22 Fundamentals of Patient Safety and Quality Improvement
Pearls
• Following the documentation of patient death and complication rate related to medical errors and preventable complications, it is now clear that an essential component of healthcare delivery is improving the safety and quality of healthcare.
• Healthcare improvement efforts prevent iatrogenic injury and maximize positive patient outcomes by incorporating the psychology of human behavior, evidence-based standards of care, and performance measurement into clinical processes and systems.
• “High-reliability” organizations have low patient morbidity and mortality, a low rate of error and complications, and they continuously learn from the analysis of potential system flaws and actual adverse events. The staff of high reliability organizations raise concerns and make decisions to support safety practices and standardize processes, and there is leadership support for this approach.
• Pediatric critical care is highly vulnerable to errors and adverse patient events, so pediatric critical care nurses must be particularly vigilant in promoting a safe work environment, and compliance with medication safety, prevention of healthcare-acquired infections, and other evidence-based methods to improve patient care outcomes.
Overview of patient safety and healthcare quality improvement
Healthcare professionals practice with the intention of promoting the well-being of their patients. Yet despite the dedication, intelligence, and education of healthcare professionals, encounters often fall short of providing safe and high-quality care. The discipline of healthcare improvement has grown out of mounting evidence that many patients are inadvertently harmed by the care that is intended to make them well and that patient outcomes are denigrated by suboptimal care delivery systems.51,56 Healthcare improvement efforts prevent iatrogenic injury and maximize positive patient outcomes by incorporating the psychology of human behavior, evidence-based standards of care, and performance measurement into clinical processes and systems. Although patient safety and quality improvement programs may be considered the domain of managers and administrators, the ultimate responsibility to deliver safe and high quality care falls squarely on front-line clinicians. Pediatric critical care nurses must understand childhood diseases and their treatment, but also must know when, how, and why their work might fail to meet the needs of patients, and they must have the tools to make care safer and more effective.
Institute of Medicine Reports: The Case for Improving Healthcare Safety and Quality
In 1999, the Institute of Medicine (IOM) report To Err is Human: Building a Safer Health System estimated that 100,000 people die every year in the United States as the result of preventable errors made by healthcare professionals.51 The statistics presented in this study ranked medical errors as the eighth leading cause of death and demonstrated that more people die because of medical errors than from motor vehicle crashes, breast cancer, or AIDS.7,18,51 The report attributed 7000 of the deaths to medication errors and estimated the annual cost of errors at $17 billion to $29 billion.51
The To Err is Human report exposed the inadequacies of healthcare systems, focused attention on patient safety, and fostered programs dedicated to measuring and improving performance. Table 22-1 outlines the recommendations for healthcare improvement that were presented in the To Err is Human report and endorsed by the IOM’s Quality of Health Care in America Committee. In a follow-up to the To Err is Human report, in 2001 the IOM published Crossing the Quality Chasm: A New Health System for the 21st Century, to more fully address quality problems related to patient-family satisfaction, treatment disparities, and resource accessibility.21,64,65 Crossing the Quality Chasm defines the ideal twenty-first century healthcare system as one that is safe, effective, patient-centered, timely, efficient, and equitable. Table 22-2 defines the six aims set forth by the IOM and highlights the recommendations of the Crossing the Quality Chasm report for achieving the ideal healthcare system.
Table 22-1 The Institute of Medicine Quality of Health Care in America Committee’s Recommendations for Health Care Improvement
| Tier | Goal | Action |
| Leadership and knowledge | Establish national leadership, research, tools, and protocols to enhance patient safety knowledge | Development of a Center for Patient Safety within the Agency for Healthcare Research and Quality |
| Identifying and learning from errors | Identify and learn through errors via mandatory and voluntary reporting efforts with an emphasis on using information to make systems safer | Development of reporting standards by the National Forum for Health Care Quality Measurement and Reporting and analysis of the data by the Center for Patient Safety |
| Requirement for state departments of health to report adverse events | ||
| Designation of federal funding for the development of reporting systems | ||
| Creation of legislation to extend peer review protections to data related to patient safety and quality improvement | ||
| Setting performance standards and expectations | Raise the standard and expectations for the improvement of safety through the actions of oversight organizations, professional organizations, and group purchasers | Inclusion of patient safety indicators in performance standards |
| Implementation of patient safety programs by regulators and accreditors, public and private healthcare purchasers and health professional licensing, certifying and credentialing agencies | ||
| Development of patient safety committees within professional societies | ||
| Increased post-marketing monitoring of drugs by the U.S. Food and Drug Administration | ||
| Implementing safety systems in healthcare organizations | Create safe systems within healthcare organizations by implementing safe practices at the delivery level | Incorporation of patient safety programs, with executive sponsorship, into all healthcare institutions |
| Adoption of safe medication practices by all healthcare institutions |
From Kohn KT, Corrigan JM, Donaldson MS: To err is human: building a safer healthcare system, Washington, DC, 1999, National Academy Press; National Academy of Sciences: To err is human: building a safer healthcare system executive summary. Available at http://www.nap.edu. Accessed October 8, 2011.
Table 22-2 Institute of Medicine Six Aims for Improvement of Health Care Systems
From Committee on Quality Health Care in America and Institute of Medicine: Crossing the quality chasm: a new health system for the 21st century. Washington, DC, 2001, National Academy Press; National Academy of Sciences: Crossing the quality chasm: a new health system for the 21st century executive summary, 2003. Available at http://www.nap.edu. Accessed October 8, 2011.
Through the implementation of the IOM recommendations and the work of organizations that have evolved to improve the safety and quality of healthcare (see the table in the Chapter 22 Supplement on the Evolve Website for a list of organizations and their focuses and websites), individual institutions have demonstrated incremental improvement in care. As late as 2005, however, national statistics had not improved substantially.2,56 Furthermore, the data compiled in 1999 may have underestimated deficiencies in patient safety.56 For example, the Centers for Disease Control and Prevention (CDC) data subsequent to the To Err is Human report suggest that healthcare-acquired blood stream infections may account for as many as 90,000 deaths every year.19,56 In light of these projections, it is clear that an essential component of contemporary healthcare delivery is improving the safety and quality of healthcare for patients of all ages.
Human Error
Refer to the Chapter 22 Supplement on the Evolve Website for a history of the industry-based theories of human error. Error theorists recommend a systems approach to improving workplace performance. Extrapolated from successful programs in the nuclear power and aviation industries, a systems approach to preventing human error starts with the notion that people are intrinsically fallible. In addition, it encourages error-proofing the systems in which people work. In a systems approach to error-proofing, layers of defensive interventions that respond to failure-prone human behaviors are used to prevent or trap errors before they cause harm. Often referred to as the Swiss Cheese Model (Fig. 22-1), Reason’s system theory79 illustrates that despite layers of defense, it is possible for holes in the safeguards to align in such a way that an error passes through the system. The model proposes that to be maximally effective, multiple defensive layers are necessary to reduce the likelihood that the holes will align and allow the system to fail. In environments such as critical care units, layers of defense are often technologic, such as redundant monitor alarms that sound at the bedside and at a central monitoring station and may also alert staff via a visual cue, such as a flashing light.79
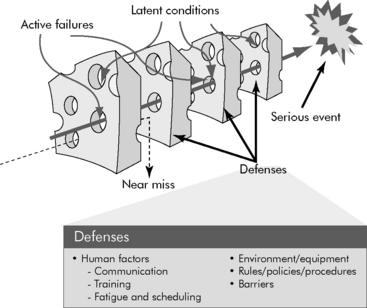
Fig. 22-1 The Swiss Cheese Model of Errors: holes in layers of defense allow errors to pass through a system.
(Adapted from Reason JT: Human error, New York, 1991, Cambridge University Press.)
