CHAPTER 86 FUNDAMENTALS OF MECHANICAL VENTILATION
Mechanical ventilation is often required to manage trauma or critical illness, whether for airway protection, administration of general anesthesia, or management of acute respiratory failure (ARF) (Table 1). New technology now provides several modes by which a patient may be ventilated, with the goals of improved gas exchange, better patient comfort, and rapid liberation from the ventilator. Moreover, noninvasive positive-pressure ventilation permits some cases of ARF to be managed without insertion of an artificial endotracheal airway, and some patients who are extubated with marginal reserves to avoid reintubation. Nearly all ventilators can be set to allow full support of the patient on the one hand, and periods of exercise on the other. Thus, the choice of ventilator settings is a matter of physician preference for the majority of patients (Table 2). Controlled ventilation with suppression of spontaneous breathing leads rapidly to respiratory muscle atrophy; therefore modes of assisted ventilation are preferred wherein machine-delivered breaths are triggered by the patient’s own inspiratory efforts. Basic modes of assisted ventilation include assist-control ventilation (ACV), synchronized intermittent mandatory ventilation (SIMV), and pressure support ventilation (PSV).
Table 1 General Indications for Mechanical Ventilation
Table 2 Glossary of Basic Terminology of Mechanical Ventilation
| Control: regulation of gas flow |
| Cycling: ventilator switching from inhalation to exhalation after volume or pressure target (or limit) has been reached |
| Triggering: causes the ventilator to cycle to inhalation |
| Breaths: cause the ventilator to cycle from inhalation to exhalation |
| Flow pattern: constant, decelerating, or sinusoidal |
| Mode (breath pattern) |
|
Controlled mechanical ventilation—controlled ventilation, without allowances for spontaneous breathing, typical of anesthesia ventilators
|
NONINVASIVE VENTILATION
PRESSURE SUPPORT VENTILATION
Positive end-expiratory pressure (PEEP, also called continuous positive airway pressure [CPAP]) is added to restore functional residual capacity (FRC) to normal for the patient. When lung volumes are low, the work of breathing during early inhalation is reduced. Noncompliant lungs require higher intrapleural pressures to inflate to a normal VT, even with CPAP. The addition of pressure support (PS) assists the patient to move up the pressure–volume curve (larger changes in volume for a given applied pressure, i.e., increased lung compliance). The term “pressure support ventilation” describes the combination of pressure support and PEEP (or CPAP). Although useful in the patient breathing spontaneously, PS may be used to assist spontaneous breaths in SIMV. Weaning may be facilitated using this combination, as the backup (SIMV) rate is weaned initially, and then the PS.
MODES OF MECHANICAL VENTILATION
Positive End-Expiratory Pressure
The ideal level of PEEP is controversial (Table 3). It may be that which prevents derecruitment of the majority of alveoli, while causing minimal overdistension; alternatively, application of PEEP is a recruitment maneuver, arguing for higher pressures to be applied to overcome alveolar collapse. Applying PEEP to put the majority of lung units on the favorable part of the pressure–volume curve will maximize gas exchange and minimize overdistention, but is easier said than done because the lower inflection point is sometimes indistinct. Undoubtedly, the combination of PEEP and low VT prevents volutrauma, but the exact amount of PEEP to apply is controversial. The reason for this is hysteresis—the tendency of the lungs, due to surfactant, to exist at higher volumes in exhalation than in inhalation.
Table 3 Protocol Summary for Institution of Mechanical Ventilation for Acute Lung Injury/Acute Respiratory Distress Syndrome
PEEP, Positive end-expiratory pressure.
Adapted from Nathens AB, Johnson JL, Mine JP, et al. Inflammation and the Host Response to Injury Investigators: Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core—standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma 59:764–769, 2005.
Routine Settings
Ventilator settings are based on the patient’s ideal body mass and medical condition. The risk of oxygen toxicity from prolonged exposure to a fraction of inspired oxygen (FIO2) greater than 60% is minimized by using the lowest FIO2 that can oxygenate arterial blood satisfactorily (e.g., arterial oxygen tension [PaO2] of 60 mm Hg or an oxygen saturation [SaO2] of 88%) (see Table 3).
