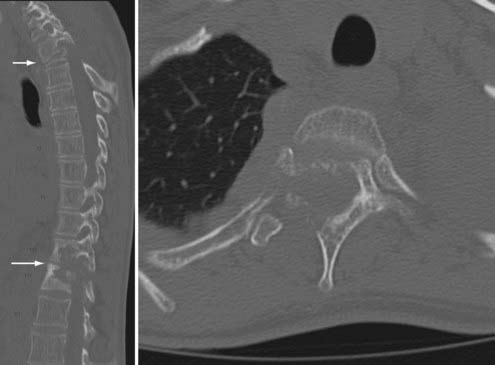
M. tuberculosis primarily infects the lungs through inhalation (Fig. 277-8) and is then spread throughout the body hematogenously. Because of their high blood flow, the vertebral bodies are particularly at risk for infection from disseminated M. tuberculosis. In fact, bone and joint infection account for as many as 10% to 35% of all cases of extrapulmonary TB, with 50% of all cases of musculoskeletal TB involving the spine.41–44 Spinal TB, also known as Pott’s disease, most often affects the lumbar and lower thoracic spine. One common complicating factor in spinal TB is tuberculous abscess (Fig. 277-9), which can often be bilateral.45,46 After primary infection there is ongoing evidence of active containment of reactivating foci by the TH1 cellular immune response, CD4 and CD8, lymphocytes, and interferon-γ.47 Certain factors such as poor nutrition, advancing age, HIV infection, or renal failure can predispose one to progression of disease, and thus active TB can develop immediately or after decades.48 In spinal TB, the focus of infection usually starts in the anterior-inferior aspect of the cancellous vertebral body, and inflammatory bone destruction and caseating necrosis ensue. Infection can then spread behind the anterior longitudinal ligament to infect the adjacent vertebral body. The infection can also spread to adjacent local structures and lead to a potentially compressive epidural abscess. Extraspinal abscesses can erode local structures such as ribs or can expand within the psoas muscle and track down as far as the groin (Fig. 277-10). The destructive capabilities of TB can lead to spinal deformity (Fig. 277-11) and thus create a further set of complications and unique need for intervention.
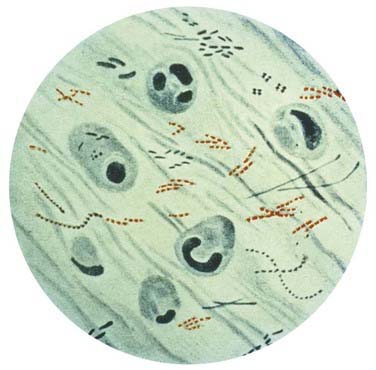
FIGURE 277-8 Photomicrograph of Mycobacterium tuberculosis from a sputum specimen viewed with Ziehl-Neelsen stain.
(Courtesy of Centers for Disease Control and Prevention, Public Health Image Library.)
Historical Perspective
TB has been recognized as an infectious disease process as far back as 4000 BC, with the features of spinal TB having been seen in Egyptian mummies dating back to that period.49 The Greco-Roman civilization knew the disease as phthisis or consumption.50 Paleopathologic evidence of TB of the bones and joints has been demonstrated.51 Hippocrates (400-300 BC) first formally reported TB of the spine with a description of gibbosity above and below the diaphragm.52 Early scientists and physicians in India also described the TB disease process as early as 3500 BC as “yakshma.”52,53 Percivall Pott then described spinal TB in 1779 as “that kind of palsy of the lower limbs which is frequently found to accompany a curvature of the spine.”53,54 Laennec, a French physician, discovered the microscopic pathogen and designated it a “tubercle.”53 Mycobacterium was discovered as the causative agent in 1870. Bacille Calmette-Guérin (BCG) vaccination was introduced in 1945, and specific antitubercular drugs came into common use between 1948 and 1951.50 Before the use of antitubercular drugs, treatment consisted of bed rest and immobilization in specialized hospitals known as “sanatoria,” with the average length of bed rest being 1 to 5 years.50 Treatment of TB of the bones and joints, including the spine, can be divided into three categories: (1) the pre–antitubercular era, where patients were treated by a variety of methods, including ancient medical/pharmacologic therapies, in addition to operative and nonoperative procedures; (2) the post–antitubercular era, where patients were universally treated by radical surgical intervention in conjunction with antitubercular drug therapy; and (3) the post–antitubercular era, where surgical intervention is reserved for those who fail nonoperative management with antitubercular drug therapy or for those with neurological or medical complications requiring surgical intervention.50
Early cases of TB allowed demonstration of the natural history and progression of the disease. For example, skeletal TB was divided into three stages. First, the “stage of onset” consisted of localized infection and a warm tender area of swelling with significant osteoporosis but minimal bone destruction. The second stage, known as the “stage of destruction,” consisted of progression of disease with bone destruction, deformity, subluxation, contracture, and abscess formation. The abscesses often developed draining sinuses that were susceptible to superimposed pyogenic infection, which significantly taxed the host immune system. Cachexia with miliary TB then often developed, as well as tubercular meningitis, thus popularizing the term consumption. Those who survived this stage entered the phase known as the “stage of repair and ankylosis,” in which the overall condition frequently improved. Unfortunately, frequent reactivation of the disease caused progression of symptoms and deformity.50 For those who underwent orthodox nonoperative treatment, only 30% to 44% were able to return to full working capacity.50 Surgery before the advent of antitubercular drugs was aimed at drainage of abscesses and almost uniformly resulted in poor outcomes, including persistent sinus formation, superimposed pyogenic infection, and death. Surgeons then began to pursue operations at sites distant from the location of infection to avoid opening the site of disease. Albee as early as 1911 and Hibbs as early as 1912 developed the technique of posterior spinal fusion in the hope of shortening the period of immobilization and bed rest by providing some degree of internal stability.50 Fusion resulted in some functional improvement in terms of stability but could not offer any benefit for those with neurological deficits.
The advent of antitubercular drugs with streptomycin in 1947, p-aminosalicylic acid in 1949, and isoniazid in 1952 signified a tremendous step forward in the fight against TB. Throughout the 1950s and 1960s, many surgeons continued to aggressively operate on spinal TB. Improved outcomes were due to the fact that antitubercular drugs prevented the dissemination of the disease at the time of surgery that was common before the use of antitubercular agents. As the use of antitubercular agents was becoming more commonplace, resolution of the draining sinuses and abscesses without surgical intervention could occur. Surgeons then began to reserve surgical intervention for prevention and correction of deformity, for patients with neurological deterioration or progressive disease, and in an attempt to improve functionality and the quality of life of severely affected patients.50
Pathophysiology
The various types of fungal and tubercular infections of the spine have variable mechanisms of entering the human host and thus progressing to infection of the spinal column. Most fungal infections that ultimately infect the spine first involve the lung. For example, Aspergillus, Cryptococcus, Coccidioides, Blastomyces, and M. tuberculosis all produce spores that can be inhaled and then spread to the spine, and these organisms can all secondarily infect the spine via hematogenous dissemination. After inhalation, Aspergillus can affect the spine by direct extension from lung tissue or by hematogenous spread. Another important source of infection for some of these pathogens is direct contamination at the time of surgery. Candida infection can occur in patients with intravenous lines or monitoring devices and during implantation of prosthetic devices.2 The difficulty in controlling some of these infections leads to the need for both medical/pharmacologic forms of treatment and possibly surgical intervention. Treatment is needed to both control the primary source and address the sequelae of destructive spine infections.