Reason notes that errors in systems are caused by either “active failures” or “latent conditions.”79 In healthcare, an active failure is an unsafe act performed by a professional who is in direct contact with a patient. A latent condition is a flaw in the design of the care delivery system and its layers of defense. In keeping with the Swiss Cheese Model, patient safety is promoted by barriers to both the active failures of professionals and the latent conditions of a system. Successful error prevention only adds defensive layers and minimizes the number of potential failures that exist in the system. A common approach to prospectively analyzing a system for potential failures is failure mode effect analysis (FMEA). Root cause analysis (RCA) is a technique for assigning a cause to errors that have actually occurred. The methodologies for FMEA and RCA are described later in this chapter. Such preoccupation with errors, their cause, and their prevention is a key characteristic of safe and high reliability industries.79
High Reliability Organizations
Reliability is the rate at which a system produces a desired effect without failure.80 High reliability organizations perform well in hazardous situations that depend on technology and human interactions.114 (Refer to the Chapter 22 Supplement on the Evolve Website for information about the study of nuclear power and aviation accidents.) By understanding the interplay between the technical and human causes of accidents, the nuclear power and aviation industries have significantly improved the safety of their practices and are now considered high reliability organizations. Regardless of the industry, all high reliability organizations share the following characteristics:
Box 22-1 lists the characteristics of high reliability organizations. In addition to a high rate of positive patient outcomes and a low rate of error and complications, evidence of reliability in a healthcare organization includes continual learning from the analysis of potential system flaws and actual adverse events, the ability of staff at any level to raise concerns and make decisions that are within the scope of their expertise, standardization of processes, and leadership support for safety practices.16,60,77
Culture of Safety
The ability of a healthcare system to be highly reliable hinges on its underlying organizational culture and leadership support for a culture of safety. To fully support a culture of safety, leadership must prioritize and build consensus on the importance of safety and quality improvement goals and must remove barriers and conflicts of interest to achieving these goals. Leadership can also promote a culture of safety by using nonpunitive approaches to investigating errors and by promoting transparency for the purpose of learning from adverse events.37,92 Evidence that a culture of safety exists in an organization includes sufficient staff and resources, a flat organizational hierarchy, open dialogue about problems without fear of repercussion, clear lines of communication and effective intradisciplinary and interdisciplinary teamwork.63,72 The Agency for Healthcare Research and Quality provides a survey that can be used to assess the culture of safety in a hospital3 (Box 22-2).
Box 22-2 Agency for Healthcare Research and Quality Hospital Survey Questions on Patient Safety Culture
1. Do people support one another in this unit?
2. Do we have sufficient staff to handle the workload?
3. When a lot of work needs to be done quickly, do we work together as a team to get the work done?
4. Do people treat each other with respect?
5. Do staff members in this unit work longer hours than is best for patient care?
6. Are we are actively doing things to improve patient safety?
7. Do we use more agency or temporary staff than is best for patient care?
8. Do staff believe that their mistakes are held against them?
9. Do mistakes lead to positive changes here?
10. Is it just by chance that more serious mistakes don’t happen around here?
11. When one area in this unit gets really busy, do others help out?
12. When an event is reported, does it seem as though the person is being written up, not the problem?
13. After we make changes to improve patient safety, do we evaluate their effectiveness?
14. Do we work in “crisis mode” trying to do too much, too quickly?
15. Is patient safety ever sacrificed to get more work done?
16. Do staff members worry that mistakes they make are kept in their personnel file?
17. Do we have patient safety problems in this unit?
18. Are our procedures and systems good at preventing errors?
19. Does my supervisor or manager say a good word when they see a job done according to established patient safety procedures?
20. Does my supervisor or manager seriously consider staff suggestions for improving patient safety?
21. Whenever pressure builds, does my supervisor or manager wants us to work faster, even if it means taking shortcuts?
22. Does my supervisor or manager overlook patient safety problems that happen repeatedly?
23. Are we are given feedback about changes put into place based on event reports?
24. Will staff members freely speak up if they see something that may negatively affect patient care?
25. Are we are informed about errors that happen in this unit?
26. Do staff members feel free to question the decisions or actions of those with more authority?
27. Do staff members discuss ways to prevent errors from happening again?
28. Are staff members afraid to ask questions when something does not seem right?
29. When a mistake is made, but is caught and corrected before affecting the patient, how often is this reported?
30. When a mistake is made, but has no potential to harm the patient, how often is this reported?
31. When a mistake is made that could harm the patient, but does not, how often is this reported?
32. Does hospital management provide a work climate that promotes patient safety?
33. Do hospital units coordinate well with each other?
34. Do things “fall between the cracks” when transferring patients from one unit to another?
35. Is there good cooperation among hospital units that need to work together?
36. Is important patient care information often lost during shift changes?
37. Is it often unpleasant to work with staff members from other hospital units?
38. Do problems often occur in the exchange of information across hospital units?
39. Do the actions of hospital management show that patient safety is a top priority?
40. Does hospital management seem interested in patient safety only after an adverse event happens?
41. Do hospital units work well together to provide the best care for patients?
42. Are shift changes problematic for patients in this hospital?
Adapted from Patient Safety culture surveys, Rockville, MD, 2009, Agency for Healthcare Research and Quality. Available at http://www.ahrq.gov/qual/patientsafetyculture/. Accessed October 1, 2009.
Crew Resource Management
Effective teamwork and communication are key features of the safety culture in high reliability organizations. Initially developed by the National Aeronautics and Space Administration Aerospace Human Factors Research Division to train flight crews, crew resource management (CRM), is essential for the effective functioning of multidisciplinary healthcare teams (Box 22-3). CRM includes team training via simulations and interactive group briefings that focus on the development of behaviors critical to decision making and performance in high-risk and high-stress environments that depend on technology, process, and interpersonal communication.72 The ultimate goal of CRM is a “shared mental model” that aligns all team members to the goals and the strategies for any team activity.96 Behaviors taught to team members via CRM include:
Box 22-3 Crew Resource Management: Core Components of Teamwork Training
In addition to teaching these core behaviors, CRM provides team members with the opportunity to develop technical proficiency and to practice avoiding and trapping errors and mitigating their consequences, and it helps participants appreciate the effects of stress, fatigue, and work overload on performance. Serial performance appraisal of team members is also an important component of CRM programs.72,96
Healthcare team training programs have gained acceptance over the past 10 years, and performance of labor and delivery, surgical, and anesthesia teams have demonstrated the positive effects of CRM.30,59,69 The Joint Commission concluded that communication failures contribute to more than half of all errors.40 In response, the Joint Commission and other patient safety interest groups have mandated the use of the structured inquiries and briefings that are central to CRM. Examples of such mandated CRM methods include the performance of “universal protocol” or “time-out” to confirm the correct patient, procedure, and site before invasive interventions, and the use of standardized templates or scripts for hand-off communication between caregivers.12,40,41,70,84,95
An appreciation for the importance of CRM has also expanded the use of simulation in healthcare. In critical care settings, the tradition of simulating resuscitations has taken new direction and is now used to teach delegation and communication skills and to reinforce clinical decision-making algorithms and procedural techniques.29,69 Simulation, which was once a tool reserved for advanced life support and crisis resource management training (such as learning to manage a difficult airway), can be used to develop virtually any aspect of critical care team performance, including noncrisis activities such as daily rounds.40,69
Healthcare improvement methodology
Model for Improvement
Theories of quality improvement and human error provide a foundation for programs in patient safety and quality improvement, but healthcare teams also need practical methods for orchestrating improvement initiatives. Developed by Associates in Process Improvement, the Model for Improvement (Fig. 22-2) is widely considered an appropriate quality improvement method for healthcare.104 The Model for Improvement is a two-step process consisting of (1) answering questions to define the improvement opportunity and (2) conducting plan-do-study-act (PDSA) cycles to test interventions that are hoped to improve performance.24,44,54,104
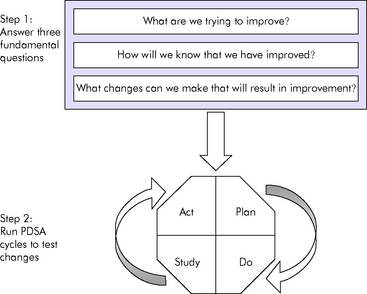
Fig. 22-2 The model for improvement.
(Adapted from Institute for Healthcare Improvement: How to improve. Available at http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/HowToImprove. Accessed April 25, 2008; Langley GJ, et al: The improvement guide: a practical approach to enhancing organizational performance, San Francisco, 1996, Jossey-Bass.)
