CHAPTER 107 Functional Restoration
Spinal rehabilitation has long aspired to be the proverbial “backbone” of spine care around the world. Application of scientific method and acumen has played an important role in overcoming fears about rehabilitation and functional capacity/limitation after injury. Spine specialists recognize that minimizing bed rest1 and maximizing early activity return are not harmful and show improved functional outcomes over “restful waiting” in 90% of patients who suffer an acute episode of low back and/or leg pain. Moreover, aggressive, quota-based rehabilitation appears to improve symptoms more rapidly than natural history in placebo-controlled trials. Even without treatment, pain symptoms improve over 90 to 120 days.2,3 Despite advances, there is still a paucity of knowledge as to appropriate triage and disposition of the 10% of spine injuries who fail all single-provider interventions (including surgery) and absorb 80% to 90% of the money spent on spine care.4,5 Since the 1980s integrated, interdisciplinary, advanced spinal rehabilitation has been recognized as the “gold standard” of care for treating patients who do not improve with other interventions.
As with most professions, best-practice philosophies and actions in spine care vary with practitioner experience when data are lacking or idiosyncratically derived. Despite a body of evidence assessing the frame-of-reference skew, surgeons often see a disproportionate number of conservative care failures and nonsurgeons see the devastation of inappropriate or poorly performed surgery.6 In the end, surgical and nonsurgical spine physicians both rely excessively on empiric data that result in inversely skewed frames of reference, allowing a significant minority of patients to become disabled failures.7
This chapter seeks to address the most contentious and interesting patients—those who fail to improve within 90 days to any single-provider modality—and who receive increasingly aggressive and invasive interventions with variably poor functional outcomes. Creating a best-practice model for this population is further complicated by the fact that pain infrequently correlates to radiologic findings nor do outcomes correlate with radiologic criteria for success.8,9
The industrial population, which still remains an important plurality of the spine-care population, poses even greater challenges for discerning best-practice treatment. For various reasons, most industrial claim patients are an excluded population from the majority of spine literature. This aberration calls into question the generalizability of much of the spine surgical literature to the industrial injury or persistent pain populations. What is generally true for spine injury (where 90 days of conservative treatment allows for 90% of the population to recover) is often untrue in work-related spine injuries. Moreover, convincing data show that delay in care beyond 6 weeks in the industrial population is a strong predictor that impairment by injury may progress to a disability.10 The paradox of the refractory 10% of spine-injury patients is that their pain has a multifactorial origin, deeply submerged, inaccessible to visual inspection, and subject to diverse influences such as psychologic stress, coping failure, social-status forfeiture, and financial collapse.
Interdisciplinary experience is not usually part of physician training with the consequence of secondary phenomena remaining unrecognized by the physician or chiropractor who has no mechanism (or “second set of eyes”) available to negotiate the dissonance and contradictory feedback from the patient who is disabled by pain.7 Greater understanding of phasic care models has progressed over the past 2 decades documenting cost and outcome success from care that has been tiered into primary, secondary, and tertiary modes of treatment. Each mode brings with it greater expense but commensurate greater functional return in exchange for the increased cost. The concepts underlying the phasic care model are identified in consensus panel documents such as provided by the North American Spine Society.11 Briefly outlined, primary care refers to modalities applied during acute injury intended to modify symptoms. These including, but are not limited to, surgery, manual techniques, early single specialty mobilization, and educational programs such as so-called “passive modalities” (e.g., immobilization after surgery, electrical stimulation, temperature modulation, possibly traction). Treatment is customarily provided by a single professional, with a limited number of treatments applied to a large number of acute-phase patients who appear clinically and radiologically appropriate. Secondary care refers to therapy provided to a smaller number of patients not responding to initial symptom-modification treatment. The postacute or postoperative symptoms often require care plans focused on reactivation with programs generally adapted to combine quota-based exercise programs with education. Occasionally, additional passive modalities are employed for symptom modification. The primary aim is to prevent/reverse late phase deconditioning that is associated with the transition from functional impairment to disability. In some cases, secondary care may have a degree of programmatic consolidation (i.e., back-school), particularly toward the end of the postacute period, but it is not required to be on site, nor is it required in the majority of cases. The lead role in secondary care is usually performed by physical and occupational therapists, with physicians, psychologists, social workers, disability managers, and/or chiropractors acting as consultants to ensure progress back to maximal function.
Although specific programmatic terms are still in flux, the concept of levels of care is increasingly accepted in clinical referral patterns and local statute.12,13 Enforced by local rule/statute in a growing number of states, evidence-based program definitions allow greater hope of achieving the goal of quality of care. It is with the best evidence available that a specific program term, functional restoration, developed at the Productive Rehabilitation Institute of Dallas for Ergonomics (PRIDE), is discussed as an exemplar of the type of tertiary care model available at several centers of excellence around the country. Functional restoration is designed for individuals with chronic pain who are also disabled from performing many activities of daily living (ADL) or work functions. By its nature, functional restoration is especially suitable for work-related injury, workers’ compensation (WC) cases, or other types of compensation injuries (long-term disability, Social Security Disability, motor vehicle accidents). However, interdisciplinary, tertiary care also has demonstrated benefit in those who have suffered a disabling injury with or without the secondary gain of compensation.14 The goal of this chapter is primarily to provide the reader a direct understanding of how an interdisciplinary program improves health outcomes for both postsurgical and nonsurgical failures of lower-level care by dissecting out various program requisites and their respective structure including the roles of members of the interdisciplinary team within a tertiary care setting.15,16 Finally, the chapter demonstrates evidence for best-practice quantitative assessment that is vital to replacing subjective, unreliable pain scores with quantitative data to follow a particular patient’s progress toward functional goals.