Clinical Findings
In both fungal and tubercular infections of the spine, the most common initial symptom is localized pain. Given that back pain is such a common complaint with an extremely varied series of causes, it is essential to perform a complete history and physical examination, including pulmonary examination in patients in whom a history of predisposing factors such as travel or immunosuppression should raise the index of suspicion for rare fungal or tubercular infections. For example, a history of previous fungemia, immunosuppression, diabetes, central venous catheterization, intravenous drug abuse, parenteral nutrition, or implanted prosthetic devices all place patients at higher risk for fungal infection of the spine in the setting of back pain.55,56 There are also multiple case reports of Aspergillus infection from contaminated air-handling systems.57 Other factors associated with increased risk for fungal osteomyelitis include diabetes, needle tracks signifying intravenous drug use, opportunistic infections, and weight loss.
Spinal TB is commonly manifested as back pain that progresses over a period of weeks to months. This pain may be associated with muscle spasm and has a mechanical nature in that it may be triggered by even the slightest of movements. This collection of symptoms has been described as causing the patient to have an “aldermanic gait,” or a rigid posture that induces the patient to walk with short deliberate steps to avoid any jarring of the spine.58 Constitutional symptoms such as fever and weight loss, though nonspecific, are present in less than 40% of patients with spinal TB.59,60 One important complication of spinal TB is cord compression. The incidence of neurological involvement in patients with spinal TB has been estimated to be 10% to 46%.61,62 The compression can result from M. tuberculosis itself during the active phase of the infection or be due to the spinal deformity created by the destructive nature of the infection (see Fig. 277-11). The neurological deficit known as Pott’s paraplegia can also be a result of arachnoiditis or vasculitis inducing an inflammatory spinal artery thrombosis.63,64 In industrialized countries, the index of suspicion for Pott’s disease is low,60 and thus late complications, including cord compression and resulting paraplegia, can often be the initial symptoms. However, even in countries in which TB is endemic, access to medical care is poor, and thus 40% to 70% of patients have symptoms of cord compression at the time of diagnosis.64 Late manifestations of Pott’s disease include psoas abscess, as well as rare late paraplegia as a result of degenerative changes at sites of previous infection.
According to Jain, two different types of neurological deficit are associated with TB of the spine: paraplegia in those with active disease (early onset) and paraplegia in those with healed disease (late onset) that appears many years after initial disease onset.62 Paraplegia in those with active disease usually develops as a result of local pressure on the spinal cord itself by tubercular abscess, granulation tissue, tubercular debris, and caseous tissue. Mechanical instability or the formation of kyphosis as a result of vertebral destruction, pathologic fracture, or subluxation can also contribute to neurological complications. The spinal cord itself may begin to display some signs of inflammation such as cord edema, or the disease burden may affect the meninges, which can trigger reactionary inflammatory changes in the cord that result in neurological compromise. In addition, vascular complications such as infective thrombosis or endarteritis of spinal vessels may lead to cord infraction and thus neurological complications. In those with neurological complications as a result of healed disease, the etiology is usually thought to be due to localized pressure on the cord by an osteophyte anterior to the cord or to inflammatory changes and resultant scarring of and around the dura. Stretching of the cord over an anterior internal gibbus can lead to spinal cord gliosis and ultimately neurological complications. It is important to point out, however, that the cord can accommodate remarkably well when the pressure placed on it is slowly progressive, as in TB. For example, Jain and coworkers found that in a case series of patients with spinal TB, the cord can often accommodate as much as 76% canal compromise without neurological deficit. Unfortunately, neurological sequelae can occur at much lesser degrees of canal compromise in the setting of vascular complication or mechanical instability.65
Tuli and associates developed a grading system for spinal TB that encompasses the following stages.66 In stage I the patient does not have any weakness but there is clumsiness of gait and a suggestion of upper motoneuron signs. In stage II the patient has weakness and clear upper motoneuron signs, but the patient is able to walk. In stage III the patient is bedridden because of total muscle weakness and maintains signs of upper motoneuron paraplegia. The patient also has less than 50% sensory loss. In stage IV patients have complete motor weakness, greater than 50% loss of sensation, loss of bowel/bladder control, or any combination of these findings, as well as probably flaccid paraplegia and possibly flexor spasm.