• Sponsors and champions: support and remove barriers to improvement and promote a culture of safety; they are often members of upper management or executive administrators
• Leaders: manage the team and the project and organize the work
• Members: determine the course or action and perform improvement actions or changes; they are subject matter experts
• Facilitators: help the team work together effectively; they are external to the issue
When the initiative includes direct patient care or operations that support patient care, the team consists of members of the clinical microsystem. The clinical microsystem is the group of people who work together on a regular basis to provide care to a discrete population of patients. Members of the clinical microsystem are best able to provide subject matter expertise and encourage the adoption of change throughout an organization.68
• Planning the improvement action, including the measures and data collection process
• Doing or performing the improvement action, as a test or small scale trial, and collecting performance data
• Studying the outcome of the test and using the data to uncover what did and did not work
• Acting on the information learned from the test to plan next steps
When an improvement effort requires multiple improvement interventions, each intervention can be tested via a separate PDSA cycle. All the related PDSA cycles are then linked to create a package of interventions that have been proven to foster improvement (Fig. 22-3). Effective improvement actions can then be spread beyond the initial test setting and population. The use of PDSA cycles to test and refine improvement actions can help to overcome an organization’s natural resistance to change.24,44,54,105
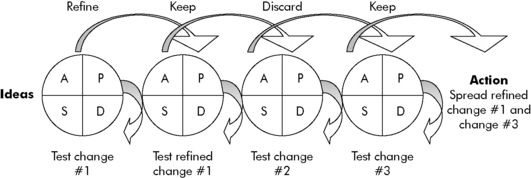
Fig. 22-3 Linking plan-do-study-act (PDSA) cycles: moving ideas to action.
(Adapted from Institute for Healthcare Improvement: How to improve. Available at http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/HowToImprove. Accessed April 25, 2008; Langley GJ, et al: The improvement guide: a practical approach to enhancing organizational performance, San Francisco, 1996, Jossey-Bass.)
Performance Measurement
• Outcome measures: measure the end result, overall performance, or outcome of an improvement effort
• Process measures: measure the rate of use and performance of a process within a comprehensive improvement effort
• Balancing measures: measure the consequences of the planned changes associated with the improvement effort44,54,104
• The outcome measure is the rate of infection.
• The process measures are the rates of use of recommended practices, such as rate of compliance with conducting and documenting a daily review for continued line necessity. An additional related process measure may also be the time to remove central lines.
• Assuming that the peripheral venous access would be used more often if central lines are removed quickly as a result of daily line necessity reviews, an appropriate balancing measure for the project is the rate of peripheral line complications, such as infiltration.
To analyze performance patterns represented by data, it is helpful to graphically display and apply statistics to the data. Run charts and control charts, which plot data over time, are two essential improvement tools. Refer to Evolve Figs. 22-1 and 22-2 in the Chapter 22 Supplement on the Evolve Website for examples of such charts.
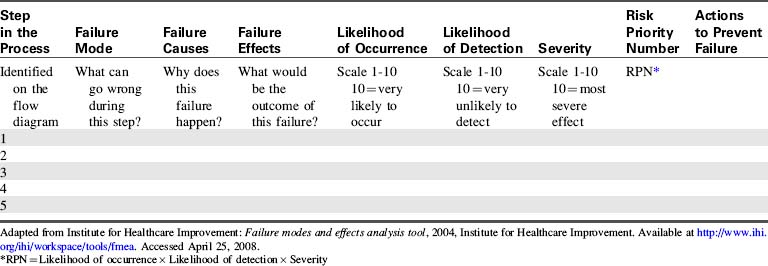
Failure Mode and Effects Analysis
1. Graphically depict a process using a flow diagram (e.g., see Evolve Fig. 22-3 in the Chapter 22 Supplement on the Evolve Website).
2. Analyze each step in the process for potential failure modes, the causes of the failure modes and their effects.
3. Assign a risk priority number (RPN) to each failure mode, based on its likelihood to occur, ability to be detected and trapped before an adverse event results, and the severity of the consequences if it does cause an adverse event.
4. Identify and prioritize the failure modes based on their RPN.
5. Propose actions to prevent the failure modes.
6. Implement the actions to prevent the failure modes (the Model for Improvement may be used to do so).
Table 22-3 provides a sample documentation grid and RPN calculation tool for conducting an FMEA. An FMEA can be used to identify new improvement projects or as a tool to further understand special cause variation demonstrated in the data generated by an existing improvement project. An improvement team that includes all key stakeholders, including members of the clinical microsystem, should be used to conduct FMEAs.43
The Institute for Safe Medication Practices has strongly encouraged the use of FMEA, and it has been shown to improve medication safety.81,91,115 In response to the overwhelming evidence that the technique contributes to patient safety, the Joint Commission mandates that healthcare organizations perform at least one FMEA per year.33
Incident Reporting
Healthcare organizations should use tools, such as FMEA, to anticipate and prevent failures before they occur. However when a failure takes place, incident reporting becomes central to the safety and quality improvement effort. Colonel John C. Flanagan, the Director of the Division of Aviation Psychology, developed the critical incident technique for military training in 1954. The critical incident technique was a structured incident reporting process that included the use of personnel directly involved in a failed process to create, at the time of discovery, a qualitative narrative of the event. Flanagan’s work also established common terminology for incident reporting that has been adapted for use in healthcare (Table 22-4).28,51,109 Healthcare incident reporting is the process by which a clinician documents adverse events, medical errors, and near-miss events, including deviations from standard procedures that could have resulted in an adverse event or error, in real time and at the point of care. Incident reports may also require clinicians to assign a perceived cause and severity grade to the event.31,42,55,109
| Term | Definition |
|---|---|
| Adverse event | An injury that was caused by medical management rather than by a patient’s underlying disease |
| Medical error | The failure of a planned action to be completed as intended or to achieve its intended goal or the use of the wrong action to achieve a goal |
| Serious error | An error that causes permanent injury or transient but potentially life-threatening harm |
| Minor error | An error that causes harm that is neither permanent nor potentially life threatening |
| Near miss | An error that could have caused harm, but did not because of timely intervention or chance |
| Sentinel event | An unexpected occurrence involving death or serious physical or psychological injury, or the risk thereof |
Adapted from Garbutt J, et al: Reporting and disclosing medical errors: pediatricians’ attitudes and behaviors. Arch Pediatr Adolesc Med 161:179-185, 2007; Joint Commission: Sentinel events. Available at http://www.jointcommission.org/SentinelEvents. Accessed April 25, 2008; Kohn KT, Corrigan JM, Donaldson MS: To err is human: building a safer healthcare system. Washington, DC, 1999, National Academy Press.
Since 1975, the U.S. Food and Drug Administration has mandated the reporting of blood transfusion reactions, and in 1995 the Joint Commission mandated reporting of all other adverse events, errors, and near misses as part of the Sentinel Event Policy. The hallmark IOM report To Err is Human called for the enhancement of voluntary and mandatory incident reporting systems in individual organizations and for public disclosure. Furthermore, and as noted previously, documenting incidents is an important characteristic of High Reliability Organizations.55,109 Despite a keen focus on incident reporting, however, there are many barriers to the use of incident reports by healthcare providers.31,97 In a 2004 survey by Taylor et al,97 34% of pediatricians and pediatric nurses surveyed indicated that they reported fewer than 20% of their own medical errors, and approximately 33% of those surveyed indicated that they reported fewer than 40% of the errors committed by their colleagues. Other studies have indicated incident reporting rates as low as 10%.51 Barriers to incident reporting can be inadequacies of reporting systems, organizational culture, or the attitudes or knowledge deficits of healthcare professionals (see Barriers to Incident Reporting in the Chapter 22 Supplement on the Evolve Website for examples).
Despite the barriers to incident reporting, pediatric healthcare providers indicate a willingness to report if and when appropriate and an interest in understanding incidents to improve care.31 Facilitating the use of incident reports is a key improvement effort of all hospitals. Effective strategies to improve incident reporting rates include:
• Dedicate the reporting system for the sole purpose of capturing clinical incidents.
• Identify the incident reporting system as a safety reporting system to emphasize its ultimate purpose.
• Include cues for clinicians about what constitutes an incident and categories of incidents on the reporting tool.
• Make the incident reporting widely accessible in the clinical environment.
• Make a secure mechanism for submitting incident reports widely accessible in the clinical environment.
• Automate the reporting system.
• Apply a consistently nonpunitive response to reported incidents and positive recognition of clinicians who report.