Functional Restoration Phases and Personnel
As outlined earlier, the goal of tertiary-level functional restoration is to use the team model to overcome the multifactorial biopsychosocial barriers that prevented patients from succeeding at lower, less expensive levels of care. A concept repeated throughout this chapter is the paradox that makes this vastly expensive 10% of the musculoskeletal-injury population different. Namely, they are disabled by pain that is ultimately self-serving, nonquantifiable, and frustrating to family, friends, and care providers who try to ameliorate it, further isolating the patient from his or her normal social role and health homeostasis. Many complex patients, requiring tertiary-level care, are self-selecting experts able to transform any attempt at objective measurement to a discussion of their degree of suffering. The unique skill of this type of patient is precisely the reason an interdisciplinary team is crucial to changing the patient’s dysfunctional trajectory. Although the rest of the chapter is devoted to the precise tools at the team’s disposal, this section focuses on mapping the roles of the team and providing an overview of an appropriate progression through a validated, peer-reviewed, outcome-substantiated program. The example and outcomes data in Table 107–1 are from more than 25 years of experience at the Productive Rehabilitation Institute of Dallas for Ergonomics (PRIDE), but the roles, progression, and tools available in this chapter are validated, generalizable, and useful in many other contexts.
TABLE 107–1 One-Year Follow-up Outcome Goals for Functional Restoration
| Outcome | Results |
|---|---|
| 1. Return to work | >90% |
| 2. Work retention (1 year) | >80% |
| 3. Post-treatment surgeries | <4% |
| 4. Percentage unsettled claims | <15% |
| 5. Spine-related medical visits (except for functional restoration or referring physician visits) | <5 visits/yr |
| 6. Rate of recurrent injury claims (lost time) in patients returned to work | <2%/yr |
Initial Evaluation
The quantitative functional capacity evaluation (FCE) is the physical assessment portion of the visit. As discussed later in the quantitative evaluation of physical function portion of this chapter, in a tertiary setting, pain and hypo-mobility fail to be accurate guides anymore. The point of assessment for an interdisciplinary program is to gather quantitative information not necessarily seen with single-specialty qualitative analysis when lower levels of care have failed. In other words, this portion of the exam is intended to “set the speedometer” of the patient’s current functional state in several physical domains. Most important is the assessment of mobility and strength around the putative weak link or dysfunctional motion segment. Building on the initial physician assessment, both the physical and occupational therapists collaborate by evaluating first range of motion, then dynamic strength, and finally endurance of the functional unit, checking each against both an internal validation method, effort factor, and an external validation method, normative database, that is adjusted for age, weight, and gender (see “Isolated Trunk Strength Assessment” later). The next domain is a whole-body performance assessment, usually the province of the occupational therapist, to give information to the team about the degree of deconditioning in noninjured segments of the body and to look for paradoxical discrepancies that portend nonfunctional, compensatory injury behaviors. Finally, cardiovascular measures give important data on deconditioning, materials handling performance, positional tolerance, effort, and gym tolerance of the prospective patient. The complexities of performing an FCE on a chronic pain patient are discussed in greater detail in an American Medical Association publication.17 All of this information is collated into a form to be interpreted by the physician when formulating the comprehensive program.
The psychologic assessment comprises a complex interplay of tests administered with the help of a staff psychologist comparing self-report scores, functional questionnaires, and affective inventories to quantify objective levels of depression, anxiety, fear, inhibition, and occasionally antisocial or manipulative traits (see “Psychologic Assessment” later). These data are collated with the structured clinical interview (SCID) to assess for barriers to participation and, more importantly, barriers to eventual functional return. Like the physical and occupational therapist, the psychologist tallies the quantified data for the physician to assess the degree of psychologic intervention and possible need for pharmacologic adjuncts in determining program length.