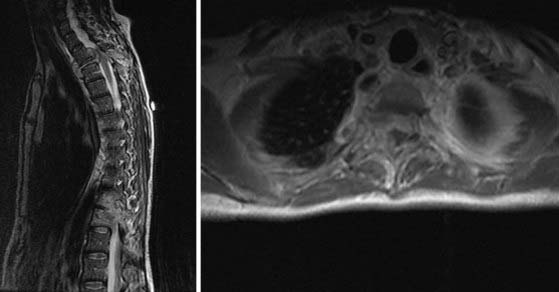
The radiographic appearance of fungal infections of the spine is much like that of the pyogenic causes of spinal osteomyelitis. For example, Williams and coauthors reported a case series of three immunocompromised patients with fungal infections of the spine.67 They described hypointensity on T1-weighted magnetic resonance imaging (MRI), which is typical of many infectious processes. They also noted a lack of hyperintensity on T2-weighted MRI, which is commonly seen in pyogenic infections. This lack of signal was thought to be the result of a depressed immune response in these immunocompromised individuals. Pyogenic infections typically show T2 hyperintensity in the intervertebral disk, whereas the absence of T2 hyperintensity in the intervertebral disk with preservation of the internuclear cleft is more typical of pathogens such as M. tuberculosis. It is also believed that the lack of hyperintensity is a result of the absence of proteolytic enzymes in Mycobacterium.68 Furthermore, involvement of the posterior elements is rare in pyogenic causes of spondylitis but is characteristic of other causes such as TB. It is important to point out that although the lack of hyperintensity with fungal infections may be the result of a blunted immune response, it may also represent the paramagnetic and ferromagnetic properties of the fungus itself, as seen in fungal sinusitis.67 The imaging characteristics of spinal TB are similar to those of a neoplasm, and thus the clinical history must be closely correlated not only to differentiate between pyogenic and nonpyogenic causes of spondylitis but also to distinguish the findings from a neoplasm. Two important characteristics that separate fungal/TB infections, as well as neoplastic processes, from pyogenic infections are sparing of the disk space on MRI and involvement of the posterior elements.69 M. tuberculosis, as well as Blastomyces, can commonly form paraspinal masses with an incidence as high as 55% to 96%.69 TB-associated paraspinal masses also tend to contain calcifications68 and exhibit thick irregular enhancement on computed tomography (CT).70 Another factor that makes tubercular infection difficult to distinguish from neoplastic disease is the fact that TB can involve three or more vertebral bodies in as many as 50% to 64% of patients.68–71 Actinomycosis can also spare the disk space and thus mimic TB or metastatic disease.68
It is important to mention that another key characteristic differentiating spinal TB from pyogenic infections is the duration of symptoms. Typically, TB develops over a period of 2 to 3 years, whereas pyogenic infections become symptomatic in days to months.69 Pyogenic osteomyelitis tends to occur in the sixth to seventh decades of life, whereas TB can develop much earlier in life. In addition, the geographic distribution of TB in less industrialized countries further underscores the importance of a thorough history from each patient.69
Radiographic evaluation of fungal infections of the spine parallels that of tubercular infections in that fungal infections tend to spare the disk space, involve the anterior vertebral body, and lead to the development of large soft tissue abscesses. However, they tend to involve adjacent ribs and posterior elements and develop draining sinuses less frequently.33,38 Paravertebral soft tissue swelling and involvement of the posterior elements are most common with Coccidioides infections.72 Vertebral body collapse and gibbus formation are common with Blastomyces infection,73 and the vertebral body lytic lesions associated with Cryptococcus infection can mimic both Coccidiomycosis infection and TB, including the formation of soft tissue abscesses.33,38,73 CT in patients with fungal osteomyelitis typically reveals hypodensities in the bone itself with small irregular bone islands, as opposed to the appearance of metastatic tumor, in which bone is completely replaced by tumor in larger segments.2
With so many radiographic similarities between fungal and tubercular infections of the spine it can be difficult to distinguish between these lesions for targeting of therapy. Scintigraphic and radionucleotide studies have not been shown to be helpful in differentiating among various pathogens, but other nonspecific markers such as the white blood cell count, erythrocyte sedimentation rate, and C-reactive protein level certainly help reveal the possibility of an infectious process.2,74,75 There are various commercially available antibody tests that have shown varying degrees of success in differentiating fungal causes, but tissue diagnosis via histopathologic examination is essential for establishing the appropriate diagnosis. Finding the correct diagnosis can be aided by other testing methods, such as the carbohydrate assimilation test for Candida and the phenol oxidase reaction for Cryptococcus.76
Tuberculin skin testing in immunocompetent patients with suspected skeletal TB reveals more than 90% to have a positive intermediate-strength skin test.77 However, a small number of immunocompetent patients will have a negative skin test, as will many immunocompromised patients. Therefore, a negative skin test does not exclude TB as a possible cause of infection. Furthermore, the tuberculin skin test is useless in countries in which the BCG vaccine is used. Patients with TB may have draining sinuses. Frequently, the draining exudate is colonized with bacteria or fungi, which when cultured may lead to an incorrect diagnosis. To further complicate diagnosis, 50% of patients with spinal TB have no signs of tubercular disease on chest radiography.44,60,78,79
Treatment
Treatment of fungal infections of the spine begins with correcting any factors that place the patient in an immunocompromised state or at risk for the development of such an infection. At the same time it is essential to begin antifungal therapy. Nonsurgical management is the mainstay of first-line treatment of some fungal infections of the spine, but several pathogens are best treated by surgical intervention for evacuation of abscesses, which yields higher clearance rates. Surgery is also required for patients with spinal instability, neurological deficit, or sepsis.80 Those who respond to antifungal agents typically have resolution of their back pain and frequently exhibit spontaneous fusion. Amphotericin B is often the first-line agent for many disseminated fungal infections. Many of those who suffer from disseminated fugal infection will require long-term antifungal therapy. The morbidity of amphotericin B therapy includes the fact that it must be given intravenously, as well as its potential for nephrotoxicity. Fortunately, the lipid formulation of amphotericin B has fewer side effects.
Candida osteomyelitis is best treated by surgical débridement and antifungal therapy.81 After surgical débridement, a 2- to 3-week course of amphotericin B (0.5 to 1 mg/kg per day), followed by fluconazole (6 mg/kg per day) for a total duration of 6 to 12 months of therapy, has been recommended.82 There are reports in the literature of successful treatment of Candida vertebral osteomyelitis with combined caspofungin and posaconazole therapy after the patient failed therapy with liposomal amphotericin B and then voriconazole.83
Successful outcomes in patients with Aspergillus osteomyelitis have been achieved with surgical débridement and treatment with amphotericin B,84,85 followed by a course of itraconazole.86 Voriconazole has also been used successfully as primary or salvage therapy after surgical débridement.87,88 Sratov and colleagues, in their review of the literature, revealed a 14% cure rate for Aspergillus osteomyelitis with amphotericin B alone and a 75% cure rate when combined with surgical débridement.88
Patients suffering from Cryptococcus osteomyelitis of the spine or other extrapulmonary cryptococcosis rarely require surgical intervention. However, because of the high preponderance of CNS infection in cryptococcosis, all patients with such an infection should undergo lumbar puncture to rule out CNS involvement.89 First-line therapy for cryptococcosis is amphotericin B in conjunction with flucytosine and ultimately fluconazole. Treatment of cryptococcosis differs depending on the patient’s HIV status. In patients with extrapulmonary or cryptococcal infection of bone, the addition of fluconazole is warranted, as it is in those who are HIV positive. Lifetime prophylaxis with fluconazole may depend on whether a patient’s immunocompromised state can be corrected. Likewise, those undergoing highly active antiretroviral therapy (HAART) for HIV should continue this therapy while receiving antifungal therapy.89
Treatment of blastomycosis depends on the severity of the infection. Unfortunately, there are no clear guidelines for grading its severity, so clinical judgment must suffice. CNS involvement also determines the form of amphotericin B to use. Current recommendations for treatment of blastomycosis include the use of either the lipid formula of amphotericin B (3 to 5 mg/kg per day) for those with CNS involvement or amphotericin deoxycholate (0.7 to 1.0 mg/kg per day) for 1 to 2 weeks or until clinical improvement is observed, followed by itraconazole (200 mg orally three times daily for 3 days and then 200 mg orally twice daily) for a total of 12 months.90
Therapy for extrapulmonary coccidioidomycosis usually begins with an oral antifungal agent such as fluconazole, itraconazole, or ketoconazole (400 mg/day); however, in particularly critical sites of extrapulmonary involvement such as the vertebral column, amphotericin B is typically needed, and in most cases of Coccidioides osteomyelitis, surgical intervention is required.91 For those who have associated meningitis, higher doses of the oral antifungal may be needed. Intrathecal therapy may also be required in certain cases.