• Continually provide feedback to clinicians about the improvement actions taken in response to reported incidents and trends in incidents.42,98,100
All hospitals should implement a system that provides timely information to front-line clinical leadership about the occurrence of and trends in incidents. Electronic incident reporting systems help to accomplish this goal.98,100 Continual feedback to clinicians about the improvement actions taken in response to incident reports is also essential to increasing reporting rates and to building a culture of transparency and learning. Often a reported event will undergo an extensive, systematic evaluation, such as an RCA, that will generate a new improvement initiative for the clinical microsystem.
Root Cause Analysis
Performing an RCA of actual adverse patient events, like FMEA and incident reporting, is a characteristic of a high reliability organization and is mandated by the Joint Commission, as well as by many state regulated incident reporting programs. Reporting programs, which are often public, typically require an RCA of a “sentinel event” or an “unexpected occurrence involving death or serious physical or psychological injury, or the risk thereof.”46 The goal of an RCA is to identify the factors that contributed to an adverse patient event in order to safeguard the system from the same event occurring again. Although an RCA may identify multiple system vulnerabilities, the emphasis of the exercise is to assign and understand the causes of the failure that resulted in death or serious morbidity or the potential for such.26 Steps for conducting an RCA include:
1. Describe the incident, include the use of a flow-diagram to illustrate the processes in which the incident occurred.
2. Compare the steps of the process that resulted in the event to the usual process used to carry out the activity.
3. Identify the human factor and other factors that contributed to the event (see Box 22-4 for categories of causes of failures and rules for assigning cause; also see Evolve Fig. 22-4 in the Chapter 22 Supplement on the Evolve Website).
4. Propose improvements to the process or system that will prevent the event from recurring.
5. Implement the actions to prevent the event from recurring; the Model for Improvement may be used.9,15,26,106
Box 22-4 Root Cause Analysis: Assigning Cause to Adverse Patient Events to Improve Systems
Rules for Assigning Cause
• Clearly show the cause-and-effect relationship
• Do not assign a quality or use a negative descriptor when identifying a cause (e.g., do not use inadequate communication or poorly written policy)
• Identify a preceding cause for each human error
• Identify a preceding cause for each procedural deviation
• Assign failure to act only when there was a preexisting duty to act
Adapted from the Veterans Administration National Center for Patient Safety: Root cause analysis. Available at http://www.va.gov/NCPS/CogAids/Triage/index.html?8. Accessed April 25, 2008.
To be effective, the approach to conducting an RCA should be consistent from one event to the next. RCA should be conducted by representatives of all stakeholders in the system in which an event occurred, including members of the clinical microsystem. To provide an objective analysis of the event, one or more members of the organization who sit outside the system under examination may also be included. In addition, a neutral facilitator should assist with the organization of the RCA and moderate related discussions and debates.9,15,107
Opportunities for improvement in pediatric critical care
Environment of Care
Pediatric critical care is highly vulnerable to errors and adverse patient events.61,85 The needs of critically ill children are complex and heterogeneous, the systems for providing care to critically ill children are tightly coupled, and the work of critical care providers is psychologically stressful and physically exhausting. Accidents related to technology, including dislodgement of invasive catheters and airways and ineffective use of monitoring alarms by staff, are prevalent in critical care, and gaps in care continuity and cognitive biases are particularly problematic when fast-paced decision making is needed.61,102,103 Elements of the critical care physical environment, such as open layouts and excessive noise, produce interruptions and distractions during care delivery. These elements are also known to impede patient safety in pediatric critical care units.61 In a study that applied an aviation “sterile cockpit” approach to critical operations, medication errors were reduced when interruptions experienced by the nurse during medication administration were minimized.61,74 Furthermore, the general effects of fatigue on clinician performance are applicable, if not amplified, in pediatric critical care, where on-call physicians have little time to sleep and nurses frequently work extended shifts. The documented effects of sleep deprivation include a 36% increase in medication and diagnostic errors and a 30% increase in surgical procedure times.53,61 Similarly, research has shown that nurses are twofold to threefold more likely to make an error after working more than 12.5 consecutive hours.61,82,87 A 2006 study conducted by the American Association of Critical-Care Nurses reported that 44% of critical care nurses are routinely scheduled to work shifts longer than 12.5 hours and that during a 1-month period, only 13% of nurses left work at the end of their assigned shift and 61% worked 10 or more overtime shifts.61,87 The ideal hospital environment culture of safety includes leadership support, teamwork, learning from mistakes, work hour restrictions, work load distribution, appropriate physical space, and decision support aids that are readily available at the point of care.37,73
Improving the hospital work environment is clearly pertinent to nursing. Leape et al56 reported that as many as 86% of harmful errors are intercepted by nurses before they reach patients. Several studies have shown that when patient-to-nurse ratios increase and when nurses spend less time with patients, there is an increase in complications such as infection, gastrointestinal bleeding, and cardiac arrest, as well as extended length of hospital stay.52,67,78 Aiken et al4,5 have published hallmark research that correlates low nursing education level and high patient-to-nurse ratio with increased patient mortality rates and increased failure to rescue patients who are experiencing physiologic instability resulting from an unexpected complication. Aiken et al estimated that for every patient receiving care beyond an assignment of four patients there is a 7% increase in the risk of a patient dying within 30 days after discharge.5 These statistics are particularly daunting in light of the estimated 29% to 40% nursing shortages predicted by the year 2020.37
In response to evidence that nursing care plays a pivotal role in patients’ outcomes, the IOM Committee for the Work Environment of Nurses and Patient Safety published a manual titled Keeping Patients Safe: Transforming the Work Environments of Nurses. The manual, intended to be a companion to the IOM report To Err is Human, provides a blueprint for the ideal work environment for nurses.73 The blueprint builds on research that demonstrates the effects of nursing education, management, staffing, work hours and processes, and the ergonomics of the work space. In addition, the blueprint links the development of a safe work environment with the evolution of a learning organization—that is, an organization that is willing to adapt over time to best meet the needs of its clients.37,38,73 The requirements set forth by the IOM for a safe and effective work environment for nurses include an organizational commitment to patient safety, effective and evidence-based management and leadership, interdisciplinary collaboration, adequate staffing and work hour restrictions, workplace design that facilitates safe care, decision support aids at the point of care, and opportunities for ongoing learning.
Failure to Rescue
The ultimate outcome of a healthy work environment is the prevention of failure-to-rescue events. Failure to rescue is any event during which a clinician is unable, because of a complication of care or a condition that was not present on admission, to save a hospitalized patient’s life.4 To recognize and intervene early when a life-threatening complication occurs, nurses use surveillance such as clinical assessment for signs and symptoms of physiologic instability, and environmental safety checks, such as verifying the settings of monitor alarms.4 A workforce and work environment that support surveillance, recognition, and early intervention by nurses are the backbone for rapid response teams (RRTs). RRTs are hospital-based teams of critical care providers who are available to assess, provide consultation, and assist with the care of a patient whose status is deteriorating prior to the need for resuscitation for cardiopulmonary arrest.116 RRTs have been successfully implemented in pediatric hospitals and have been shown to reduce the rate of cardiopulmonary arrests that occur outside of critical care units and reduce patient mortality. The use of RRTs is strongly encouraged by the Joint Commission and other patient safety interest groups.48,88,90,116
Role for Information Technology
There is great promise that evolving information technology will enable safe environments of care. Electronic health record (EHR) and computerized provider order entry (CPOE) systems offer benefits to clinicians, including expanded access to patients’ health information, particularly during and after transitions of care, and decision support such as drug-allergy interaction checking and dose calculation.99,112 By offering identification and tracking applications, electronic information systems can also enhance patient identification and patient flow.76 Furthermore, electronic capture of clinical information facilitates data mining for clinical research and measurement of performance. The IOM, the Joint Commission, the Center for Medicare and Medicaid Services, the Institute for Safe Medication Practices (ISMP), public interest groups, and many private insurers include electronic information systems in their healthcare improvement requirements and pay-for-performance plans.6,34
It is important to note that there are risks associated with EHR and CPOE systems. Because EHR and CPOE systems require changes in routine clinical operations and processes, it will be important to verify that clinical workflow is safe and effective after implementation. Other risks include lack of information security, alert fatigue, downtime, inefficiency, and the evolution of new types of medical errors, such as keystroke mistakes.11,110,112
In addition to the generic risks of clinical information systems, there is national concern that commercially available EHR and CPOE systems do not accommodate the unique needs of pediatric healthcare.6,89,93,110,112 The Joint Commission mandated that information system vendors develop functionality to help prevent pediatric medication errors,46,47 and the American Academy of Pediatrics has developed position statements on pediatric requirements for clinical information technology.6,93 Table 22-5 outlines the pediatric-specific requirements for maximal safety and effectiveness of EHR and CPOE systems.