Outcome Tracking Phase
This important program phase allows continued evaluation of long-term effectiveness of the program, both from the patient’s point of view and for the facility’s continuous quality improvement (CQI) initiative. A long-term care plan (LTCP) may be offered to the patient for quarterly visits to the supervising physician, who can provide medications to assist in meeting program goals (i.e., work return, home exercise, and decreased health utilization). The opportunity to return, as needed, for symptom exacerbation for focused interventions consistent with functional restoration philosophy is important for maintaining cost effectiveness. The patient is given the opportunity to consolidate gains, obtain feedback, and maintain his or her physical capacity plateau by repeated quantitative FCEs performed at regular prescribed intervals. A patient also has the opportunity to present himself or herself for team feedback at one full-team staffing after the intensive phase. Telephone outcome tracking is administered at 3-, 6-, and 12-month graduation anniversaries by the staff, with assistance sought from treating doctors, adjusters, and attorneys whenever needed if new problems arise (see Table 107–1). Effort is made during this phase to reinforce independence in the patient so that return visits are minimized to evaluations only, unless a new crisis requires a few “refresher sessions.” An added benefit for retaining contact is the willingness of team members to help with problems that arise after the program. Patient perception of an environment of care that extends beyond their graduation leads to a high rate of compliance in repeat testing and telephone tracking. This high contact rate, in turn, allows performance of 1- and 2-year follow-up studies such as has been performed at PRIDE.18–20
In addition to the Level II to III evidence for Therapeutic Studies cited earlier, many research publications of prognostic interest have been provided through the PRIDE Research Foundation. Since 1993, all entering and completing patients provide a large database of prospectively collected data on physical function, psychosocial function, occupational issues, and demographics. Data are updated through repeat tests/interviews on program completion and ultimately compared with the objective outcomes on work, health utilization, and recurrent injury status. To date, more than 5000 program completers and 2000 noncompleters have been studied and have become the focus of numerous prognostic cohort studies delivering Level I scientific evidence. These studies have examined the effect of demographic factors such as length of disability, age, or gender,21–23 as well as a comparison of patients with neck and upper extremity problems with those with lumbar injuries.24–26 They have also compared lumbar discectomy with fusion27 and investigated prerehabilitation psychologic status.28–31 Finally, the effect of program behaviors including final ratings of pain/disability and health utilization have provided Level I scientific evidence.32–37
Quantitative Evaluation of Physical and Functional Capacity
For this book’s audience, the main principle to understand in tertiary level, interdisciplinary, functional restoration is that the usual way we assess activity tolerance (namely by endurance and pain) is, in this self-selected population, unreliable and counterproductive to functional return. As such, validated, reproducible quantitative measures of physical and psychologic dysfunction, termed by the authors as weak links, must be employed to track functional gains. The FCE has become a popular term that subsumes such a variable set of methods and tests under its nominal rubric that it now has no specificity, potentially rendering a general FCE poorly diagnostic/prognostic for patients with impaired insight to their own function. Moreover, many of the developers of certification in this area engage in a speculative turf war by teaching and marketing opposing principles while simultaneously criticizing competitors as invalid. Additionally, few of the FCE methodologies use a quantitative approach but instead employ qualitative, observational methodology that is traditional in physical therapy. Key principles of quantification, using physics-based terminology, are poorly understood by most therapists and physicians. As stated earlier, functional restoration requires true quantification of function because the absence of numeric data leaves physically inhibited patients and their care team enslaved by the cliché, “If it hurts too much, don’t do it.” Greater understanding of accuracy, precision, and sources of error in any quantitative methodology is necessary.38 In the PRIDE model of functional restoration, physical capacity assessment implies the assessment of the injured musculoskeletal spinal region, generally involving quantification through reproducibility of mobility, strength, and endurance of a given functional unit or around a given joint. On the other hand, the term functional capacity assessment implies measurement of a functional unit’s change from a normative data curve in the context of whole-person performance—taking compensatory strategies into account in tasks that are specifically designed to stress the injured body part (e.g., weight lifted, running speed, sitting tolerance time). Recent literature has focused on prefunctional and postfunctional restoration longitudinal measurements of patients, as well as on development of specific normative databases.39–45 Aided by a new generation of quantitative tools and an evidence-based consensus on techniques for FCE, a reproducible, prognostic FCE is now possible, even for the difficult chronic pain patients.17,46 This innovation permits an individual patient’s comparison with both normative data and an absolute job requirement for materials handling and positional tolerances.
As implied earlier, the demonstrable success of a sports medicine approach to rehabilitation and reconditioning is not possible without specific quantification techniques. In the 65 years since Delorme’s groundbreaking work in 1945, we have learned much about the secondary physical changes accompanying immobilization and disuse in the spine and extremities.47,48 Spontaneous healing or physician intervention may produce maximum recovery of disrupted soft tissues (ligaments, tendons, joint capsules, muscles, and discs) or osseous tissue in a relatively short 6- to 12-week time period. In cases of severe injury, permanent tissue changes often remain after maximal recovery in the form of distorted bony structures, soft tissue defects, or scar, which may possess strong but more easily reaggravated fibrous properties. In the musculoskeletal system, these changes may create further biomechanical derangements of normal functioning through stress risers, instability, and degenerative joint changes. These problems are to some extent permanent derangements, and they can never be completely fixed. Efforts of acute conservative care, surgery, and manipulation may be directed toward correcting or ameliorating these derangements to the greatest extent possible. However, the province of the tertiary rehabilitation team is to instead tap the body’s inherent, evolutionarily engineered, redundancy; coaxing forth functional compensatory mechanisms in the face of permanent structural change.