First-line therapy for TB consists of combination therapy with isoniazid, rifampin, pyrazinamide, and ethambutol. Multidrug resistance in skeletal TB is a serious concern and is beginning to parallel the resistance profiles for pulmonary TB. Provision of appropriate medical therapy for TB has to overcome a variety of challenges, including poor compliance in the most at-risk patient populations and unreliable drug delivery, which generates greater resistance profiles. In some countries, directly observed treatment with fixed-dose multidrug combinations has resulted in improved results.92–98
Surgical intervention for fungal and tubercular infections of the spine is often required in patients with spinal cord and nerve root compression causing neurological deficit, in the setting of instability or deformity, and for the treatment of overwhelming infection. Moreover, the diagnosis is frequently difficult to made without open surgical débridement and culture of the offending pathogen. Obtaining a tissue diagnosis is essential for targeted antimicrobial therapy (Fig. 277-12). The goals of surgical intervention include aggressive débridement, relief of neural compression, and return of spinal stability. Advancements in imaging modalities have improved the ability to diagnose earlier stages of infections of various causes and have made nonoperative management more effective.99 If progressive disease develops as evidenced by new radiographically apparent disease burden or clinical deterioration, surgical intervention must be considered. Studies have shown that both nonoperative and operative management strategies have produced similar favorable results in those with one- to two-level disease.100 However, it is important to point out that in many developing countries, many patients may initially be seen with an advanced and extensive disease burden because of lack of access to medical care. Instrumented fusion has helped improve some of these outcomes, but access to the resources to perform these operations is limited in many of the countries in which fungal and tubercular infections of the spine are most common. Although many surgeons recommend immediate surgical intervention for all patients with spinal infection and neurological deficits, a 30% to 40% rate of neural recovery while waiting and preparing for surgery has been reported.65,101,102
Spinal stability in general can be difficult to define in patients with nontraumatic pathologies. In the setting of spinal TB, Jain and Sinha defined spinal instability as injury to at least two columns, as with trauma.103 In the setting of an infection such as spinal TB, ongoing and chronic inflammation superimposes an active healing process on a chronic fracture, and thus a fracture attributable to spinal TB may not be inherently unstable. However, an acute pathologic fracture in such a setting may indeed render the spine unstable. Likewise, disease burden involving both the vertebral body and posterior elements renders the spine at least moderately unstable and could thus allow the spinal column to translate.104 Long-segment disease involving more than three levels is also considered to be potentially unstable because of the possibility of the development of significant kyphosis and potential neurological injury.104
Spinal cord compression is often viewed as one of the indications for surgical decompression. It is important to point out, however, that the cord itself has been shown to be able to tolerate as much as 76% canal encroachment and yet maintain full neurological function.65 Furthermore, the neurological deficits in patients with spinal infections are often multifactorial in that the neurological dysfunction may be a result of not only physical compression on the spinal cord by abscess, granulation tissue, caseous tissue, or sequestered bone but also vascular compromise secondary to infective thrombosis or endarteritis. Other causes include intrinsic cord abnormalities such as edema or myelomalacia. Jain and coauthors reported that patients who have MRI evidence of edema or myelitis within the spinal cord and whose compressive lesion is predominantly fluid in the extradural space will respond well to nonoperative therapy.65 Conversely, patients with findings of extradural compression from a lesion that appears to be mostly granulation or caseous tissue, one that compresses the cord circumferentially, cord edema, myelitis, or myelomalacia are more likely to be candidates for early surgical intervention.104
In the setting of paralysis, known as Pott’s paraplegia in patients with tubercular infection, surgery may be required to provide the best opportunity for neurological improvement. It is generally thought that chemotherapy alone is inappropriate for Pott’s paraplegia in that a patient’s neurological status can improve rapidly after adequate decompression.64,105 Anterior or posterior decompression (or both) of the spinal cord in the setting of Pott’s paraplegia can frequently improve one’s neurological function, but such improvement is influenced by many factors. The likelihood of neurological recovery is influenced by the patient’s age; general medical condition; status of the spinal cord; the level, duration, and severity of paraplegia; the time from onset to the initiation of therapy; the type of treatment; and drug sensitivity.106,107 Conditions that have been found to be less responsive to surgical intervention in terms of neurological recovery include paralysis lasting longer than 6 months, late-onset paralysis with inactive disease and significant deformity, paralysis as a result of vascular injury to the spinal cord, and an atrophic-appearing spinal cord seen on MRI.108 Adults with severe spinal deformity also had worse return of neurological function than did those with milder deformity.107,109 The full imaging armamentarium should be used when possible, including CT, MRI, scoliosis radiographs, myelography, and even spinal angiography, when evaluating such patients to properly counsel them regarding the likelihood of neurological recovery.108 It is important to point out, however, that the incidence of paraplegia with residual spinal deformity is nearly 20% once the spine is infected with TB. This figure has not changed significantly, even with improving knowledge of the disease process.110
The purpose of surgery is to fully decompress the spinal cord and reconstruct the spinal column when necessary. Although surgical intervention can serve many purposes, an essential element is débridement, which has been defined as the removal of purulent material, granulation tissue, caseous tissue, and loose sequestered bone from the lesions.104 Conversely, Jain and colleagues have shown a need for caution in undertaking surgical intervention for such an infection in that radical resection and less aggressive débridement have been associated with similar rates of neurological improvement in short-segment disease.104 However, Upadhyay and coworkers have shown that the more extensive approach, radical resection and the use of bone grafting, can provide better correction of deformity than possible with débridement alone.111 It is thus important to tailor each operation to the individual patient and extent of disease.
The choice of surgical approach is very much an individual surgeon’s preference. Jain and Dhammi suggested that anterior vertebral lesions should be approached anteriorly.104 They justified this notion by pointing out the destructive consequences of disrupting the posterior elements that provide the only stability in such a case, as well as the limited morbidity associated with the use of video-assisted thoracoscopic decompression.112,113 However, when patients are anemic, have extensive disease, or suffer from compromised pulmonary function because of pulmonary disease or paralyzed intercostal muscles, the risk associated with transthoracic surgery is greatly increased.114,115 Posterior-only approaches can often avoid the pulmonary consequences of a transthoracic approach. The approach for surgical correction depends on the surgeon but must address both the anterior pathology and the need for adequate débridement, including possible corpectomy, as well as the fact that over time the spinal column shortens with bone destruction and the spinal cord accommodates accordingly. Posterior correction must take this into account and shorten the posterior portion of the spine to prevent stretching the cord and risking neurological injury.116 There are a variety of options for approaching both the anterior and posterior columns, with comparable rates of success in both single- and multiple-stage procedures. Anterolateral-only, posterior-only, and combined anterior and posterior approaches have all been used successfully in single-stage or multistage procedures, with each having their own set of advantages and disadvantages (Fig. 277-13).110,116,117 A surgeon should select an approach in which the goals of débridement of infection, neurological decompression, spinal column stabilization, and correction of deformity with the least morbidity for the patient can be accomplished.