Table 22-5 Requirements for Safe and Effective Pediatric Electronic Information Systems
| Requirement | Electronic Health Record | Computerized Provider Order Entry and Prescribing |
|---|---|---|
| Immunizations | Ability to record immunization data and link to state immunization registries | Immunization decision support with reminders regarding immunization schedules and electronic entry of immunization orders |
| Ability to print an immunization record for families | ||
| Growth documentation and tracking | Automated growth charts with alerts when children fall outside of growth parameters for age, including gestational and adjusted age for premature infants | Weight displays on all order screens |
| Ability to document gestational age | Reminders to document a patient’s weight | |
| Accommodation of normative values that change specific to the child’s age (e.g., vital sign norms) | Stopping functions that prevent providers from ordering medications without a weight entry in the system | |
| Medication management | Presence of medication lists and the ability to view medication history and a medication administration record | Dosing by body weight or body mass index |
| Electronic medication administration record with decision support prompts for nurses | Dose range checking | |
| Rounding to safe and convenient doses | ||
| Age-based (including gestational age) dosing decision support | ||
| Indication-based dosing decision support | ||
| Drug allergy checking, drug-drug interaction checking, drug-food interaction checking, and duplicate medication alerts | ||
| Patient identification | Availability of two patient identifiers on all screens and printed reports | Availability of two patient identifiers on all order screens and printed copies of orders |
| Mechanism for newborn infant identification before a name or Social Security number has been assigned | ||
| Ability to store prenatal data for an identified fetus and connect this data with the patient after birth, without relying on maternal identifiers | ||
| Ability to change an infant’s name | ||
| Ability to assign unknown or ambiguous sex | ||
| Privacy | Ability to flag and limit access to protected information, including adolescent health issues as dictated by state laws | Ability to flag and limit access to orders reflecting protected information, including adolescent health issues as dictated by state laws (e.g., pregnancy test) |
| Ability to accurately and confidentially identify foster and custodial care |
Adapted from American Academy of Pediatrics Council on Clinical Information Technology, Gerstle RS: Electronic prescribing systems in pediatrics: the rationale and functionality requirements. Pediatrics 119:1229-1231, 2007; Joint Commission: Preventing pediatric medication errors. Sentinel Event Alert, 39, April 2008. Available at http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_39.htm. Accessed April 25, 2008; Spooner SA: Council on Clinical Information Technology, American Academy of Pediatrics. Special requirements of electronic health record systems in pediatrics. Pediatrics 119:631-637, 2007.
Additional technologies, such as bar code identification systems and programmable infusion pumps, also have the potential to improve patient safety in pediatric critical care units. Despite somewhat conflicting evidence in the literature, it appears that bar code technology reduces medication administration and lab labeling errors20,35,62 and that programmable or “smart” infusion pumps reduce duplicate infusion and infusion rate errors.71 Additional research is needed to determine the most appropriate uses and the true effects of such technologies.20,58
Common Pediatric Critical Care Unit Improvement Opportunities
There are unique impediments to improving patient safety in pediatric critical care. Many of the safety and quality improvement efforts launched by regulatory and public interest groups primarily focus on adult populations and may not match the safety risks specific to critically ill children.85 In light of the high risk for iatrogenic complications and the special needs of sick children, pediatric nurses and physicians must identify the key safety and quality indicators for pediatric critical care.85 A number of professional organizations, such as the National Initiative for Children’s Health Care Quality (NICHQ), the Child Health Corporation of America (CHCA), and related work groups have been formed to define and prioritize pediatric specific safety and quality initiatives and measures. Box 22-5 lists targeted pediatric safety and quality issues; it can be used by pediatric critical care providers to develop their own unit-specific improvement goals. Medication safety and healthcare-acquired infections are the two most consistent themes of all proposed pediatric safety and quality improvement programs.
Box 22-5 Common Opportunities for Clinical Improvement in Pediatric Critical Care
• Compliance with hand hygiene
• High-risk medication management
• Prevention of healthcare-acquired infections
• Prevention of skin breakdown
• Venous thromboembolism prophylaxis
• Patient flow efficiency (admission through discharge)
• Implementation of rapid response teams
• Implementation and enhancement of electronic health records
Pediatric Medication Errors and Adverse Drug Events
Medication errors and adverse drug events (ADE) are the largest subset of medical errors occurring in hospitals.36,51 A medication error is an error in drug ordering, transcribing, dispensing, administering, or monitoring. An ADE is an injury from a medication that is either unexpected or caused by inappropriate use of the medication or that results from lack of administration of a necessary medication. A potential ADE is an error that had the potential to result in significant injury, but was deflected before drug administration or did not produce the adverse consequence.8 Children, particularly children younger than 2 years, are up to threefold more likely than adults to experience a drug error or ADE.39,49,50,57,113 Approximately 3% of all hospitalized children experience an ADE, and 4% of the occurrences are classified as harmful.36 As many as 28% of medication errors and ADEs are preventable.13
There are many reasons why children experience more drug errors and ADEs than adults. Children are often incapable of questioning the appropriateness of a medication. In addition, calculations of drug dose are complicated by weight- or body surface area-based doses and by complex or incomplete information about drug pharmacokinetics and pharmacodynamics in children.36,39,49,50 The rate of pediatric medication errors and ADEs increases with extended length of stay, and it is higher in units that care for a complex mix of patients and in critical care units.36,39,49 The drugs most commonly associated with pediatric medication errors and ADEs are analgesics, sedatives, anticoagulants, antimicrobials, antineoplastic agents, corticosteroids, insulin, and vasoactive drugs. In addition, errors are commonly made with intravenous fluid and electrolyte therapy.36,39,49 For additional information about pharmacokinetics and drug monitoring in children, see Chapter 4.
In general, medication errors and ADEs are more frequently associated with intravenous drugs than with other routes of administration.36 More than 75% of pediatric medication errors and ADEs are associated with prescribing, and improper dose is reported as the most common type of prescribing error.36,39,49 Approximately 4% of dose prescribing errors are attributed to a missing or inaccurate patient weight.49
The second most common pediatric ADE is failure to administer a necessary medication, with antimicrobial agents being the drugs most frequently omitted.36 Omissions of medication are often associated with shift changes and transfers between units,36 and as many as 30% of all medication plans are incomplete or inaccurate at the time of discharge.105 Frequent failures in medication management during periods of patient transition have led to the regulatory mandate for medication reconciliation, in which planned medications are compared and deliberate actions to continue or discontinue drugs on the list are documented before and after transitioning care.75,86,105 Additional nursing errors have been reported related to improper infusion rates and errors in pump programming in pediatric critical care units.36
• Using CPOE and electronic medication administration records32,110,112 (see Chapter 23)
• Providing immediate access to patient medical history, allergies, and relevant laboratory data at the time of medication order entry and administration
• Having a unit-based pharmacist actively participate in the care of patients
• Restricting access to drugs until orders are checked and profiled by pharmacists
• Reconciling medications when patients transition between settings
• Standardizing drug concentrations, particularly for continuous medication infusions
• Storing medications in patient-specific storage bins
• Identifying and specially managing high-risk medications
• Removing concentrated electrolytes from nursing units
• Using two patient identifiers and potential bar-code identification before every drug administration
• Prohibiting the use of error-prone medication abbreviations
• Prohibiting the use of trailing zeros and encouraging the use of leading zeros
• Using verbal orders for medications only in urgent or emergent situations; when necessary, verbal orders should be written down and read back to the prescribing clinician
• Using tall-man letters to identify look-a-like or sound-a-like medications and separately storing look-a-like or sound-a-like medications
• Implementing vigilant monitoring protocols for patients receiving analgesics and sedatives 13,22,36,83,111–113
Healthcare-Acquired Infections
Prevention of healthcare-acquired infections is a common focus of safety improvement efforts. The National Nosocomial Infections Surveillance System (NNIS) is a voluntary reporting system that integrates with the CDC to capture data about healthcare-acquired infections in the U.S. Research using NNIS data has shown that bloodstream infections are the most common type of healthcare-acquired infection in children and that 91% of bloodstream infections are associated with central venous catheters.66,94 Indwelling airways and urinary catheters also contribute to healthcare-acquired infections. Most (95%) healthcare-acquired pneumonia occurs in children who are receiving mechanical ventilation, and 77% of urinary tract infections are associated with indwelling bladder catheters.66,94,101 NNIS data and related research have also identified the following risk factors for all types of pediatric healthcare-acquired infections:
• Young age, particularly less than 1 year
• Immunosuppression, caused by underlying disease or immunosuppressant drugs
• Presence of invasive devices, including central venous catheters, bladder catheters, and endotracheal tubes
• Medical diagnosis, except for cardiac and neurosurgical diagnoses
Healthcare-acquired infections increase length of stay and contribute significantly to patient morbidity and mortality, and thus are the target of improvement efforts launched by the Joint Commission and the Institute for Health Care Improvement, as well as by the Pediatric Affinity Group, a pediatric critical care consortium sponsored by the NICHQ and CHCA.94 Collectively these organizations have focused on preventing the two types of infection most pertinent to pediatric critical care: central line-associated blood stream infections and ventilator-associated pneumonia.56,94,108
Preventing Central Line-Associated Blood Stream Infections
Because critically ill children are often dependent on vascular access for monitoring and for fluid, blood product, and medication administration, catheter line-associated blood stream infections are a significant patient safety risk in pediatric critical care units. In pediatric critical care units more than 90% of blood stream infections are associated with central venous catheters; most are caused by skin bacterial flora such as coagulase-negative staphylococcus.94,101 Mortality from central line-related blood stream infections can be as high as 18%.10 Substantial reduction in central line-associated blood stream infections is possible through focused quality improvement efforts such as the application of the Institute for Healthcare Improvement’s (IHI) Central Line Bundle of recommended practices and the related Pediatric Affinity Group recommended “change package” of interventions proven to reduce blood stream infections.1,14,108
Practices to reduce the incidence of central line-associated blood stream infections include:
The Central Line Bundle recommended by the IHI is described further in Box 22-6.