The normal lung (e.g., during general anesthesia) may be ventilated safely with VT 8–10 ml/kg for prolonged periods. Historically, critically ill patients with acute lung injury (ALI)/acute respiratory distress syndrome (ARDS) have been ventilated with VT 10–15 ml/kg of ideal body mass, which is now considered inappropriate due to convincing data from experiments indicating that alveolar overdistention can produce endothelial, epithelial, and basement membrane injuries associated with increased microvascular permeability and iatrogenic lung injury (VILI). Direct monitoring of alveolar volume is not feasible. A reasonable substitute is to estimate peak alveolar pressure as obtained from the plateau pressure (Pplat) measured in a relaxed patient by occluding the ventilator circuit briefly at end-inspiration. In patients with pulmonary dysfunction, there is a growing tendency to reduce the VT delivered to 4–6 ml/kg or less in order to achieve a Pplat no higher than 35 cm H2O. The incidence of VILI increases markedly when Pplat is high. Low VT ventilation may lead to an increase in PaCO2. Acceptance of elevated CO2 tension in exchange for controlled alveolar pressure is termed “permissive hypercapnia.” It is important to focus on pH rather than PaCO2 if this approach is employed. If the pH falls below 7.25, increase VE or administer NaHCO3.
Sedation
Most patients who require mechanical ventilation will require sedation, but only a minority (∼10%) will also require neuromuscular blockade. A panoply of agents are available for both (Table 4), so the choice of agent can be individualized for the patient, but caution must be exercised so that patients receive only what they need and are not oversedated. Titration of sedation such that patients are comfortable is facilitated by ordering sedation to be titrated to a sedation score of 3–4 points on the Ramsay or Riker scale (Table 5). Intermittent doses of sedatives are preferred to continuous infusions, also to attempt to minimize the amount of sedation. Neuromuscular blockade should be avoided whenever possible.
Table 4 Selected Formulary for Analgesia, Anesthesia, and Sedation in the Intensive Care Unit
| Agent | Initial IV Adult Dose | Comments |
|---|---|---|
| Induction Agents | ||
| Etomidate | ≥6 mg | Maintains CO and BP. Reduces ICP but maintains CPP. Short T½; use infusion for maintenance. Possible adrenal suppression. |
| Ketamine | 1–2 mg/kg | R apid-onset, short-duration agent for induction of anesthesia. Can be given by maintaining continuous infusion, and at lower dose for sedation without anesthesia. Transiently increases BP and HR, raises ICP and intraocular pressure. Usually does not depress breathing. Generally safe in pregnancy and for neonates and children. Concurrent narcotics or barbiturates may prolong recovery. Can cause anxiety, disorientation, dysphoria, and hallucinations, which may be reduced by a short-acting benzodiazepine during emergence. Atropine pretreatment is recommended to decrease secretions, but may increase incidence of dysphoria. Hepatic metabolism. |
| Propofol | 1.5–2.5 mg/kg | Provides no analgesia. Potent amnestic effect. Causes apnea and loss of gag reflex. Can cause marked low BP. Infuse at 0.05–0.3 mg/kg/min for prolonged sedation. Minimal accumulation (hepatic insufficiency) facilitates rapid elimination. Account for 1 kCal/ml (lipid infusion) in nutrition prescription. Use of same vial >12 hours associated with bacteremia. Safety for children still debated. |
| Intravenous Sedatives/Analgesics | ||
| Midazolam | 0.5–4 mg | Short T½, but accumulates during infusion owing to active metabolites. Only benzodiazepine with potent amnestic effect. Can cause low BP and loss of airway. Primary use is short-term sedation for ICU procedures. Renal elimination. |
| Lorazepam | 1–4 mg | Effective anxiolytic. Preferred agent for continuous infusion of benzodiazepine (starting dose 1 mg/hr). Can cause low BP, especially with hypovolemia, and paradoxical agitation. Hepatic elimination. |
| Morphine | 2–10 mg | Analgesic and sedative effects. Can cause low BP, CO, and apnea. Tolerance and withdrawal possible after long-term use. Can be given as IV infusion or by PCA for analgesia or to facilitate prolonged mechanical ventilation or withdrawal of care. Hepatic elimination. |
