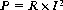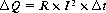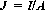Chapter 39 Fundamentals of minimally invasive radiofrequency applications in ear, nose and throat medicine
Ear, nose and throat (ENT) medicine currently has several different systems at its disposal for minimally invasive treatments which apply high frequency electric current to achieve a therapeutic effect. This chapter is intended to provide a survey of the technical fundamentals and tissue effects of such systems.
1 SYSTEM OVERVIEW
1.1 TYPE OF APPLICATION
Four types of application of high-frequency energy can be distinguished:
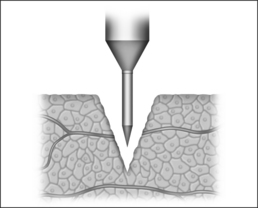
1.1.1 SUBMUCOUS COAGULATION
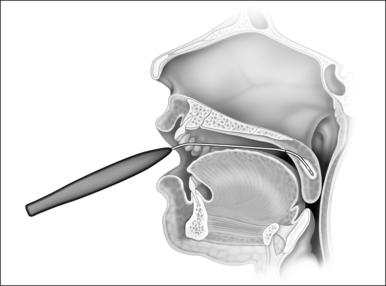
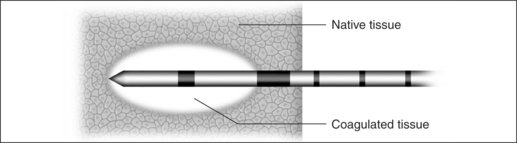
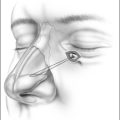
In the process of submucous coagulation, needle-shaped electrodes puncture the surface of the organ and are subsequently positioned inside the organ (Fig. 39.1). When energy is applied, thermally induced coagulation builds up around the electrode, usually of ellipsoid shape, depending on the construction of the electrode. If positioning and energy dose are correct, the organ’s surface is conserved and the application is almost entirely free of pain. The body’s own decomposition and discharge of the necrotic tissue leads to a reduction in volume in the region treated.
1.1.2 SUPERFICIAL VAPORIZATION
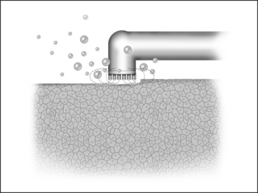
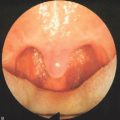
The reduction in size of an organ, e.g. a tonsil, may also be accomplished by means of direct tissue ablation via vaporization (tissue vaporization) on the surface (Fig. 39.2).
1.1.3 SUBMUCOUS VAPORIZATION
In the process of submucous vaporization, so-called ‘channeling’, channels are bored in the affected organ. For this purpose, a special bipolar electrode is necessary, at the tip of which a plasma ignition process takes place. When the tip comes into contact with tissue, the latter vaporizes immediately. A channel is generated by pushing the electrode forward in the tissue. More details on so-called plasma applications are to be found in Section 2.4.3.
1.2 THERAPY EFFECT
1.2.1 VOLUME SHRINKAGE BY THE BODY’S OWN DISCHARGE OF COAGULATION NECROSIS/TISSUE STABILIZATION BY SCAR FORMATION AFTER COAGULATION NECROSIS
Following thermo-coagulation several phases take effect in the volume of tissue involved.