Box 22-6 Practices to Reduce the Incidence of Central Line-Associated Blood Stream Infections, Adapted from the Institute for Health Care Improvement Central Line Bundle*
Hand Hygiene
• Before and after inserting and handling catheter insertion site
• Before and after touching catheter insertion site
• Touch only before antisepsis for placement or with aseptic technique
• Before and after inserting, replacing, accessing, repairing, or dressing a CVC
• When hands are obviously soiled or contaminated
Maximal Barrier Precautions
• Use a central line checklist that includes barrier precautions
• Keep equipment, including barriers, stocked in a central line cart that is readily accessible
• Prevent unnecessary interruption of barriers to get supplies
• Create a culture in which clinicians remind each other to maintain precautions
Chlorhexidine Skin Antisepsis for Children Older Than 2 Months
Prepare skin for line insertion with a solution of 2% chlorhexidine, 70% isopropyl alcohol
Adapted from the Institute for Healthcare Improvement: 5 Million lives campaign: how-to guide—prevent central line infections. Copyright 2007, Institute for Healthcare Improvement. Available at: http://www.ihi.org/explore/CentralLineInfection/Pages/default.aspx. Accessed October 8, 2011.45
Ventilator-Associated Pneumonia
Along with central line-associated blood stream infections, the IHI and the NICHQ-CHCA Pediatric Affinity Group identify ventilator-associated pneumonia (VAP) as a critical patient safety improvement effort for pediatric critical care units.23,94,108 VAP is defined as pneumonia that evolves at least 48 h after endotracheal intubation and the start of mechanical ventilation. Approximately 5.1% of children receiving mechanical ventilation develop VAP.27 Risk factors for VAP in children include antibiotic administration, underlying chronic condition, gastroesophageal reflux, supine position, presence of a nasogastric tube, immobility, and surgery of the head, neck, or upper abdomen.23,94 The criteria for diagnosing VAP in children were adapted by Curley et al in 2006 from CDC criteria and include23:
• Mechanical ventilation for more than 48 h, fever (≥ 38° C), and leukopenia (< 4000 white blood cells [WBCs] per mm3) or leukocytosis (≥ 12,000 WBCs/mm3) with no other recognized source
There are additional customized criteria for children younger than 1 year, between 1 and 12 years old, 13 years or older, and for immunocompromised children.23 Interventions to prevent VAP have also been extrapolated from adult research and initiatives. They include:
The effect of daily sedation holidays in children and the appropriateness of venous thromboembolism prophylaxis have not been studied as well in children and should be enacted only after thoughtful consideration and an analysis of the risks and benefits.23 The IHI and Pediatric Affinity Group recommendations for the prevention of VAP are more fully described in Box 22-7.
Additional Care
• Provide oral hygiene (using chlorhexidine if patient is older than 2 months)
• Use in-line suction catheters or single use only open suction catheter systems and store suction devices in a clean environment
• Drain ventilator circuit away from patient every 2-4 h
• Change ventilator circuits and in-line suction catheters only when malfunctioning or visibly soiled
Adapted from Curley MA, et al: Tailoring the Institute for Health Care Improvement 100,000 Lives Campaign to pediatric settings: the example of ventilator-associated pneumonia. Pediatr Clin North Am 53:1231-1251, 2006; Stockwell JA: Nosocomial infections in the pediatric intensive care unit: affecting the impact on safety and outcome. Pediatr Crit Care Med 8(Suppl 2):S21-S37, 2007.
1 Aboelela S.W., Stone P.W., Larson E.L. Effectiveness of bundled behavioural interventions to control healthcare-associated infections: a systematic review of the literature. J Hosp Infect. 2007;66:101-108.
2 Agency for Healthcare Research and Quality. National healthcare quality report. Rockville, MD: U.S. Department of Health & Human Services; 2004.
3 AHRQ Hospital Survey on Patient Safety Culture. Available at http://www.ahrq.gov/qual/hospculture, 2008. Accessed on April 25
4 Aiken L.H., et al. Educational levels of hospital nurses and surgical patient mortality. J Am Med Assoc. 2003;290:1617-1623.
5 Aiken L.H., et al. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. J Am Med Assoc. 2002;288:1987-1993.
6 American Academy of Pediatrics Council on Clinical Information Technology, Gerstle R.S., Lehmann C.U. Electronic prescribing systems in pediatrics: the rationale and functionality requirements. Pediatrics. 2007;119(6):1229-1231.
7 American Hospital Association. Chicago;Hospital statistics: 1999.
8 American Society of Health System Pharmacists. Suggested definitions and relationships among medication misadventures, medication errors, adverse drug events, and adverse drug reactions. Am J Health Syst Pharm. 1998;55(2):165-166.
9 Apostolakis G., Barach P. Reporting and preventing medical mishaps: safety lessons learned from nuclear power. In: Youngberg B.J., Hatlie M., editors. The patient safety handbook. ed 1. Boston: Jones and Bartlett; 2004:205-224.
10 Armenian S.H., Singh J., Arrieta A.C. Risk factors for mortality resulting from bloodstream infections in a pediatric intensive care unit. Pediatr Infect Dis J. 2005;24(4):309-314.
11 Ash J.S., et al. The extent and importance of unintended consequences related to computerized provider order entry. J Am Med Informat Assoc. 2007;14(4):415-423.
12 Backster A., et al. Transforming the surgical “time-out” into a comprehensive “preparatory pause.”. J Card Surg. 2007;22(5):410-416.
13 Buckley M.S., et al. Direct observation approach for detecting medication errors and adverse drug events in a pediatric intensive care unit. Pediatr Crit Care Med. 2007;8:145-152.
14 Byrnes M.C., Coopersmith C.M. Prevention of catheter-related blood stream infection. Curr Opin Crit Care. 2007;13(4):411-415.
15 Canadian Root Cause Analysis Framework: A Tool for Identifying and Addressing the Root Causes of Critical Incidents in Healthcare. Available at http://www.patientsafetyinstitute.ca/English/toolsResources/rca/Pages/default.aspx. Accessed October 8, 2011
16 Carroll J.S., Rudolph J.W. Design of high reliability organizations in health care. Qual Saf Health Care. 2006;15(Suppl 1):i4-i9.