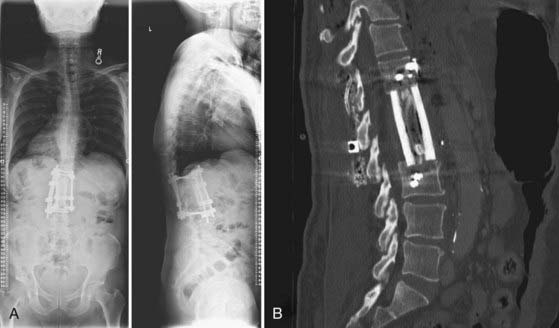
FIGURE 277-13 A, Standing anteroposterior (left) and lateral (right) radiographs after anterior L1-2 corpectomy, evacuation of vertebral/spinal and psoas abscesses, T12-L3 instrumented anterior spinal fusion through a left-sided retroperitoneal approach, and T12-L3 instrumented posterior spinal fusion with bone morphogenic protein, locally harvested autograft, and allograft cancellous chips. Note also the correction of prior kyphosis. B, Sagittal computed tomography scan of an instrumented construct demonstrating a combined autograft (harvested rib from the lateral approach) and allograft (cadaveric femur) interbody graft. The rib is placed inside the femoral marrow channel. Compare with the preoperative images seen in Figures 277-10 and 277-11.
Correction of kyphosis in patients with Pott’s disease is a difficult issue and is indicated for those in whom neurological deterioration has resulted from progressive kyphosis or in situations in which the kyphosis has created cardiopulmonary complications or painful costopelvic impingement. Late-onset paraplegia is more commonly seen in patients with long-standing healed kyphosis of 60 degrees or greater.118 Unfortunately, in patients with long-standing spinal disease the spinal cord itself has probably undergone multifactorial intrinsic changes that may not be as responsive to correction of kyphosis in terms of neurological function.62,118,119 Given the poor results associated with long-standing disease, it is important to prevent severe kyphosis as early as possible, and thus correction should be performed in the early, active stage of the disease (Figs. 277-14 and 277-15).65 Correction of kyphosis for cosmesis alone should not be done because it is associated with a high complication rate and poor durability.106 There are a few radiographic indicators of high risk for progressive deformity and thus a potential need for surgical intervention, including subluxation or dislocation of the facet joint at the apex of the kyphosis, the presence of retropulsion, translation of vertebrae in the coronal plane, and the presence of the posterior toppling sign (a line drawn along the anterior border of the distal healthy vertebra in the coronal plane that intersects with the upper half of the proximal healthy vertebra signifies instability).120 If two or more of these signs are present, the spine is potentially unstable and thus the kyphosis is probably progressive and should be corrected.120 Progressive radiographic kyphosis should also be considered for surgical correction.
The use of segmental instrumentation has greatly enhanced the ability of the spine surgeon to effectively correct the deformity resulting from destructive long-segment vertebral disease (see Fig. 277-13). It is important to mention that the use of instrumentation in the setting of bacterial osteomyelitis has been deemed safe, and thus similar principles may apply to the use of instrumented correction of deformity in patients with fungal and tubercular infections of the spine. Oga and coworkers have shown that is also safe to use instrumentation in the setting of TB.121 Hsieh and colleagues outlined several strategies for the treatment of bacterial osteomyelitis that are probably applicable to fungal and tubercular infections of the spine.122 For example, ultimate clearance of infection may best be facilitated with titanium instrumentation rather than stainless steel because of the porous nature of titanium, which allows attachment of vascularized soft tissue and thus the delivery of antimicrobial agents. Stainless steel has been shown to develop an encasing glycocalyx pseudocapsule that may harbor infective pathogens.122 They also discussed the advantages of adequate drainage at the time of closure, as well as the importance of using vascularized pedicled soft tissue flaps to ensure appropriate closure, in addition to providing blood supply to devitalized tissue.122
Other studies have shown the beneficial effects of using recombinant human bone morphogenetic protein-2 (rhBMP-2) for pyogenic osteomyelitis, which may have similar applications in fungal and tubercular infections of the spine. O’Shaughnessy and associates demonstrated high rates of fusion, as well as eradication of infection, with the use of rhBMP-2 for bacterial osteomyelitis.123 Although these studies are in reference to predominantly bacterial causes, they may have some application to the treatment of other causes of vertebral osteomyelitis. It should also be noted that the use of osteobiologic agents in the setting of infection is off label and contraindicated on the package labeling.
Bick K. Classics of Orthopedics. Philadelphia: JB Lippincott; 1976.
Chapman S. Blastomyces dermatitidis. In: Principles and Practice of Infectious Disease. New York: Churchill Livingstone; 1995.
Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801.
Daniel TM. The history of tuberculosis. Respir Med. 2006;100:1862.
Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658.
Hsieh PC, Wienecke RJ, O’Shaughnessy BA, et al. Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg Focus. 2004;17(6):E4.
Jain AK. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop Relat Res. 2002;398:75.
Jain AK, Aggarwal A, Mehrotra G. Correlation of canal encroachment with neurological deficit in tuberculosis of the spine. Int Orthop. 1999;23:85.
Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39.
Jain AK, Maheshwari AV, Jena S. Kyphus correction in spinal tuberculosis. Clin Orthop Relat Res. 2007;460:117.
Kim CW, Perry A, Currier B, et al. Fungal infections of the spine. Clin Orthop Relat Res. 2006;444:92.
Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, 6th ed. Philadelphia: Elsevier; 2005.
Moon MS. Tuberculosis of the spine. Controversies and a new challenge. Spine. 1997;22:1791.
Moon MS, Moon JL, Moon YW, et al. Pott’s paraplegia in patients with severely deformed dorsal or dorsolumbar spines: treatment and prognosis. Spinal Cord. 2003;41:164.
Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83:243.
O’Shaughnessy BA, Kuklo TR, Ondra SL. Surgical treatment of vertebral osteomyelitis with recombinant human bone morphogenetic protein-2. Spine. 2008;33:E132.
Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161.
Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710.
Smith AS, Weinstein MA, Mizushima A, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol. 1989;153:399.