| Hydromorphone | 0.5–2.0 mg | Hydrated ketone of morphine with similar use and risk profiles. Approximately eight-fold more potent than morphine. Hepatic elimination. |
| Fentanyl | 50–100 mcg | Approximately 50-fold potency compared with morphine, but less likely to cause low BP in appropriate dosage (less histamine release). Versatile for ICU use given IV or by epidural infusion or PCA. Less potent than local anesthetics for epidural analgesia or abrogation of surgical stress response. Can cause truncal rigidity and apnea with inability to ventilate by hand (use neuromuscular blockade to facilitate intubation in that setting). Hepatic elimination. |
| Neuromuscular Blocking Agents | ||
| Succinylcholine | 0.75–1.5 mg/kg | Only dep olarizing NBMA (occupies ACh receptor). Rapid onset, effect dissipates within 10 minutes of single dose. Causes hyperkalemia. Can precipitate malignant hyperthermia. Increases ICP and intraocular pressure. Contraindicated in TBI, spinal cord injury, neuromuscular disease, and burns. Metabolized by plasma cholinesterase; absence of enzyme (relatively common) causes prolonged paralysis. |
| Atracurium | 0.2–0.5 mg/kg | Short-acting nondepolarizing NMBAs (competitive inhibitor of ACh). |
| Cisatracurium | 0.2–0.5 mg/kg | Relatively slow in onset (also competitive inhibitor of ACh). Atracurium and cisatracurium are similar, except that the former causes histamine release and can cause high HR and low BP. Cisatracurium now used preferentially. Short acting, requires IV infusion for prolonged effect. Effect potentiated by hypokalemia. Many drug interactions. Metabolized by Hoffman elimination/ester hydrolysis, and is thus used for patients with renal/hepatic insufficiency. |
| Pancuronium | 0.05–0.1 mg | Rapid onset, prolonged effect. Causes increased BP and HR. Used for induction of neuromuscular blockade, but should be converted to a drug such as continuousinfusion cisatracurium for maintenance. Renal/hepatic elimination, accumulates in organ dysfunction. |
| Vecuronium | 0.08–0.10 mg/kg | Nondepolarizing NMBA with rapid onset and short duration of action. Less potential for histamine release. Can cause malignant hyperthermia. Metabolized by liver. |
| Miscellaneous Agents | ||
| Dexmedetomidine | 1 mcg/kg load, then 0.2–0.7 mcg/kg/hr | Central selective α2 agonist used for short-term (<24 hours) sedation. Sympatholysis lowers HR and BP. Can achieve light sedation, does not depress respirations. No anamnestic effect. Useful for drug/alcohol withdrawal and sedation when liberation from mechanical ventilation is imminent. Expensive. |
| Haloperidol | 2–5 mg | Used commonly for anxiolysis (often over lorazepam), especially when respiratory depression is undesirable. IV administration, not FDA-approved, is commonplace. Antidopaminergic properties contraindicate use in Parkinson disease. Causes extrapyramidal effects. Hepatic elimination. |
| Ketorolac | 0.5–1.0 mg/kg | Parenteral NSAID used in lieu of opioids or for opioid-sparing effect in combination. Irreversible platelet dysfunction; can cause incisional or GI hemorrhage and acute renal failure. Use strictly limited to less than 5 days in postoperative period. |
| Reversal Agents | ||
| Flumazenil | 0.1–0.2 mg | Benzodiazepine antagonist. Rapid onset and short duration. Adverse effect of benzodiazepine can persist after drug wears off. Repeated doses of up to 0.8 mg can be used. Abrupt antagonism of chronic benzodiazepine use can precipitate seizures. |
| Naloxone | 0.4 mg | Opioid antagonist. Rapid onset and short duration. Often diluted 0.4 mg/10 ml and titrated 0.04–0.08 mg at a time to reverse undesirable side effects while preserving analgesia. Repeated doses of up to 0.4 mg or continuous IV infusion can be used. Abrupt opioid antagonism can precipitate hypertension, increased HR, pulmonary edema, or myocardial infarction. |
| Edrophonium with atropine |
ACh, Acetylcholine; BP, blood pressure; CO, cardiac output; CPP, cerebral perfusion pressure; ICP, intracranial pressure; FDA, U.S. Food and Drug Administration; GI, gastrointestinal; HR, heart rate; IV, intravenous; NBMA, neuromuscular blocking agent; NSAID, nonsteroidal anti-inflammatory drug; PCA, patient-controlled analgesia; T½, elimination half-life; TBI, traumatic brain injury; VO2, oxygen consumption.