Range-of-Motion Assessment
Trunk motion is a compound movement combining intersegmental spine and hip motion components. A patient with a completely fused spine can often bend forward to perform toe touches using hip motion alone. Although it is difficult to measure intersegmental motion nonradiologically, inclinometers may be used to separate the hip motion component from the lumbar spine motion component and derive valuable information.49,50 The basic information on inclinometry comes from British rheumatology, and the system has been used, in one form or another, in Europe for nearly 40 years.51 As in all physical capacity measures, range-of-motion information is only useful when compared with a normative database and contextualized by an identifiable effort factor. For lumbar range of motion, the effort factor is the comparison between the hip motion component and the spine straight leg raise test measurement.50,52 These measurements are isolated anatomic/physiologic physical capacity measurements, assessing the capabilities of a single functional unit of the body. It is these isolated functional unit measurements that ultimately supply context to general functional or whole body measurements taken when a synthetic task such as bending, climbing, or lifting is performed.
In addition to sagittal movement of the spine, coronal movement can be assessed by rotating the inclinometer 90 degrees in the axial plane. Rotation may also be assessed where it is important, namely in the cervical and thoracic spines, through relatively simple techniques.53 The greater the limitation of ROM in any given direction, the less reliable the test. However, if done in a standardized fashion with good effort, these quantitative measurement techniques are highly reproducible, giving both the patient and the interdisciplinary team insight into the weak link.
The multi-inclinometer measurement techniques can demonstrate the actual ROM in the T12-S1 segment in the sagittal and coronal planes, as well as the T1-T12 sagittal/torsional movement, or the occiput-T1 cervical movement in all three planes. Functional assessment aids the rehabilitation process, and in cases of arbitrating disputed compensation it can be used for impairment evaluation purposes. As stated earlier, these tests can also be performed at multiple time intervals to document progress. Suboptimal effort in this portion should lead to careful scrutiny of other components of the functional capacity test battery and trigger greater team involvement. However, even in the presence of poor effort, the determination of “normal” or “abnormal” motion can be made by comparing the spine and hip motion ratios. In the normally mobile lumbar spine the sequence of forward bending generally involves spinal flexion before initiation of the hip flexion component, which proceeds until the spine is “hanging on its ligaments.” At this point, hip motion increases while further spine motion is constrained.50 If, even in the presence of suboptimal effort, a normal spine/hip ratio exists, the clinician can usually conclude that normal spine mobility would have been present if the patient had provided sufficient effort. However, in the presence of an abnormal spine/hip ratio, with or without good effort, actual limitation of spine mobility is likely present (postfusion ankylosis or postoperative/disuse scarring or stiffness). The technique is also important in the work capacity evaluation whether or not the patient completes the interdisciplinary functional restoration program. ROM assessment is usually included as one part of the quantitative functional evaluation, a specific battery of quantitative, internally validated physiologic tests for mobility, strength, endurance, and synthetic task performance.20,52,54 There may be initial resistance to use of measurement techniques by the interdisciplinary team because of concerns about the testing being time consuming, equipment intensive, and cumbersome. With mechanical devices (such as inexpensive carpenter’s levels), two inclinometers must be used simultaneously and the calculations done by hand after measurements are taken. The advantage of versatility and internal calculation (at greater expense) is provided by computerized devices. Once mastered, both techniques are less time consuming than obtaining blood pressure but are far more useful in this population where physician measurements replace the overlimiting, dysfunctional, inhibitory belief that stems from the patient’s perception of pain. Many medical specialists will prefer to perform the tests themselves, though these measurements are often competently performed by well-trained therapists, nurses, or technicians.
As in all other physiologic measurements, there is variation within the asymptomatic population. Interestingly, our normative data show that mean true lumbar motion is almost the same in males and females, even though females tend to have greater hip and straight leg raising mobility components. Patient values are expressed as a “percent normal” as related to mean scores of the symptomatic subject population, normalized for variables like age, gender, and body weight (when applicable).41–43 The system allows the clinician to judge the significance of small variations from the anticipated value and, more importantly, to track the progress of the rehabilitation process between examinations.
Isolated Trunk Strength Assessment
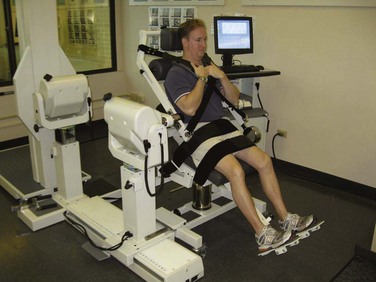
Several devices are commercially available for assessing isometric, isotonic, or isokinetic trunk strength in various planes of motion. Most involve some type of pelvic stabilization with application of force through a line projecting between sternum and scapulae and thus represent trunk strength as torque (torsional force) around a pelvic fulcrum with a lever arm individualized to a subject’s height. Cervical dynamic strength measurement devices have been seen in prototype form, but are not currently available, leaving isometrics as the only alternative. Isokinetic devices stabilize the variables of acceleration and velocity in order to provide torque as the primary independent variable. Isokinetic testing narrows the Gaussian distribution of values by limiting the number of independent variables, which, in our opinion, provides a more valid test. Other available devices may be purely isometric or isoinertial. For historic interest, isometric test models employing strain gauges have been used for more than 60 years. Though commercially available, dynamic, isokinetic trunk strength testing has only been available since 1985, there is abundant literature demonstrating efficacy in identifying isolated motion-segment dysfunction (differentiating weakness and/or decreased endurance) and quantifying outcome improvements. Only a few commercially available isometric or isokinetic devices still exist. PRIDE currently uses a dynamometer connected to a sagittal semi-seated torso testing device detailed in the latter half of Chapter 5 in which lumbar muscle anatomy and function are discussed (Fig. 107–1).