17 Cavalcante S.S., et al. Risk factors for developing nosocomial infections among pediatric patients. Pediatr Infect Dis J. 2006;25(5):438-445.
18 Centers for Disease Control and Prevention (National Center for Health Statistics. Births and deaths: preliminary data for 1998. Natl Vital Stat Rep. 1999;47(25):6.
19 Centers for Disease Control and Prevention. Monitoring hospital acquired infections to promote patient safety—United States, 1990-1999. Morbidity & Mortality Weekly Report. 2000;49:149-153.
20 Cochran G.L., et al. Errors prevented by and associated with bar-code medication administration systems. Joint Comm J Qual Patient Saf. 2007;33(5):293-301. 245
21 Committee on Quality Health Care in America, Institute of Medicine. Crossing the quality chasm: a new health system for the 21st century. Washington, DC: National Academy Press; 2001.
22 Conroy S., et al. Interventions to reduce dosing errors in children: a systematic review of the literature. Drug Saf. 2007;30(12):1111-11125.
23 Curley M.A., et al. Tailoring the Institute for Health Care Improvement 100,000 Lives Campaign to pediatric settings: the example of ventilator-associated pneumonia. Pediatr Clin North Am. 2006;53(6):1231-1251.
24 Deming W.E. The new economics for industry, government, education, ed 2. Cambridge, MA: MIT Press; 2000.
25 Deshpande K.S., et al. The incidence of infectious complications of central venous catheters at the subclavian, internal jugular, and femoral sites in an intensive care unit population. Crit Care Med. 2005;33(1):13-20. discussion 234-235
26 Duwe B., Fuchs B.D., Hansen-Flaschen J. Failure mode and effects analysis application to critical care medicine. Crit Care Clin. 2005;21(1):21-30. vii
27 Elward A.M., Warren D.K., Fraser V.J. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics. 2002;109(5):758-764.
28 Flanagan J. The critical incident technique. Psychol Bull. 1954;51:327-385.
29 Flin R., Maran N. Identifying and training non-technical skills for teams in acute medicine. Qual Saf Health Care. 2004;13(Suppl 1):i80-i84.
30 France D.J., et al. An observational analysis of surgical team compliance with perioperative safety practices after crew resource management training. Am J Surg. 2008;195(4):546-553.
31 Garbutt J., et al. Reporting and disclosing medical errors: pediatricians’ attitudes and behaviors. Arch Pediatr Adolesc Med. 2007;161(2):179-185.
32 Gerstle R.S., Lehmann C.U., American Academy of Pediatrics Council on Clinical Information Technology. Electronic prescribing systems in pediatrics: the rationale and functionality requirements. Pediatrics. 2007;119(6):e1413-e1422.
33 Grissinger M., Rich D. JCAHO: meeting the standards for patient safety. Joint Commission on Accreditation of Healthcare Organizations. J Am Pharmaceut Assoc. 2002;42(5 Suppl 1):S54-S55.
34 Hackbarth G., Milgate K. Using quality incentives to drive physician adoption of health information technology. Health Aff. 2005;24(1):1147-1149.
35 Hayden R.T., et al. Computer-assisted bar-coding system significantly reduces clinical laboratory specimen identification errors in a pediatric oncology hospital. J Pediatr. 2008;152(2):219-224.
36 Hicks R.W., Becker S.C., Cousins D.D. Harmful medication errors in children: a 5-year analysis of data from the USP’s MEDMARX program. J Pediatr Nurs. 2006;21(4):290-298.
37 Hinshaw A.S. Navigating the perfect storm: balancing a culture of safety with workforce challenges. Nurs Res. 2008;57(Suppl 1):S4-S10.
38 Holden J. How can we improve the nursing work? Matern Child Nurs. 2006;1(31):34-38.
39 Holdsworth M.T., et al. Incidence and impact of adverse drug events in pediatric inpatients. Arch Pediatr Adolesc Med. 2003;157(1):60-65.
40 Hunt E.A., et al. Simulation: translation to improved team performance. Anesthesiol Clin. 2007;25(2):301-319.
41 Hunter J.G. Extend the universal protocol, not just the surgical time out. J Am Coll Surg. 2007;205(4):e4-e5.
42 Iedema R., et al. Narrativizing errors of care: critical incident reporting in clinical practice. Soc Sci Med. 2006;62:134-144.
43 Institute for Healthcare Improvement: Failure modes and effects analysis tool. Available at http://www.ihi.org/knowledge/Pages/Tools/FailureModesandEffectsAnalysisTool.aspx. Accessed October 8, 2011
44 Institute for Healthcare Improvement: How to improve. Available at http://www.ihi.org/IHI/Topics/Improvement/ImprovementMethods/HowToImprove. Accessed April 25, 2008
45 Institute for Healthcare Improvement: 5 Million lives campaign: how-to guide: prevent central line infections. Copyright 2007 Institute for Healthcare Improvement. Available at http://www.ihi.org/NR/rdonlyres/0AD706AA-0E76-457B-A4B0-78C31A5172D8/0/CentralLineInfectionsHowtoGuide.doc. Accessed April 25, 2008
46 Joint Commission: Sentinel Events. Available at http://www.jointcommission.org/SentinelEvents. Accessed April 25, 2008
47 Joint Commission: Preventing pediatric medication errors. Sentinel Event Alert 39, April 2008. Available at http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_39.htm. Accessed April 25, 2008
48 Jolley J., et al. Rapid response teams: do they make a difference? Dimens Crit Care Nurs. 2007;26(6):253-260.
49 Kaushal R., et al. Medication errors and adverse drug events in pediatric inpatients. J Am Med Assoc. 2001;285(16):2114-2120.
50 Kaushal R., et al. Pediatric medication errors: what do we know? What gaps remain? Ambul Pediatr. 2004;4(1):73-81.
51 Kohn K.T., Corrigan J.M., Donaldson M.S. To err is human: building a safer healthcare System. Washington, DC: National Academy Press; 1999.
52 Kovner C., et al. Nurse staffing and postsurgical adverse events: an analysis of administrative data from a sample of U.S. hospitals, 1990-1996. Health Serv Res. 2002;37(3):611-629.
53 Landrigan C.P., et al. Effect of reducing interns’ work hours on serious medical errors in intensive care units. New Engl J Med. 2004;351(18):1838-1848.
54 Langley G.J., et al. The improvement guide: a practical approach to enhancing organizational performance. San Francisco, CA: Jossey-Bass; 1996.
55 Le Duff F., et al. Monitoring incident report in the healthcare process to improve quality in hospitals. Int J Med Informat. 2005;74(2–4):111-117.
56 Leape L.L., Berwick D.M. Five years after to err is human: what have we learned? J Am Med Assoc. 2005;293(19):2384-2390.
57 Leape L.L., et al. Systems analysis of adverse drug events. J Am Med Assoc. 2005;274(1):35-43.
58 McDonald C.J. Computerization can create safety hazards: a bar-coding near miss. Ann Intern Med. 2006;144(7):510-516.
59 McGreevy J.M., Otten T.D. Briefing and debriefing in the operating room using fighter pilot crew resource management. J Am Coll Surg. 2007;205(1):169-176.
60 McKeon L.M., Oswaks J.D., Cunningham P.D. Safeguarding patients: complexity science, high reliability organizations, and implications for team training in healthcare. Clin Nurse Spec. 2006;20(6):298-304.
61 Montgomery V.L. Effect of fatigue, workload, and environment on patient safety in the pediatric intensive care unit. Pediatr Crit Care Med. 2007;8(Suppl 2):S11-S16.
62 Mulder D. Minimizing mistakes. Beloit Memorial Hospital is focused on bedside bar coding to help eliminate medication errors. Healthc Informat. 2007;24(9):52-53.
63 Nance J.J. Admitting imperfection: revelations from the cockpit for the world of medicine. In: Youngberg B.J., Hatlie M., editors. The patient safety handbook. ed 1. Boston: Jones and Bartlett; 2004:187-203.
64 National Academy of Sciences. To err is human: building a safer healthcare system executive summary. Available at http://www.nap.edu2003. Accessed April 25, 2008
65 National Academy of Sciences. Crossing the quality chasm: a new health system for the 21st century executive summary. Available at http://www.nap.edu2003. Accessed April 25, 2008
66 National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Contr. 2004;32(8):470-485.
67 Needleman J., et al. Nurse-staffing levels and the quality of care in hospitals. New Engl J Med. 2002;346(22):1715-1722.