Table 5 Sedation Scales in Common Usage
| Value | Clinical Correlate | |
|---|---|---|
| Ramsay Sedation Score | ||
| Awake scores 1–3 | 1 | Anxious, agitated, or restless |
| 2 | Cooperative, oriented, tranquil | |
| 3 | Responsive to commands | |
| Asleep scores 4–6 | 4 | Brisk response to stimulus a |
| 5 | Sluggish response to stimulus | |
| 6 | No response to stimulus | |
| Riker Sedation-Agitation Scale | ||
| Dangerous agitation | 7 | Pulling at catheters, striking staff |
| Very agitated | 6 | Does not calm to voice, requires restraint |
| Agitated | 5 | Anxious, responds to verbal cues |
| Calm and cooperative | 4 | Calm, awakens easily, follows commands |
| Sedated | 3 | Awakens to stimulus |
| Very sedated | 2 | Arouses to stimulus, does not follow commands |
| Unarousable | 1 | Minimal or no response to noxious stimulus |
a Stimulus is light glabellar tap or loud auditory stimulus.
Adapted from Ramsay M, Savege T, Simpson B, et al: Controlled sedation with alphaxalon-alphadolone. BMJ 2:656–659, 1974; and Riker RR, Picard JT, Fraser GL: Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med 27:1325–1329, 1999.
MONITORING
Capnography
Other information is acquired from capnography as well. Prognostically, an ETCO2-PaCO2 gradient of 13 mm Hg or more after resuscitation has been associated with increased mortality in trauma patients. A sudden decrease or even disappearance of ETCO2 can be correlated with potentially serious pathology or events, such as a low cardiac output state, disconnection from the ventilator, or pulmonary thromboembolism. A gradual increase of ETCO2 can be seen with hypoventilation; the converse is also true. Another cause of gradually decreasing ETCO2 is hypovolemia.
INVASIVE HEMODYNAMIC MONITORING
Arterial Catheterization
Arterial catheters may be placed in any of several locations. The catheter should be a special-purpose thin-walled catheter to maintain fidelity of the waveform and to avoid obstructing the vessel lumen; a standard intravenous cannula should not be used. The radial artery at the wrist is the most commonly used site; although the ulnar artery is usually of larger diameter, it is relatively inaccessible percutaneously. Careful confirmation of a patent collateral circulation to the hand is mandatory before cannulation of an artery at the wrist, to minimize potential tissue loss from arterial occlusion or embolization. In neonates, the umbilical artery may be catheterized; intestinal ischemia is a rare complication. The axillary artery is relatively spared by atheromata, supported by good collaterals at the shoulder, and easy to cannulate percutaneously, making it a suitable choice. The superficial femoral artery may also be used, but is not a location of choice because the burden of plaque (and therefore the risk of distal embolization) is higher, as is the infection rate. The superficial temporal artery is difficult to cannulate because of small caliber and tortuosity. The dorsalis pedis artery is accessible, but should be avoided in patients with peripheral vascular disease. The brachial artery should be strictly avoided, because the collateral circulation around the elbow is poor and the risk of ischemia of the hand or forearm is high. Severe peripheral vasoconstriction due to vasopressor therapy may necessitate a longer catheter at a more central location (e.g., axillary, femoral) in order to place the catheter tip into an artery in the torso that would be less affected. Nosocomial infection of arterial catheters is unusual, provided that basic tenets of infection control are honored and femoral artery catheterization is avoided. Other complications from arterial catheterization include bleeding, hematoma, and pseudoaneurysm.
CLINICAL USE OF THE PULMONARY ARTERY CATHETER
Many indications have been championed for the PAC, but no studies have demonstrated unequivocally that PAC use decreases morbidity or mortality. Pulmonary artery catheters may still be useful in cardiomyopathy, shock of various etiologies, suspected pulmonary hypertension, or an unpredicted or poor response to conventional fluid therapy. Transesophageal echocardiography is an alternative, but it is not widely available outside cardiac surgery operating rooms. Critically ill patients receiving inotropic agents despite resuscitation with large volumes of fluid may also benefit from monitoring by PAC. However, a recent ARDSnet trial demonstrated no differences in outcome when ALI/ARDS was managed by PAC versus CVP monitoring.