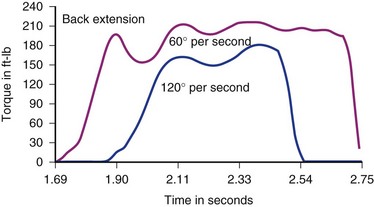
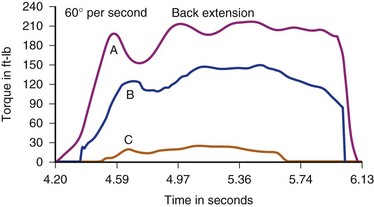
Results of normal subject testing have been compared with chronic spine disorder (CSD) patients, with and without prior surgery.42,43,55–58 Substantial differences have been shown between these groups, initially with incremental trunk strength improvement demonstrated during rehabilitation of chronically disabled spine pain patients (Figs. 107-2 and 107-3).18–2057 The intent of all of the devices is to isolate and challenge the trunk strength component of the thoracolumbar functional unit by stabilizing above and below the area to be tested. The isolation of the vulnerable “weak link” portion of the vertebral biomechanical chain linking the shoulder girdle to the pelvis is intended to assess muscle strength and endurance, just as measuring quadriceps and hamstrings is of prima facie importance to knee function. For any of the devices to be useful, the dynamometer must give accurate and reproducible measurements and the testing protocol must conform to the one employed when the normative database was created. Moreover, such a database must be available to express the individual’s results as a “percent of normal” and the clinician must have a method for assessing effort-validity of each test.
Whole Body Task Performance Assessment (Functional Capacity)
The whole body task performance assessment, usually the province of the occupational therapist (OT), requires a skilled eye and a highly burnished insight. Whereas the quantification of the weak link has multiple internal checks, the whole-body performance component is subject to the intrusion of all manner of biopsychosocial baggage—the precise reason the patient graduated to a tertiary-level care in the first place. As an example, a “rigid spine,” like one might see with complete thoracolumbar to sacral fusion, is actually a biomechanically sound construct. Its functional utility is reliant on biomechanical redundancies to accommodate for diminished efficiency, as in gait modification in lumbar constructs.59 The whole body performance assessment is divided into two major subsections that can be classified as materials handling and positional tolerance. Because of the complex reciprocal action of deconditioning-mediated physical limitations (to both the injured functional unit and other body parts, see earlier discussion) with fear-related and belief-related inhibition, the histogram of “normal” is widened. Therefore when obtaining “real-world” information, different methods of executing the same task using different postures and metrics are employed to quantify consistency, as well as performance.
Lifting capacity has long been the “gold standard” for spine-related, materials-handling functional capacity. Because of its perceived importance as the mode of industrial injury, lifting capacity is still the measurement of greatest concern to those medical and nonmedical personnel who judge the patient’s work capacity or vocational suitability. The isometric lift task employed in the NIOSH (National Institute of Occupational Safety and Health) guidelines is still in wide use and has a large comparative database that does not quite rectify the inherent nongeneralizability of isometric measurements. To give the full picture of any materials-handling task, several measures including isometric,60 isokinetic,61 and isoinertial62–65 must be combined. Specifically, though isokinetic devices can also be used for isometric measures, their results are not interchangeable and neither gives the picture of real-world lifting as does an isoinertial test.61 Moreover, only limited correlation exists between isokinetic and isoinertial, dynamic-lifting measures, just as only limited correlation exists between lifting capacity measures and trunk strength measures because of substitution in performing whole body tasks. With such confusing data underlying the fear-related issues of a patient who was educated by another health provider to “never lift anything more than a beer can,” it is difficult to get a sense of capacity for task performance.