1.3 EQUIPMENT TECHNOLOGY
1.3.1 MONOPOLAR AND BIPOLAR APPLICATION TECHNOLOGY
Monopolar technology
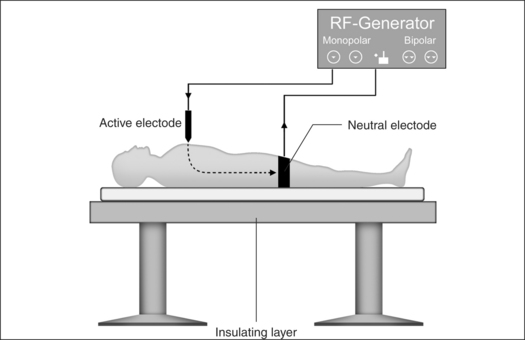
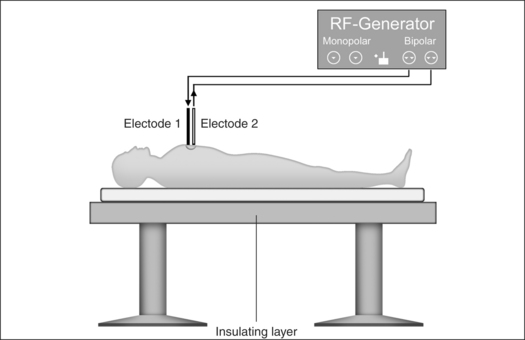
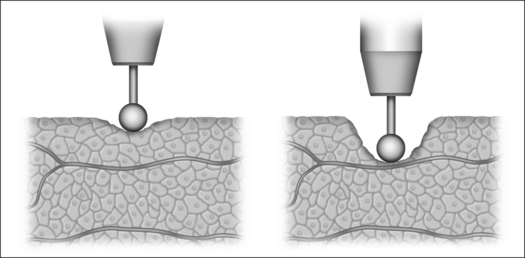
Monopolar technology, where one of the two electrodes required to close the circuit is connected to the patient as a large-surface return path, is most commonly used in radiofrequency surgical applications to date. The actual working or active electrode is in the shape of a small-surface surgical instrument, e.g. in the form of a needle, a lancet or a ball. It is from the latter electrode that the radiofrequency alternating current from the generator is conducted into the patient, producing the desired surgical effect as a result of high current density at the point where the tissue is touched. The radiofrequency current disperses rapidly in the tissue and flows with lower current density through the body of the patient to the neutral electrode, whence it flows back to the generator (Fig. 39.4).
A considerable number of complications may arise from the use of radiofrequency surgery, which are excluded when bipolar technology is applied.1
Problem: neutral electrode
Attachment of a neutral electrode to the patient is necessary when monopolar technology is used. In this context, attention must be paid to the certainty of low contact resistance. The current density has to be evenly distributed over the entire surface of the neutral electrode. Perspiration, hairs or fatty substances between skin and the electrode surface may mean the current is unable to utilize the entire transfer surface, leading to current densities which are too high in certain places and which may in turn lead to burns.2
Problem: current flow through the body between active and neutral electrode
Thermal tissue damage may occur in parts of the body between the active and neutral electrodes where the cross- section available for current flow is small and electrical resistance high.2 The current flow is particularly problematic in the region of head and neck: the greater part of the volume concerned here consists of bone as well as air-filled nasal and oral cavities or paranasal sinuses. In certain places only the thin mucous membrane layer remains as conducting residual cross-section.
The application of monopolar high-frequency technology for patients with cardiac pacemakers or catheters may only be carried out in exceptional cases. The specialist literature contains different documented opinions and research results on the interaction between radiofrequency current and pacemakers.3
Bipolar technology
Although bipolar technology has been known for some considerable time,4 it was only in the mid-1980s that renewed efforts were made to press ahead and further develop bipolar radiofrequency technology for reasons of safety, both for coagulation and cutting purposes.
Bipolar application technology (Fig 39.5) is characterized in that both electrodes, integrated in an application handset, are brought as close together as possible. The current only passes immediately between the two electrodes, meaning that secondary thermal damage to the patient, both internal and external, caused by leakage currents or marked changes in impedance (cross-sections with a high percentage of bone or fat and poorly conducting residual cross-sections) can be avoided. Since the attachment of a neutral electrode is not required in bipolar radiofrequency surgery, and the current flow is restricted to the point of surgical intervention, the risks involved in monopolar technology as described above can principally be avoided (Fig. 39.6).
1.3.2 PROCESS MONITORING AND POWER REGULATION
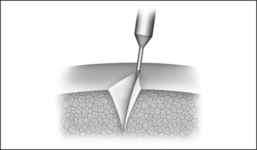
The size of a submucous coagulation (Fig. 39.7) depends on numerous parameters. Worth mentioning in this case are the electrode geometry, the power and the application time. Tissue resistance, tissue temperature or the energy input provide information on the tissue changes achieved.
Determination of energy input
A correlation exists between energy input and coagulation volume in the process of submucous tissue coagulation. If a certain quantity of electrical energy is introduced into the tissue under treatment, using a particular electrode geo-metry, a reproducible coagulation volume is the result. This only applies, however, as long as the marginal conditions of the coagulation process do not change. A change in the electrode position (distance from tissue surface or from larger vessels), in the temperature of the tissue or in the blood perfusion rate has the result that a different coagulation volume is produced from a certain quantity of energy.