LIBERATION FROM MECHANICAL VENTILATION
Some patients do not separate readily from the ventilator, which may be due to disease- or therapy-related reasons. Most clinical cases of failed liberation from the ventilator are multifactorial, but respiratory muscle fatigue is a common factor, in that the load on the respiratory system exceeds the capacity to breathe (Table 6). The increased load may take the form of a demand for increased VE, or increased work of breathing. Increased VE may result from increased CO2 production, increased dead space (VD) ventilation, or increased ventilatory drive. Increased CO2 production may be caused by a catabolic state, or excess carbohydrate administered during nutritional support. Increased VD (ventilation of unperfused or under-perfused lung) may be caused by decreased Q, pulmonary embolism, pulmonary hypertension, severe ALI, or iatrogenically from positive-pressure ventilation. Increased ventilatory drive may occur from muscle fatigue or failure, stimulation of pulmonary J receptors (usually by lung inflammation or parenchymal hemorrhage), or lesions of the central nervous system. Psychological stress is also an important factor that may manifest itself as tachypnea, hypoxemia, or agitation or delirium. Stress may be caused by inadequate analgesia or sedation, or untreated delirium. Acute alcohol or drug withdrawal is a major factor in some patients.
Patients who “fight” the ventilator technically have the syndrome of patient–ventilator dyssynchrony. The cause can usually be found and must be sought; to sedate the patient more deeply (or administer neuromuscular blockade) before correctable causes are identified and remedied is incorrect and may be catastrophic if an unstable airway is the cause. A systematic approach to evaluation is advocated; recognizing that the patient and the ventilator are supposed to be working in concert facilitates an understanding that the problem may be the patient or the ventilator. The cause may be found anywhere on the continuum from the alveolus to the power outlet or the source of respiratory gases, and must be sought systematically (Table 7). The first step is always to ensure that the patient has a patent airway that is positioned properly.
Table 7 Therapies to Reverse Ventilatory Failure
| Improve muscular function | |
| Reduce respiratory load | |
| Airway resistance | Ensure airway patency/adequate caliber |
| Compliance (elastance) | Treat pneumonia |
| Treat pulmonary edema | |
| Identify/reduce intrinsic PEEP (auto-PEEP) | |
| Drain large pleural effusions | |
| Evacuate pneumothorax | |
| Treat ileus (promotility agents) | |
| Decompress abdominal distention/treat abdominal compartment syndrome | |
| Position patient 30 degrees head up | |
| Minute ventilation | Treat sepsis |
| Antipyresis (temperature > 40° C | |
| Avoid overfeeding | |
| Correct metabolic acidosis | |
| Identify/reduce intrinsic PEEP (auto-PEEP) | |
| Bronchodilators | |
| Maintain least possible PEEP | |
| Resuscitate shock/correct hypovolemia | |
| Identify and treat pulmonary embolism | |
PEEP, Positive end-expiratory pressure.
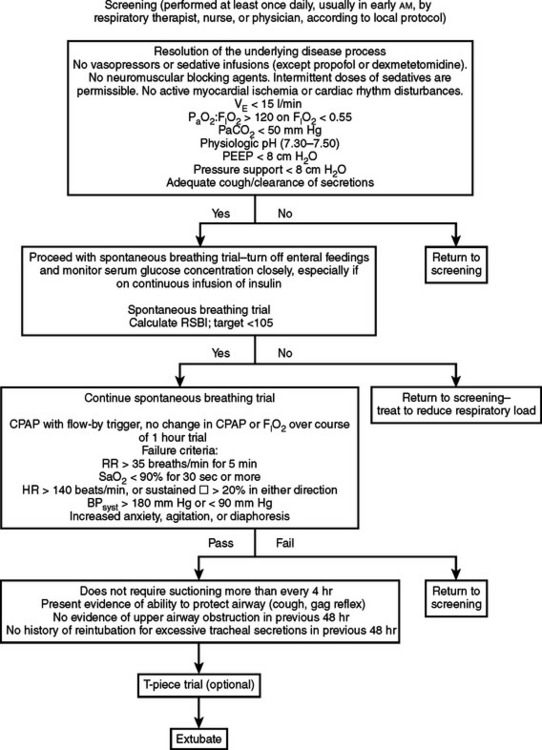
Liberation from mechanical ventilation can be easy in patients requiring short-term support. However, as many as 25% of patients will experience respiratory distress such that ventilation has to be reinstituted; patients recovering from acute respiratory failure, necrotizing pneumonia, or major torso trauma can be especially challenging. Patients who cannot be weaned have a characteristic response to trials of spontaneous breathing: there is an almost immediate increase in respiratory rate and decrease in VT. As the trial of spontaneous breathing continues over 30–60 minutes, work of breathing increases substantially by four- to seven-fold. Increased oxygen demand is met by increased oxygen extraction, which eventually causes decreased DO2 and arterial hypoxemia. Pulmonary compliance decreases, and gas trapping from lengthened I:E doubles measured auto-PEEP. The rapid, shallow breathing pattern causes CO2 retention because of increased dead space ventilation despite increased VE. There is considerable cardiovascular stress also, with pulmonary and systemic hypertension and increased afterload on both ventricles, likely from the extreme changes in intrathoracic pressure generated by the struggling patient.