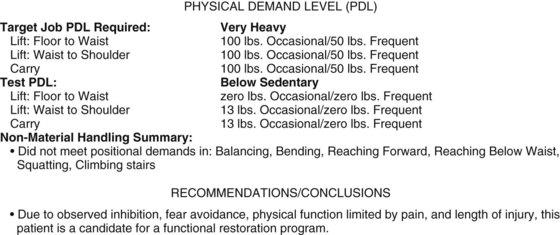
Tests are standardized in a way to provide interpretable, quantifiable information to assess the “speedometer” of the patient and the proximity to job-specific task performance, thereby keeping the patient accountable to a specific outcome of choice. Within the domain of whole body task performance, the subdomains of materials handling (e.g., floor-to-waist lifting, waist-to-shoulder lifting, carrying, pushing, pulling) and nonmaterials handling or postural tolerance (e.g., sitting, standing, balance, stair/ladder climbing, and decent, fitness testing) each have to be assessed and collated for the physician team leader. Repeated longitudinal testing adds validity to programmatic compliance and credibility to the physician’s attestation of task-specific suitability to the eventual employer. In addition, longitudinal studies demonstrating sufficiently large differences in prerehabilitation and postrehabilitation performance measures are an effective justification of the program’s relevance and validity. When quantified preprogram and postprogram data are combined with outcomes such as employment retention (see Table 107–1), stakeholders appreciate a program’s effectiveness in treating a variable and complex patient population and may act to influence the referral pattern.40,57 Additionally, some large employers including state and federal agencies that require further documentation of task-specific preparedness do not need any further attestation than the data acquired during the program. A quantitative FCE has been developed for use in or after the PRIDE program, and a typical summary for a prerehabilitation chronic pain patient (15 months total disability and 11 months after L4 discectomy) is demonstrated in Figure 107–4. The principles underpinning an FCE in a chronic pain population are discussed in an American Medical Association book on FCE by Genovese and Galper.17
Psychologic Assessment
Even though severe disability is the unifying characteristic of CSD patients that brings them to the attention of employers, attorneys, insurance companies, and vocational rehabilitation personnel, the patients originally enter the medical care system with pain and are initially indistinguishable from any other injured patient. Chronic pain is a complex and interactive psychophysiologic behavior pattern that cannot be broken down into distinct, independent physiologic and physical components. Therefore general psychologic health is less important than is identifying the various psychosocial barriers to functional recovery that may impede a patient’s rehabilitation program or their eventual reintegration to resuming their desired societal role. Rates of psychiatric illness in the CSD population (e.g., major depression, substance abuse, anxiety disorders, personality disorders, childhood trauma) are much higher than for the U.S. population in general.66–68 Assessment uses a number of tests that, when combined, can assist the clinician in identifying these barriers, rather than “curing” premorbid psychiatric distress. Like the physical assessment, the goal is to discover therapeutic interventions the psychiatric team member can use to assist the patient to overcome the barriers to vocational, avocational, familial, and societal productivity. At this time, no single, comprehensive instrument developed specifically for chronic spinal pain has been developed or validated. Thus multiple tests are used to evaluate, assist in the counseling, and guide patients through functional restoration. These tests fall into several categories.
Self-Report Tests
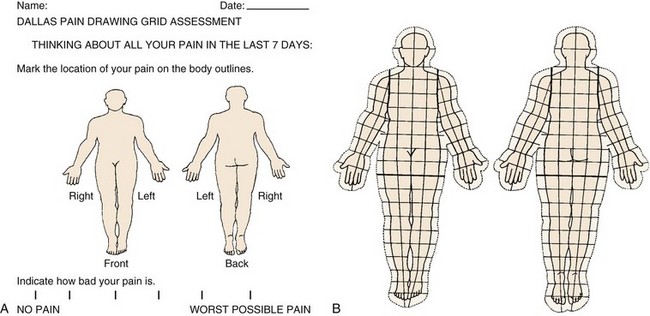
Quantified Pain Drawing
For more than a decade, quantified pain drawings have been used to give an assessment of pain location, severity, and subjective characteristics in a nonverbal communication by the patient.69 An overlay, over the pain drawing itself, has been devised to give a numeric count of the trunk and extremity area covered, as well as whether pain is limited within the bodily confines or is outside the body. The overlay is on a transparent plastic sheet permitting easy scoring by office personnel (Fig. 107–5A).70 There is also a visual analog score (VAS) to suggest whether the patient perceives the problem to be more axial or extremity in origin and to gauge the severity of pain (Fig. 107–5B).
Function/Disability Questionnaires
One of the most important developments of the past decade is the use of self-report scores for assessing treatment outcomes by using the same self-report tool prerehabilitation and postrehabilitation. Several validated tools are available. One commonly used tool is the Oswestry Disability Index (ODI), which has been present for many years.71 The self-report scores have also been used with the concept of a minimum clinically important difference (MCID) in a number of recent studies.72,73 The MCID concept can be used with both anchor-based and distribution-based approaches.74,75 The fallacy of comparing one self-report measure with another, as well as other pitfalls of the MCID concept, are being raised as the MCID concept gains greater popularity.76,77
The Million Visual Analog Scale (VAS) is a validated instrument useful not only for the lumbar spine like the ODI but for any spinal disorder and consists of 15 questions.78 It describes both pain and disability, expressed over 10 cm, spanning a gamut of responses from the minimum to the maximum possible activity on each item.