1.4 THERAPY SYSTEMS CURRENTLY ON THE MARKET
1.4.1 CELON AG MEDICAL INSTRUMENTS
1.4.4 DIVERSE RADIOFREQUENCY SURGICAL EQUIPMENT
2 BASICS OF RADIOFREQUENCY SURGERY
Reidenbach has defined radiofrequency surgery as follows5: ‘radiofrequency surgery is understood as the application of radiofrequency energy for the purpose of changing or destroying tissue cells and of cutting through or removing tissue in combination with mechanical operation techniques’.
2.1 HISTORICAL RETROSPECTIVE
In the middle of the 19th century, the so-called ‘Paquelin burner’ was developed as a thermocauter (instrument for thermocautery) and the so-called ‘galvanic cauter’ as an electrocauter. The first of these consisted of a metal pin heated to over 1000°C by a fuel/air mixture and used for the destruction of tumorous tissue. The electrocauter, on the other hand, made it possible to separate or slough off biological tissue by means of a knife or platinum sling raised to red heat by direct current. Prior to the introduction of high-frequency technology at the beginning of the 20th century, such ‘cauterization’ was the method usually applied in the field of surgery.
In 1891, the French physicist d’Arsonval reported on thermal effects induced in biological tissue by using alternating current at frequencies above 250 kHz without stimulation of muscles or nerves (so-called ‘Faradic effect’).6 At the beginning of the 20th century needle electrodes which were inserted into the tissue (so-called ‘electro-desiccation’) were used by Clark for the first time. Between 1907 and 1910 ‘coagulation’ was developed as a method for tissue destruction, particularly against cancer, by Doyen, Czerny and Nagelschmidt.4,7–9 In 1929, radiofrequency technology found its way into brain surgery with the development of suitable tube generators by Gulke and Heymann.10 At this time, a physicist, Bovie, and a surgeon, Cushing, were mainly responsible for the development of one of the first radiofrequency generators for the cutting and coagulation of tissue.11,12
2.2 PHYSICAL AND TECHNICAL BASICS
2.2.1 FARADIC EFFECT
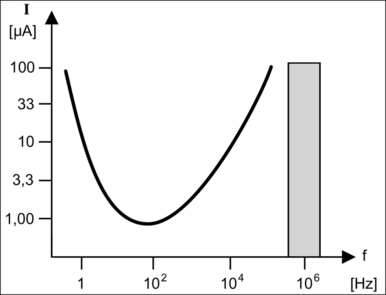
The Faradic effect is apparent using alternating currents of lower and middle frequency between 10 Hz and 10 kHz, which induce motor stimulation of muscle and nerve cells. Figure 39.8 shows the stimulation threshold curve where current is plotted against frequency. It can be seen that the threshold current possesses a minimum between 50 and 100 Hz, meaning that very low currents are enough to cause serious damage to humans within the range of the usual power frequencies. At 50 Hz even small currents may cause ventricular fibrillation.
Nernst13 has defined the ‘physiological stimulation’ of alternating currents as a function of the electric current I and the frequency f with the equation:
The Faradic stimulation effect increases with increasing current at constant frequency. If the current is constant and the frequency increases (from approximately 100 Hz onwards), the stimulation effect diminishes. The cellular ionic concentration gradient is reduced since ever smaller amounts of electricity can be transported within the individual half-waves of the alternating current. For frequencies in the range f>200…300 kHz, freedom from stimulation is assumed according to studies carried out by Gildemeister.14 If high-frequency alternating currents in the range between 300 kHz<f<2 MHz are used, the Faradic stimulation effect can be eliminated in the widest sense, even at higher currents. At frequencies of >2 MHz, uncontrolled ‘vagabond activity’ has to be reckoned with as a result of capacitive leakage currents, even over widely insulated stretches and beyond. The arising cable and radiation problems come into the foreground and should be avoided for safety reasons.
2.2.2 ELECTROLYTIC EFFECT
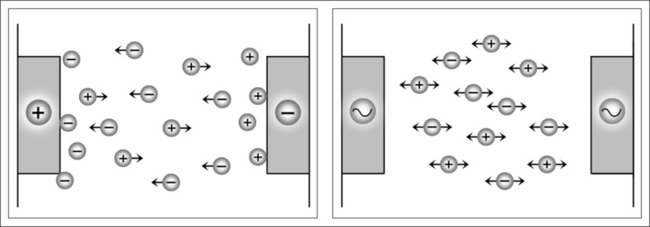
The electrolytic effect occurs mainly when direct current and low-frequency alternating currents are used. In such cases, intracellular as well as transcellular ionic migration occurs, with movement towards both electrodes, depending on the ionic charge. Cell membranes possess different permeability towards different electrolytes, with the effect that concentration tailbacks accumulate in these zones. Congestion of this nature destroys isotonia among the cells on the one hand and may lead to serious cell and tissue damage, such as tissue burns, on the other (Fig. 39.9).