The process of weaning begins by determining patient readiness (Figure 1). Patients should be screened carefully for hemodynamic stability, cooperative mental status, respiratory muscle strength, consistent and adequate wakefulness, ability to manage secretions, nutritional repletion and normalization of acid–base and electrolyte status, and an artificial airway of adequate size. Particular attention should be given to acceptance of hypercapnia if chronically present and avoidance of new metabolic alkalosis. Finally, ensure normality of electrolytes affecting muscle function (e.g., calcium, phosphate, and potassium). If the aforementioned conditions are addressed, weaning may be attempted.
Acton RD, Hotchkiss JR, Dries DJ. Noninvasive ventilation. J Trauma. 2002;53:593-601.
Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med. 1998;339:429-435.
ARDS Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301-1308.
Arroliga A, Frutos-Vivar F, Hall J, et al. International Mechanical Ventilation Study Group. Use of sedatives and neuromuscular blockers in a cohort of patients receiving mechanical ventilation. Chest. 2005;128:496-506.
Auriant I, Jallot A, Hervé P, et al. Noninvasive ventilation reduces mortality in acute respiratory failure following lung resection. Am J Respir Crit Care Med. 2001;164:1231-1235.
Banner MJ, Kirby RR, MacIntyre NR. Patient and ventilator work of breathing and ventilatory muscle loads at different levels of pressure support ventilation. Chest. 1991;100:531-533.
Bouderka MA, Fakhir B, Bouaggad A, et al. Early tracheostomy versus prolonged endotracheal intubation in severe head injury. J Trauma. 2004;57:251-254.
Brochard L, Pluskwa F, Lemaire F. Improved efficacy of spontaneous breathing with inspiratory pressure support. Am Rev Respir Dis. 1987;136:411-415.
Brochard L. Inspiratory pressure support. Eur J Anaesthesiol. 1994;11:29-36.
Brochard L, Rauss A, Benito S, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. Am J Respir Crit Care Med. 1994;150:896-903.
Consales G, Chelazzi C, Rinaldi S, De Gaudio AR. Bispectral Index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol. 2006;72:329-336.
Cook DJ, Meade MO, Hand LE, McMullin JP. Toward understanding evidence uptake: semirecumbency for pneumonia prevention. Crit Care Med. 2002;30:1472-1477.
Dodek P, Keenen S, Cook D, et al. Evidence-based clinical guideline for the prevention of ventilator-associated pneumonia. Ann Intern Med. 2004;141:305-313.
Eachempati SR, Barie PS. Monitoring respiratory function and weaning from the ventilator. In: Bland K, editor. The Practice of General Surgery. Philadelphia: WB Saunders; 2001:144-150.
Ely EW, Baker AM, Dunagan DP, et al. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864-1869.
Ely EW. Weaning from mechanical ventilatory support. In: Murray MJ, Coursin DB, Pearl RG, Prough DS, editors. Critical Care Medicine: Perioperative Management. Baltimore, Lippincott: Williams & Wilkins; 2002:460-474.
Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186-192.
Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345-350.
Freeman BD, Isabella K, Lin N, Buchman TG. A meta-analysis of prospective trials comparing percutaneous and surgical tracheostomy in critically ill patients. Chest. 2000;118:1412-1418.
Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416.