Tuli SM. Management and Results, 2nd ed. New Delhi, India: Jaypee Brothers Medical Publishers; 1997.
Volmink J, Garner P. Systematic review of randomised controlled trials of strategies to promote adherence to tuberculosis treatment. BMJ. 1997;315:1403.
Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327.
1 VandenBergh MF, Verweij PE, Voss A. Epidemiology of nosocomial fungal infections: invasive aspergillosis and the environment. Diagn Microbiol Infect Dis. 1999;34:221.
2 Kim CW, Perry A, Currier B, et al. Fungal infections of the spine. Clin Orthop Relat Res. 2006;444:92.
3 Cha JG, Hong HS, Koh YW, et al. Candida albicans osteomyelitis of the cervical spine. Skeletal Radiol. 2008;37:347.
4 Khazim RM, Debnath UK, Fares Y. Candida albicans osteomyelitis of the spine: progressive clinical and radiological features and surgical management in three cases. Eur Spine J. 2006;15:1404.
5 Waldvogel FA, Papageorgiou PS. Osteomyelitis: the past decade. N Engl J Med. 1980;303:360.
6 Jain M, Sharma S, Jain TS. Cryptococcosis of thoracic vertebra simulating tuberculosis: diagnosis by fine-needle aspiration biopsy cytology—a case report. Diagn Cytopathol. 1999;20:385.
7 Kuzo RS, Goodman LR. Blastomycosis. Semin Roentgenol. 1996;31:45.
8 McGahan JP, Graves DS, Palmer PE. Coccidioidal spondylitis: usual and unusual radiographic manifestations. Radiology. 1980;136:5.
9 Salvalaggio PR, Bassetti M, Lorber MI, et al. Aspergillus vertebral osteomyelitis after simultaneous kidney-pancreas transplantation. Transpl Infect Dis. 2003;5:187.
10 Tack KJ, Rhame FS, Brown B, et al. Aspergillus osteomyelitis. Report of four cases and review of the literature. Am J Med. 1982;73:295.
11 Kushwaha VP, Shaw BA, Gerardi JA, et al. Musculoskeletal coccidioidomycosis. A review of 25 cases. Clin Orthop Relat Res. 1996;332:190.
12 Arias F, Mata-Essayag S, Landaeta ME, et al. Candida albicans osteomyelitis: case report and literature review. Int J Infect Dis. 2004;8:307.
13 Gathe JCJr, Harris RL, Garland B, et al. Candida osteomyelitis. Report of five cases and review of the literature. Am J Med. 1987;82:927.
14 Miller DJ, Mejicano GC. Vertebral osteomyelitis due to Candida species: case report and literature review. Clin Infect Dis. 2001;33:523.
15 Fidel PLJr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999;12:80.
16 Calvo JM, Ramos JL, Garcia F, et al. Pyogenic and non-pyogenic vertebral osteomyelitis: descriptive and comparative study of a series of 40 cases. Enferm Infecc Microbiol Clin. 2000;18:452.
17 McHenry MC, Easley KA, Locker GA. Vertebral osteomyelitis: long-term outcome for 253 patients from 7 Cleveland-area hospitals. Clin Infect Dis. 2002;34:1342.
18 Rodriguez D, Pigrau C, Almirante B, et al. Vertebral osteomyelitis due to Candida spp. Enferm Infecc Microbiol Clin. 2003;21:568.
19 Vinas FC, King PK, Diaz FG. Spinal Aspergillus osteomyelitis. Clin Infect Dis. 1999;28:1223.
20 Dayan L, Sprecher H, Hananni A, et al. Aspergillus vertebral osteomyelitis in chronic leukocyte leukemia patient diagnosed by a novel panfungal polymerase chain reaction method. Spine J. 2007;7:615.
21 Son JM, Jee WH, Jung CK, et al. Aspergillus spondylitis involving the cervico-thoraco-lumbar spine in an immunocompromised patient: a case report. Korean J Radiol. 2007;8:448.
22 van Ooij A, Beckers JM, Herpers MJ, et al. Surgical treatment of Aspergillus spondylodiscitis. Eur Spine J. 2000;9:75.
23 Hummel M, Schuler S, Weber U, et al. Aspergillosis with Aspergillus osteomyelitis and diskitis after heart transplantation: surgical and medical management. J Heart Lung Transplant. 1993;12:599.
24 Beaudoin MG, Klein L. Epidural abscess following multiple spinal anaesthetics. Anaesth Intensive Care. 1984;12:163.
25 Hendrix WC, Arruda LK, Platts-Mills TA, et al. Aspergillus epidural abscess and cord compression in a patient with aspergilloma and empyema. Survival and response to high dose systemic amphotericin therapy. Am Rev Respir Dis. 1992;145:1483.
26 Wagner DK, Varkey B, Sheth NK, et al. Epidural abscess, vertebral destruction, and paraplegia caused by extending infection from an aspergilloma. Am J Med. 1985;78:518.
27 Witzig RS, Greer DL, Hyslop NEJr. Aspergillus flavus mycetoma and epidural abscess successfully treated with itraconazole. J Med Vet Mycol. 1996;34:133.
28 Diamond RD, Bennett JE. Disseminated cryptococcosis in man: decreased lymphocyte transformation in response to Cryptococcus neoformans. J Infect Dis. 1973;127:694.
29 Sorensen RU, Boehm KD, Kaplan D, et al. Cryptococcal osteomyelitis and cellular immunodeficiency associated with interleukin-2 deficiency. J Pediatr. 1992;121:873.
30 Al-Tawfiq JA, Ghandour J. Cryptococcus neoformans abscess and osteomyelitis in an immunocompetent patient with tuberculous lymphadenitis. Infection. 2007;35:377.
31 Singh N, Gayowski T, Marino IR. Successful treatment of disseminated cryptococcosis in a liver transplant recipient with fluconazole and flucytosine, an all oral regimen. Transpl Int. 1998;11:63.
32 Liu PY. Cryptococcal osteomyelitis: case report and review. Diagn Microbiol Infect Dis. 1998;30:33.
33 Goldman AB, Freiberger RH. Localized infectious and neuropathic diseases. Semin Roentgenol. 1979;14:19.
34 Chapman S. Blastomyces dermatitidis. In: Principles and Practice of Infectious Disease. New York: Churchill Livingstone; 1995.
35 Bradsher RW. Blastomycosis. Clin Infect Dis. 1992;14(suppl 1):S82.
36 Hadjipavlou AG, Mader JT, Nauta HJ, et al. Blastomycosis of the lumbar spine: case report and review of the literature, with emphasis on diagnostic laboratory tools and management. Eur Spine J. 1998;7:416.