2.2.3 THERMAL EFFECT
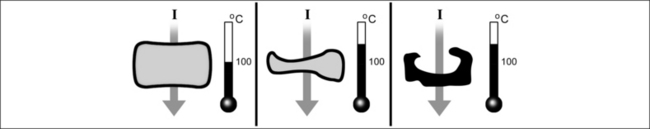
Radiofrequency surgery uses the thermal effect based on Joule’s law. Joule’s law states that the overall electrical output power P is released in the form of heat when an electric current I flows through an electrical resistance R. The relationship between power, resistance and current is as follows:
The quotient of current I and surface A is designated as electric current density J, where:
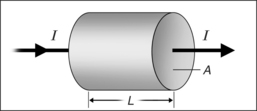
The electrical resistance R of a cylinder-shaped tissue element as shown in Figure 39.10 can be calculated from the specific resistance ρ, the surface area A through which the current passes and the cylinder length L, where:
By setting equations (4) and (3) into equation (2) and taking into account that the cylindrical volume is given by V=A 3 L, the heat generated per volume and time unit can be calculated from ΔQ/(V 3 Δt). This tissue heating is directly proportional to the product of the specific resistanceρ and the square of the electric current density J.
Equation (5) also shows that the heating is larger, the larger the specific resistance ρ of the conductor through which the current passes. In other words, if an electric current flows from a metal electrode into biological tissue, the heating takes place almost exclusively in the tissue itself since the specific resistance of the metal is many times smaller than that of the tissue.
2.3 TISSUE EFFECTS IN RADIOFREQUENCY CURRENT APPLICATIONS
No cell damage arises from heating the tissue to about 40°C. Further heat input and increasing temperature lead to reversible tissue damage, depending on the duration of exposure. Temperatures above 49°C lead to irreversible cell damage as a result of protein denaturation. This thermal destruction process is markedly dependent on time. The actual coagulation effect starts at a temperature between 60°C and 70°C. Macroscopically viewed, coagulation is accompanied by a white/yellow discoloration and shrinkage of the affected tissue matrix (Fig. 39.11).
If the temperature is raised to 100°C in a short period of time, the vaporization process is accelerated. The internal cell pressure increases to such an extent that the cell bursts, with subsequent tissue dissipation (Fig. 39.12).
At temperatures around 200°C, carbonization of the tissue takes place (Fig. 39.13). The carbonized layer has the effect of a thermal insulation shield, leading to a reduction of convective thermal conductivity after coagulation at too high a power level, and to inadequate hemostasis as a consequence. The wound healing process is considerably delayed in the carbonized tissue. At this location a ‘foreign body reaction’ occurs. The mutagenic effect of carbonized tissue is also a subject of discussion.
2.4 TYPES OF RADIOFREQUENCY CURRENT APPLICATION
2.4.1 ELECTROTOMY
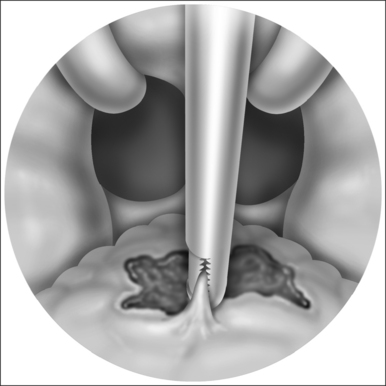
In electrotomy (Fig. 39.14), the cutting of tissue, a high current density is generated by means of small-surface electrodes at the point of transfer to the tissue. The electric power required for this purpose must be sufficient to heat the tissue above 100°C in the shortest time. As a result of this rapid heating or vaporization of the cell fluid, cell rupture, the actual cutting effect, occurs. In the above process, the electric power introduced generates a coagulation seam of greater or lesser depth at the cutting edges, which in turn seals smaller vessels and leads to a reduction of bleeding as a result.