68 Nelson E.C., et al: Microsystems in health care: Part 1. Learning from high-performing front-line clinical units. [see comment].: Joint Comm J Qual Improv. 2002;28(9):472-493
69 Nishisaki A., Keren R., Nadkarni V. Does simulation improve patient safety? Self-efficacy, competence, operational performance, and patient safety. Anesthesiol Clin. 2007;25(2):225-236.
70 Norton E. Implementing the universal protocol hospital-wide. AORN J. 2007;85(6):1187-1197.
71 Nuckols T.K., et al. Programmable infusion pumps in ICUs: an analysis of corresponding adverse drug events. J Gen Intern Med. 2008;23(Suppl 1):41-45.
72 Oriol M.D. Crew resource management: applications in healthcare organizations. J Nurs Admin. 2006;36(9):402-406.
73 Page A., editor. Keeping patients safe: transforming the work environments of nurses. Washington, DC: National Academies Press, 2004.
74 Pape T.M. Applying airline safety practices to medication administration. MEDSURG Nurs. 2003;12(2):77-93. quiz 94
75 Poole D.L., et al. Medication reconciliation: a necessity in promoting a safe hospital discharge. J Healthc Qual. 2006;28(3):12-19.
76 Prince S.B., Herrin D.M. The role of information technology in healthcare communications, efficiency, and patient safety: application and results. J Nurs Admin. 2007;37(4):184-187.
77 Pronovost P.J., et al. Creating high reliability in health care organizations. Health Serv Res. 2006;41(4 Pt 2):1599-1617.
78 Pronovost P.J., et al. Intensive care unit nurse staffing and the risk for complications after abdominal aortic surgery. Effect Clin Pract. 2001;4(5):199-206.
79 Reason J.T. Human error: models and management. Br Med J. 2000;320:768-770.
80 Resar R.K. Making noncatastrophic health care processes reliable: learning to walk before running in creating high-reliability organizations. Health Serv Res. 2006;41(4 Pt 2):1677-1689.
81 Robinson D.L., Heigham M., Clark J. Using Failure Mode and Effects Analysis for safe administration of chemotherapy to hospitalized children with cancer. Joint Comm J Qual Patient Saf. 2006;32(3):161-166.
82 Rogers A.E., et al. The working hours of hospital staff nurses and patient safety. Health Aff. 2004;23(4):202-212.
83 Roman N. Innovative solutions: standardized concentrations facilitate the use of continuous infusions for pediatric intensive care unit nurses at a community hospital. Dimens Crit Care Nurs. 2005;24(6):275-278.
84 Sandlin D. Improving patient safety by implementing a standardized and consistent approach to hand-off communication. J Perianesth Nurs. 2007;22(4):289-292.
85 Scanlon M.C., Mistry K.P., Jeffries H.E. Determining pediatric intensive care unit quality indicators for measuring pediatric intensive care unit safety. Pediatr Crit Care Med. 2007;8(Suppl 2):S3-S10.
86 Schwarz M., Wyskiel R. Medication reconciliation: developing and implementing a program. Crit Care Nurs Clin North Am. 2006;18(4):503-507.
87 Scott L.D., et al. Effects of critical care nurses’ work hours on vigilance and patients’ safety. Am J Crit Care. 2006;15(1):30-37.
88 Sebat F., et al. Effect of a rapid response system for patients in shock on time to treatment and mortality during 5 years. Crit Care Med. 2007;35(11):2568-2575.
89 Shamliyan T.A., et al. Just what the doctor ordered. Review of the evidence of the impact of computerized physician order entry system on medication errors. Health Serv Res. 2008;43(1 Pt 1):32-53.
90 Sharek P.J., et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a Children’s Hospital. J Am Med Assoc. 2007;298(19):2267-2274.
91 Sheridan-Leos N., Schulmeister L., Hartranft S. Failure mode and effect analysis: a technique to prevent chemotherapy errors. Clin J Oncol Nurs. 2006;10(3):393-398.
92 Singer S., et al. Workforce perceptions of hospital safety culture: development and validation of the patient safety climate in healthcare organizations survey. Health Serv Res. 2007;42(5):1999-2021.
93 Spooner S.A., Council on Clinical Information Technology, American Academy of Pediatrics. Special requirements of electronic health record systems in pediatrics. Pediatrics. 2007;119(3):631-637.
94 Stockwell J.A. Nosocomial infections in the pediatric intensive care unit: affecting the impact on safety and outcome. Pediatr Crit Care Med. 2007;8(2 Suppl):S21-S37.
95 Sullivan E.E. Hand-off communication. J Perianesth Nurs. 2007;22(4):275-279.
96 Sundar E., et al. Crew resource management and team training. Anesthesiol Clin. 2007;25(2):283-300.
97 Taylor J.A., et al. Use of incident reports by physicians and nurses to document medical errors in pediatric patients. Pediatrics. 2004;114(3):729-735.
98 Taylor J.A., et al. Evaluation of an anonymous system to report medical errors in pediatric inpatients. J Hosp Med (Online). 2007;2(4):226-233.
99 Taylor J.A., et al. Medication administration variances before and after implementation of computerized physician order entry in a neonatal intensive care unit. Pediatrics. 2008;121(1):123-128.
100 Tepfers A., Louie H., Drouillard M. Developing an electronic incident report: experiences of a multi-site teaching hospital. Healthc Q. 2007;10(2):117-122.
101 Urrea M., et al. Prospective incidence study of nosocomial infections in a pediatric intensive care unit. Pediatr Infect Dis J. 2003;22(6):490-494.
102 Valentin A., Bion J. How safe is my intensive care unit? An overview of error causation and prevention. Curr Opin Crit Care. 2007;13(6):697-702.
103 Valentin A., et alResearch Group on Quality Improvement of European Society of Intensive Care Medicine, Sentinel Events Evaluation Study Investigators:. Patient safety in intensive care: results from the multinational Sentinel Events Evaluation (SEE) study. Intensive Care Med. 2006;32(10):1591-1598.
104 Varkey P., et al. Multidisciplinary approach to inpatient medication reconciliation in an academic setting. Am J Health Syst Pharm. 2007;64(8):850-854.
105 Varkey P., Reller M.K., Resar R.K. Basics of quality improvement in healthcare. Mayo Clinic Proc. 2007;82(6):735-739.
106 Veterans Administration National Center for Patient Safety 1: Root cause analysis. Available at http://www.va.gov/NCPS/rca.html. Accessed April 25, 2008
107 Veterans Administration National Center for Patient Safety 2: Root cause analysis. Available at http://www.va.gov/NCPS/CogAids/Triage/index.html?8. Accessed April 25, 2008
108 Wachter R.M., Pronovosta P.J. The 100,000 Lives Campaign: a scientific and policy review. Joint Comm J Qual Patient Saf. 2006;32(11):621-627.
109 Wald H., Shojania K.G. Incident reporting. In: Agency for Health Care Research & Quality: Making health care safer: a critical analysis of patient safety practices. Rockville, MD: AHRQ; 2001:41-51.
110 Walsh K.E., et al. Medication errors related to computerized order entry for children. Pediatrics. 2006;118(5):1872-1879.
111 Walsh K.E., Kaushal R., Chessare J.B. How to avoid paediatric medication errors: a user’s guide to the literature. Arch Dis Child. 2005;90(7):698-702.
112 Walsh K.E., et al. Effect of computer order entry on prevention of serious medication errors in hospitalized children. Pediatrics. 2008;121(3):e421-e427.
113 Wang J.K., et al. Prevention of pediatric medication errors by hospital pharmacists and the potential benefit of computerized physician order entry. Pediatrics. 2007;119(1):e77-85.
114 Weick K., Sutcliff K. Managing the unexpected: assuring high performance in and age of complexity. San Francisco: Jossey-Bass; 2001.
115 Wetterneck T.B., et al. Using failure mode and effects analysis to plan implementation of smart i.v. pump technology. Am J Health Syst Pharm. 2006;63(16):1528-1538.
116 Zenker P., et al. Implementation and impact of a rapid response team in a children’s hospital. Joint Comm J Qual Patient Saf. 2007;33(7):418-425.

 Be sure to check out the supplementary content available at
Be sure to check out the supplementary content available at