37 Riegler HF, Goldstein LA, Betts RF. Blastomycosis osteomyelitis. Clin Orthop Relat Res. 1974;100:225.
38 Pritchard DJ. Granulomatous infections of bones and joints. Orthop Clin North Am. 1975;6:1029.
39 Winter WGJr, Larson RK, Zettas JP, et al. Coccidioidal spondylitis. J Bone Joint Surg Am. 1978;60:240.
40 Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149.
41 Fanning A. Tuberculosis: 6. Extrapulmonary disease. CMAJ. 1999;160:1597.
42 Sharma SK, Mohan A. Extrapulmonary tuberculosis. Indian J Med Res. 2004;120:316.
43 Teo HE, Peh WC. Skeletal tuberculosis in children. Pediatr Radiol. 2004;34:853.
44 Watts HG, Lifeso RM. Tuberculosis of bones and joints. J Bone Joint Surg Am. 1996;78:288.
45 Lifeso RM, Weaver P, Harder EH. Tuberculous spondylitis in adults. J Bone Joint Surg Am. 1985;67:1405.
46 Weaver P, Lifeso RM. The radiological diagnosis of tuberculosis of the adult spine. Skeletal Radiol. 1984;12:178.
47 Kaufmann SH, Cole ST, Mizrahi V, et al. Mycobacterium tuberculosis and the host response. J Exp Med. 2005;201:1693.
48 Ellner JJ. Review: the immune response in human tuberculosis—implications for tuberculosis control. J Infect Dis. 1997;176:1351.
49 Daniel TM. The history of tuberculosis. Respir Med. 2006;100:1862.
50 Tuli SM. Tuberculosis of the spine: a historical review. Clin Orthop Relat Res. 2007;460:29.
51 Lichtor J, Lichtor A. Paleopathological evidence suggesting pre-Columbian tuberculosis of the spine. J Bone Joint Surg Am. 1957;39:1398.
52 Duraiswami PK, Orth M, Tuli SM. 5000 years of orthopaedics in India. Clin Orthop Relat Res. 1971;75:269.
53 Bick K. Classics of Orthopedics. Philadelphia: JB Lippincott; 1976.
54 Tuli SM. Tuberculosis of the Skeletal System, 3rd ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2004.
55 Friedman BC, Simon GL. Candida vertebral osteomyelitis: report of three cases and a review of the literature. Diagn Microbiol Infect Dis. 1987;8:31.
56 O’Connell CJ, Cherry AV, Zoll JG. Letter: Osteomyelitis of cervical spine: Candida guilliermondii. Ann Intern Med. 1973;79:748.
57 Lutz BD, Jin J, Rinaldi MG, et al. Outbreak of invasive Aspergillus infection in surgical patients, associated with a contaminated air-handling system. Clin Infect Dis. 2003;37:786.
58 Lifeso R. Atlanto-axial tuberculosis in adults. J Bone Joint Surg Br. 1987;69:183.
59 Hodgson SP, Ormerod LP. Ten-year experience of bone and joint tuberculosis in Blackburn 1978-1987. J R Coll Surg Edinb. 1990;35:259.
60 Nussbaum ES, Rockswold GL, Bergman TA, et al. Spinal tuberculosis: a diagnostic and management challenge. J Neurosurg. 1995;83:243.
61 Hodgson AR, Skinsnes OK, Leong CY. The pathogenesis of Pott’s paraplegia. J Bone Joint Surg Am. 1967;49:1147.
62 Jain AK. Treatment of tuberculosis of the spine with neurologic complications. Clin Orthop Relat Res. 2002;398:75.
63 Janssens JP, de Haller R. Spinal tuberculosis in a developed country. A review of 26 cases with special emphasis on abscesses and neurologic complications. Clin Orthop Relat Res. 1990;257:67.
64 Hsu LC, Leong JC. Tuberculosis of the lower cervical spine (C2 to C7). A report on 40 cases. J Bone Joint Surg Br. 1984;66:1.
65 Jain AK, Aggarwal A, Mehrotra G. Correlation of canal encroachment with neurological deficit in tuberculosis of the spine. Int Orthop. 1999;23:85.
66 Tuli SM. Management and Results, 2nd ed. New Delhi, India: Jaypee Brothers Medical Publishers; 1997.
67 Williams RL, Fukui MB, Cidis Meltzer C, et al. Fungal spinal osteomyelitis in the immunocompromised patient: MR findings in three cases. AJNR Am J Neuroradiol. 1999;20:381.
68 Chapman M, Murray RO, Stoker DJ. Tuberculosis of the bones and joints. Semin Roentgenol. 1979;14:266.
69 Smith AS, Weinstein MA, Mizushima A, et al. MR imaging characteristics of tuberculous spondylitis vs vertebral osteomyelitis. AJR Am J Roentgenol. 1989;153:399.
70 Whelan MA, Naidich DP, Post JD, et al. Computed tomography of spinal tuberculosis. J Comput Assist Tomogr. 1983;7:25.
71 Raininko RK, Aho AJ, Laine MO. Computed tomography in spondylitis. CT versus other radiographic methods. Acta Orthop Scand. 1985;56:372.
72 Govender S, Mutasa E, Parbhoo AH. Cryptococcal osteomyelitis of the spine. J Bone Joint Surg Br. 1999;81:459.
73 Gehweiler JA, Capp MP, Chick EW. Observations on the roentgen patterns in blastomycosis of bone. A review of cases from the Blastomycosis Cooperative Study of the Veterans Administration and Duke University Medical Center. Am J Roentgenol Radium Ther Nucl Med. 1970;108:497.
74 Frazier DD, Campbell DR, Garvey TA, et al. Fungal infections of the spine. Report of eleven patients with long-term follow-up. J Bone Joint Surg Am. 2001;83:560.
75 Rothman SL. The diagnosis of infections of the spine by modern imaging techniques. Orthop Clin North Am. 1996;27:15.
76 O’Shaughnessy EM, Shea YM, Witebsky FG. Laboratory diagnosis of invasive mycoses. Infect Dis Clin North Am. 2003;17:135.
77 Berney S, Goldstein M, Bishko F. Clinical and diagnostic features of tuberculous arthritis. Am J Med. 1972;53:36.
78 Davidson PT, Horowitz I. Skeletal tuberculosis. A review with patient presentations and discussion. Am J Med. 1970;48:77.
79 Vohra R, Kang HS, Dogra S, et al. Tuberculous osteomyelitis. J Bone Joint Surg Br. 1997;79:562.
80 Eismont FJ, Bohlman HH, Soni PL, et al. Pyogenic and fungal vertebral osteomyelitis with paralysis. J Bone Joint Surg Am. 1983;65:19.
81 Hendrickx L, Van Wijngaerden E, Samson I, et al. Candidal vertebral osteomyelitis: report of 6 patients, and a review. Clin Infect Dis. 2001;32:527.