A newer and more generally usable functional assessment tool, derived from the Million VAS, is the Pain Disability Questionnaire (PDQ).34 This tool has been expanded to cover not only lumbar spine functional assessment (such as the ODI) but all spinal and other musculoskeletal conditions in a general way. In addition, it includes a psychosocial component, vital in the assessment of chronic spinal pain patients. Its responsiveness has been demonstrated, and it is now being used in the most updated version of the American Medical Association Guides to the Evaluation of Permanent Impairment (Fig. 107–6).30,79
Mood Disorder Inventories
Many techniques are available for measuring depression, both self-reported measures and clinician-administered tools. Although we briefly discuss the most common self-report instrument (Beck Depression Inventory or BDI),80 other self-report measures include the Zung and RIDS.81–83 Clinician-administered tools include the Hamilton-D.84 The BDI consists of 21 questions with a cumulative scoring system that looks at manifestations of depression including sleep disturbance, weight change, irritability, sexual dysfunction, and anhedonia. It was developed by Beck (1967) as a means of assessing the cognitive components of depression. Depression, like anxiety, frequently coexists with long-term spine dysfunction and disability. Although it is unclear whether depression precedes or follows the onset of low back symptoms in the majority of cases, knowledge of its presence can be quite helpful. Offering depressed patients pharmacologic treatment may encourage greater patient motivation and compliance, as well as reduce barriers to recovery.85
Less well known, but increasing in popularity, are scales that measure anxiety as it relates to fear of movement (kinesophobia) and avoidance of activity (fear avoidance). Several instruments are available for this assessment beginning with the Pain Anxiety Symptom Scale (PASS), a 20-item questionnaire.86 A more popular scale, but with a limited focus, is the Fear-Avoidance Beliefs Questionnaire (FABQ), which consists of 16 items and provides two subscales on work and physical activity.85,87
Quality-of-Life Questionnaire
The SF-36 (Short Form-36) is a 36-item validated self-report test currently achieving wide acceptance as an outcome measure in many areas of medicine.88 It provides multiple dimensions of health status and is used in longitudinal studies of Quality Adjusted Life Years (QALYs) for many diseases. Eight dimensions of physical and emotional function/coping document self-perception of health status, with two global, comprehensive scores summarizing physical and mental health.
More Extensive Psychologic Measures
Minnesota Multiphasic Personality Inventory
The Minnesota Multiphasic Personality Inventory (MMPI) is the grandfather of the tests used in chronic pain patients and revised as the MMPI II. It has more than 500 self-report items but has low patient acceptance. The main reason for its use at present appears to be its long-term and common usage. By itself, it does not offer much help in choosing among treatment options. However, as part of a comprehensive evaluation using several other assessment tools, the MMPI can add valuable information.31
Of the 10 major clinical scales by which the MMPI is classified, the Hysteria Depression and Hypochondriasis Scales are the most frequently associated with a chronic pain patient. Elevation of the Hysteria and Hypochondriasis Scales with a normal depression scale provides a so-called “Conversion V.” This test profile was thought to be associated with pain that has a large psychologic component. However, it has been shown that, in reality, Conversion V is a stress indicator, and it returns to a more normal profile after resolution of disability from whatever cause.89 For years, certain clinical profiles were felt to be consistent with chronic pain behaviors, which also included the Neurotic Triad and Hypochondriasis profiles. However, recent work has demonstrated that, by far, the most frequently detected pattern in a chronic pain/disability population is the disability profile, which occurs in a majority of patients, whereas the other profiles occur in only a small minority.30
Psychiatric Assessment
The Structured Clinical Interview for DSM-IV diagnosis (SCID) has a standard interview format specifically designed to provide a psychiatric diagnosis (if present) for the patient being assessed.90 Because the rate of psychiatric illness is so high in chronically disabled patients, whether lifetime or secondary to the effects of disability, this test has become vital for the assessment of CSD patients. The SCID provides information on both Axis I and Axis II diagnoses but is clinician-administered and therefore somewhat costly and time consuming. For this reason, the Patient Health Questionnaire (PHQ) was developed.91 The PHQ is self-administered by the patient, easy to perform in a physician office, and closely correlated to the Axis I and II diagnoses in the SCID.
Functional Restoration Approach to Rehabilitation
Sports Medicine Concepts and Physical Training
The physical part of the interdisciplinary approach is centered around a quantitatively based, quota-driven, sports medicine approach to comprehensive rehabilitation. The principles of sports medicine have evolved from narrowly meaning rehabilitation of the competitive athlete to a conceptual and methodologic framework that connotes active treatment protocols for all individuals who want to return to high levels of function. Its component parts are shown in Box 107–1. Much of the initial work and literary evidence was done with rehabilitation of injured limbs, but these concepts are clearly applicable to the spine as well.
A characteristic of the musculoskeletal injury that may carry beyond the three tissue-healing phases is the tendency to splint and protect the injured area that started with a reflexive neuromuscular response.92 Unfortunately, in the long term, splinting leads to decreased stress and shear across the injured tissue, which translates to delayed maturation of collagen,59,93 muscle atrophy,94 adhesions and deficits in joint lubrication,95 ligament atrophy,93 and bone loss.96 Subsequently, changes in endurance and aerobic fitness create a feed-forward process (vicious cycle) of physiologic and behavioral changes termed deconditioning syndrome. Deconditioning syndrome is marked by progressively declining physical capacity with each subsequent pain episode, increasing the patient’s fear, which in turn encourages a greater degree of disuse, feeding forward to further increase inactivity and decrease physical capacity.