Habashi N, Andrews P. Ventilator strategies for posttraumatic acute respiratory distress syndrome: airway pressure release ventilation and the role of spontaneous breathing in critically ill patients. Curr Opin Crit Care. 2004;10:549-557.
Hillberg RE, Johnson DC. Noninvasive ventilation. N Engl J Med. 1997;337:1746-1752.
Jaeschke RZ, Meade MO, Guyatt GH, et al. How to use diagnostic test articles in the intensive care unit: diagnosing weanability using f/VT. Crit Care Med. 1997;25:1514-1521.
Keenan SP, Sinuff T, Cook DJ, Hill NS. Does noninvasive positive pressure ventilation improve outcome in acute hypoxemic respiratory failure? A systematic review. Crit Care Med. 2004;32:2516-2523.
Keroack MA, Cerese J, Cuny J, et al. The relationship between evidence-based practices and survival in patients requiring prolonged mechanical ventilation in academic medical centers. Am J Med Qual. 2006;21:91-100.
Kollef MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567-574.
Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471-1477.
Liesching T, Kwok H, Hill NS. Acute applications of noninvasive positive pressure ventilation. Chest. 2003;124:699-713.
MacIntyre NR. Respiratory function during pressure support ventilation. Chest. 1986;89:677-683.
MacIntyre NR, Cook DJ, Ely EWJr, et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians, the American Association for Respiratory Care, and the American College of Critical Care Medicine. Chest. 2001;120:375S-395S.
Marelich GP, Murin S, Batistella F, et al. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118:459-467.
Mascia ME, Koch M, Medicis JJ. Pharmacoeconomic impact of rational use guidelines on the provision of analgesia, sedation, and neuromuscular blockade in critical care. Crit Care Med. 2000;28:2300-2306.
McCulloch TM, Bishop MJ. Complications of translaryngeal intubation. Clin Chest Med. 1991;12:507-521.
McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123-1133.
Meduri GU. Noninvasive positive-pressure ventilation in patients with acute respiratory failure. Clin Chest Med. 1996;17:513-553.
Nathens AB, Johnson JL, Mine JP, et al. Inflammation and the Host Response to Injury Investigators: Inflammation and the host response to injury, a large-scale collaborative project: patient-oriented research core—standard operating procedures for clinical care. I. Guidelines for mechanical ventilation of the trauma patient. J Trauma. 2005;59:764-769.
Nieszkowska A, Combes A, Luyt CE, et al. Impact of tracheotomy on sedative administration, sedation level, and comfort of mechanically ventilated intensive care unit patients. Crit Care Med. 2005;33:2527-2533.
Pieracci FM, Barie PS, Pomp A. Critical care of the bariatric patient. Crit Care Med. 2006;34:1796-1804.
Ramsay M, Savege T, Simpson B, et al. Controlled sedation with alphaxalon-alphadolone. BMJ. 1974;2:656-659.
Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation scale for adult critically ill patients. Crit Care Med. 1999;27:1325-1329.
Schuerer DJ, Whinney RR, Freeman BD, et al. Evaluation of the applicability, efficacy, and safety of a thromboembolic event prophylaxis guideline designed for quality improvement of the traumatically injured patient. J Trauma. 2005;58:731-739.
Squadrone V, Coha M, Cerutti E, et al. Continuous positive airway pressure for treatment of postoperative hypoxemia: a randomized controlled trial. JAMA. 2005;293:589-595.
Straus C, Louis B, Isabey D, et al. Contribution of the endotracheal tube and the upper airway to breathing workload. Am J Respir Crit Care Med. 1998;157:23-30.
Tobin MJ. Advances in mechanical ventilation. N Engl J Med. 2001;344:1986-1996.
Tobin MJ, Van de Graaff WB. Monitoring of lung mechanics and work of breathing. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. New York: McGraw-Hill; 1994:967-1003.
Wheeler AP, Bernard GP, Thompson BT, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. N Engl J Med. 2006;354:2213-2224.
Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324:1445-1450.
Young CC, Prielipp RC. Sedative, analgesic, and neuromuscular blocking drugs. In: Murray MJ, Coursin DB, Pearl RG, Prough DS, editors. Critical Care Medicine: Perioperative Management. Baltimore, Lippincott: Williams & Wilkins; 2002:147-167.