82 Pappas PG, Rex JH, Sobel JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38:161.
83 Schilling A, Seibold M, Mansmann V, et al. Successfully treated Candida krusei infection of the lumbar spine with combined caspofungin/posaconazole therapy. Med Mycol. 2008;46:79.
84 Kirby A, Hassan I, Burnie J. Recommendations for managing Aspergillus osteomyelitis and joint infections based on a review of the literature. J Infect. 2006;52:405.
85 Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327.
86 Tang TJ, Janssen HL, van der Vlies CH, et al. Aspergillus osteomyelitis after liver transplantation: conservative or surgical treatment? Eur J Gastroenterol Hepatol. 2000;12:123.
87 Mouas H, Lutsar I, Dupont B, et al. Voriconazole for invasive bone aspergillosis: a worldwide experience of 20 cases. Clin Infect Dis. 2005;40:1141.
88 Stratov I, Korman TM, Johnson PD. Management of Aspergillus osteomyelitis: report of failure of liposomal amphotericin B and response to voriconazole in an immunocompetent host and literature review. Eur J Clin Microbiol Infect Dis. 2003;22:277.
89 Saag MS, Graybill RJ, Larsen RA, et al. Practice guidelines for the management of cryptococcal disease. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:710.
90 Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:1801.
91 Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658.
92 Results of directly observed short-course chemotherapy in 112,842 Chinese patients with smear-positive tuberculosis. China Tuberculosis Control Collaboration. Lancet. 1996;347:358.
93 Blumberg HM, Leonard MKJr, Jasmer RM. Update on the treatment of tuberculosis and latent tuberculosis infection. JAMA. 2005;293:2776.
94 Floyd K, Wilkinson D, Gilks C. Comparison of cost effectiveness of directly observed treatment (DOT) and conventionally delivered treatment for tuberculosis: experience from rural South Africa. BMJ. 1997;315:1407.
95 Jacobs RF. Multiple-drug-resistant tuberculosis. Clin Infect Dis. 1994;19:1.
96 Morse DI. Directly observed therapy for tuberculosis. BMJ. 1996;312:719.
97 Moulding T, Dutt AK, Reichman LB. Fixed-dose combinations of antituberculous medications to prevent drug resistance. Ann Intern Med. 1995;122:951.
98 Volmink J, Garner P. Systematic review of randomised controlled trials of strategies to promote adherence to tuberculosis treatment. BMJ. 1997;315:1403.
99 Fam AG, Rubenstein J. Another look at spinal tuberculosis. J Rheumatol. 1993;20:1731.
100 A controlled trial of six-month and nine-month regimens of chemotherapy in patients undergoing radical surgery for tuberculosis of the spine in Hong Kong. Tenth report of the Medical Research Council Working Party on Tuberculosis of the Spine. Tubercle. 1986;67:243.
101 Jain AK, Aggarwal PK, Arora A, et al. Behaviour of the kyphotic angle in spinal tuberculosis. Int Orthop. 2004;28:110.
102 Jain AK, Kumar S, Tuli SM. Tuberculosis of spine (C1 to D4). Spinal Cord. 1999;37:362.
103 Jain AK, Sinha S. Evaluation of systems of grading of neurological deficit in tuberculosis of spine. Spinal Cord. 2005;43:375.
104 Jain AK, Dhammi IK. Tuberculosis of the spine: a review. Clin Orthop Relat Res. 2007;460:39.
105 Martin NS. Pott’s paraplegia. A report on 120 cases. J Bone Joint Surg Br. 1971;53:596.
106 Moon MS. Tuberculosis of the spine. Controversies and a new challenge. Spine. 1997;22:1791.
107 Moon MS, Ha KY, Sun DH, et al. Pott’s paraplegia—67 cases. Clin Orthop Relat Res. 1996;323:122.
108 Moon MS, Moon JL, Moon YW, et al. Pott’s paraplegia in patients with severely deformed dorsal or dorsolumbar spines: treatment and prognosis. Spinal Cord. 2003;41:164.
109 Pattisson PR. Pott’s paraplegia: an account of the treatment of 89 consecutive patients. Paraplegia. 1986;24:77.
110 Moon MS, Woo YK, Lee KS, et al. Posterior instrumentation and anterior interbody fusion for tuberculous kyphosis of dorsal and lumbar spines. Spine. 1995;20:1910.
111 Upadhyay SS, Sell P, Saji MJ, et al. Surgical management of spinal tuberculosis in adults. Hong Kong operation compared with debridement surgery for short and long term outcome of deformity. Clin Orthop Relat Res. 1994;302:173.
112 Huang TJ, Hsu RW, Chen SH, et al. Video-assisted thoracoscopic surgery in managing tuberculous spondylitis. Clin Orthop Relat Res. 2000;379:143.
113 Kapoor SK, Agarwal PN, Jain BKJr, et al. Video-assisted thoracoscopic decompression of tubercular spondylitis: clinical evaluation. Spine. 2005;30:E605.
114 Vidyasagar C, Murthy HK. Management of tuberculosis of the spine with neurological complications. Ann R Coll Surg Engl. 1994;76:80.
115 Vidyasagar C, Murthy HK. Spinal tuberculosis with neurological deficits. Natl Med J India. 1996;9:25.
116 Jain AK, Maheshwari AV, Jena S. Kyphus correction in spinal tuberculosis. Clin Orthop Relat Res. 2007;460:117.
117 Laheri VJ, Badhe NP, Dewnany GT. Single stage decompression, anterior interbody fusion and posterior instrumentation for tuberculous kyphosis of the dorso-lumbar spine. Spinal Cord. 2001;39:429.
118 Tuli SM. Severe kyphotic deformity in tuberculosis of the spine. Int Orthop. 1995;19:327.
119 Hsu LC, Cheng CL, Leong JC. Pott’s paraplegia of late onset. The cause of compression and results after anterior decompression. J Bone Joint Surg Br. 1988;70:534.
120 Rajasekaran S. The natural history of post-tubercular kyphosis in children. Radiological signs which predict late increase in deformity. J Bone Joint Surg Br. 2001;83:954.
121 Oga M, Arizono T, Takasita M, et al. Evaluation of the risk of instrumentation as a foreign body in spinal tuberculosis. Clinical and biologic study. Spine. 1993;18:1890.
122 Hsieh PC, Wienecke RJ, O’Shaughnessy BA, et al. Surgical strategies for vertebral osteomyelitis and epidural abscess. Neurosurg Focus. 2004;17(6):E4.
123 O’Shaughnessy BA, Kuklo TR, Ondra SL. Surgical treatment of vertebral osteomyelitis with recombinant human bone morphogenetic protein-2. Spine. 2008;33:E132.