As a result of the almost explosive nature of cell fluid vaporization immediately after starting the flow of current, an electrically insulating steam cushion is created which effectively lifts the cutting electrode away from the tissue. At sufficiently high generator voltage, this steam layer is penetrated by an electric arc. The arc concentrates the total electrical energy on a point in such a way that the tissue in the vicinity of the arc’s impact point either vaporizes or burns. In this process, more steam is created at the said location with the result that the insulating layer becomes much thicker here and the distance between electrode and tissue correspondingly larger. The result of this is that another point on the electrode reaches higher field strength and the spark flashover now takes place at another location. Over the entire length of the contact zone, tiny arcs are set off wherever the distance between electrode and tissue is momentarily least. In this way, the overall effective tissue contact surface is scanned by flashovers during the cutting process. Such flashovers occur predominantly in the cutting direction, making tissue excision possible as a result of the circumstances described above.
2.4.2 COAGULATION
In the process of electrocoagulation large-surface (several mm2) electrodes of cylindrical or conical shape are used as a rule (Fig. 39.15). The contact surfaces between electrode and tissue are larger and the high-frequency voltage smaller than those used in electrotomy, with the result that lower current densities are involved. It is a matter of good sense that the current densities worked with are such that the tissue is gradually heated above the coagulation temperature (approximately 60°C) and do not lead to vaporization (>100°C). The objective of coagulation is hemostasis by the closure of vessels, superficial coagulation or in-depth coagulation, by means of which the large-volume destruction of benign or malignant tumor tissue is usually carried out.
2.4.3 ‘PLASMA’ SURGERY
2 THE ELECTRODE IS LOCATED INSIDE THE TISSUE OR IS SURROUNDED BY A CONDUCTIVE FLUID
With this arrangement, a gas discharge may occur if a radio-frequency generator with sufficient power is available and impedances are low. The conductive fluid has to be heated in the shortest time by high currents to such an extent that it vaporizes. In this dehydrated zone at sufficiently high field strength a gas discharge occurs as described in the previous section. Once the spark has been ignited, tissue may be effectively vaporized as a result of the very high temperatures at its impact point. The electric current can continue to flow in order to maintain the spark as a result of the electrical conductivity of the plasma. The spark is only extinguished again if the generator is deactivated or too large a distance arises between electrode and tissue.
Mueller (1993) Mueller W. The advantage of laparoscopic assisted bipolar high-frequency surgery. Endosc Surg Allied Technol. 1993;1:91-96.
Reienbach (1993) Reienbach HD. Fundamentals of bipolar high-frequency surgery. Endosc Surg Allied Technol. 1993;1:85-90.
Jung (1995) Jung W, Neubrand M, Lüderitz B. Einsatz von Hochfrequenzstorm in der Endoskopie bei Patienten mit Herzschrittmachern. Endopraxis. 1995;2:22-25.
Doyen (1908) Doyen E. Bipolaire Voltaisation. Münch Med Wochenschr. 1908;48:2516.
Reidenbach (1983) Reidenbach HD. Hochfrequenz und Lasertechnik in der Medizin. Berlin: Springer-Verlag; 1983.
d’Arsonval (1891) d’Arsonval A. Action physiologique des courants alternatifs. C R Soc Biol. 1891;43:283-286.
Doyen (1910) Doyen E. Traitment local des cancers accessibles par l’action de la chaleur au-dessus de 55°C. Rev Thér Méd Chir. 1910;77:551-577.
Czerny (1910) Czerny V. Über Operationen mit dem Lichtbogen und Diathermie. Dtsch Med Wochenschr. 1910;35(11):489-493.
Nagelschmidt (1909) Nagelschmidt F. Über Hochfrequenzströme. Fulguration und Transhermie. Z Phys Diat Ther. 3, 1909.
Heymann (1930) Heymann E. Chirurgische Eingriffe mit Hochfrequenzströmen. Med Klin. 1930;15:539-545.
Bovie (1928) Bovie WT. New electro-surgical unit with preliminary note on new surgical current generator. Surg Gynecol Obstet. 1928;47:751-784.
Cushing (1928) Cushing H. Electrosurgery as an aid to the removal of intracranial tumors. Surg Gynecol Obstet. 1928;47:751-784.
Nernst (1908) Nernst W. Zur Theorie des elektrischen Reizes. Pflügers Archiv. 1908;122:275-315.
Gildemeister (1912) Gildemeister M. Über die im tierische Körper bei elektrischer Durchströmung entstandenen Gegenkräfte. Pflügers Archiv. 1912;149:389-400.