Strength in the physical portion of functional restoration may be restored after injury in a variety exercise modalities, all of which should roughly coincide with the underlying principles of DeLorme, namely, resistance progression to muscle failure. Initially, soon after injury, when continued immobilization may be necessary, isometric exercise may be the only type that can be performed by the patient. This involves repetitive muscle contraction against fixed resistance without accompanying joint motion. Although isometric exercises may be done in a cast, splint, or brace, they have many drawbacks. First, isometric exercise is the most fatiguing and least effective type of exercise.97 The specificity of strength training to the corresponding activity is determined by muscle fiber length, which means that isometric exercise is poorly generalizable to any activity except splinting. Additionally, endurance and agility necessary for dynamic activities are not produced by isometric exercise. During the early phase after injury, isometric exercise can serve as a placeholder to maintain muscle tone and resist the body’s tendency to atrophy, but the physician should progress the patient to mobilization as soon as possible. Electrical muscle stimulation in combination with isometric exercise has been suggested to be of benefit, but electrical stimulation alone shows no gains in functional strength when the patient is mobilized.98
As stated earlier in this chapter, dynamic muscle training has been shown to be the most efficient method of training. It involves distinct subcategories: isotonic, isokinetic, eccentric, and isoinertial, sometimes termed psychophysical (free weights).99 Isotonic exercises are those in which the same force is applied throughout the dynamic range and is often inappropriately used for exercises in which a changing lever arm actually alters the applied torque. This type of exercise is most often associated with the variable resistance devices, using a cam to equalize muscular demands throughout the dynamic range of motion. Isokinetic training devices require a sophisticated dynamometer that limits the speed to a preset value. In this mode, speed and acceleration are controlled, allowing almost unlimited torque around a central axis, which in turn eliminates the effect of acceleration on work. These devices accommodate a force application that provides injury protection, at least in the concentric (muscle shortening while contracting) type of contraction. Unlike variable resistance devices, however, high-speed training is possible for development of agility. Pure eccentric training is far to the right on the force-speed curve and can produce rapid strength gains with a correspondingly higher likelihood of injury at high forces or high speeds with little ability to provide external control for injury prevention. Finally, isoinertial (psychophysical) strength training, using free weights, is limited to those postures in which weight can be attached to the body or held in the hands, usually against gravity. The method is occasionally termed psychophysical because the subject self-selects the amount of weight that is acceptable and recruits the appropriate neuromuscular units (agonists and antagonists) to progress the muscles surrounding a discrete joint to the failure point (hopefully stopping short of the injury point). Although this closely approximates real-world muscle loading, the maximum weight that can be handled is limited by both the weakest link in the dynamic ROM and the dynamically changing lever arm. Further, the inability to effectively coordinate agonists and antagonists around a recovering functional unit creates potential for reinjury to that unit. Exercise that specifically simulates motions and loads of a sport or work activity has been shown in multiple studies to be an effective training tool that is robustly protective against reinjury.100 Psychophysical and variable resistance lifting devices enable both concentric and eccentric contraction capability. This is more difficult with greater potential for injury than with isokinetic devices, though computerized dynamometers are currently available to act as a “governor” for this type of training.
Thus the specific exercise programs that combine stretching exercises and strengthening exercises and improve cardiovascular fitness, endurance, and agility show the best and most consistent functional outcomes with less risk of future injury.101 The application of these exercises is individualized on the basis of quantitative testing of function (noted earlier in this chapter), with the specificity and intensity of exercise changing to minimize the likelihood of injury and to maximize improvement in specific deficits to function. A variety of weight equipment is used in the program (Fig. 107–7). Finally, a fitness maintenance program must be established and tailored to the physical and psychologic makeup of the patient to ensure compliance when building on the initial gains made after graduating the intensive, supervised program.
Psychosocial Interventions in Functional Restoration
Just like the physical interventions seen earlier, psychosocial interventions take integrated, quantified test results to set the speedometer for the degree and amount of psychologic intervention needed within a particular program. Contrary to popular patient and payer opinion, the tertiary program is not designed to cure premorbid, longstanding psychosociopathy. On the other hand, it must be acknowledged by all stakeholders that the patients who fail lower levels of care were functioning adequately before their injury. The patient undergoing tertiary-level spinal rehabilitation outwardly suffers from physical complaints that are perpetuated by prolonged disability, failed coping skills, intrusive psychosocial stressors, and exposure or development of affective or anxiety disorders.28 A significant proportion of this population are “chemical copers,” who use a cocktail of opioids, centrally acting muscle relaxants, and alcohol to escape from intense feelings of hopelessness, helplessness, shame, fear, and despondency.102 Patients with a somatic focus tend to deal with anger, anxiety, and underlying unresolved emotional conflict with increased psychophysiologic responses. Traditional treatment approaches in pain management programs tend to teach patients about coping with pain and how to reduce self-defeating thoughts and behaviors but fail because of the self-defeating nature of pain as a functional guide. The traditional multidisciplinary and interdisciplinary pain clinic often add a mix of education, individual psychotherapy, supportive group therapy, group relaxation, individual biofeedback, and group cognitive behavioral therapy. Medication reduction may only be a secondary goal of treatment. The essential flaw in this type of treatment has been the continued primary emphasis on the patient’s self-report of pain. This approach is ultimately self-serving in terms of disability management (being inherently difficult to quantify and subjective) and often allows the patient to be rewarded for counterproductive choices and behaviors.
Pharmacotherapy
An awareness of common comorbid pain and neuropsychiatric disorders is necessary for more effective pharmacotherapeutic treatment of the spinal pain patient in the functional restoration setting. Chronic widespread pain or fibromyalgia may be premorbid or appear as a result of a traumatic injury.103 Treatment agents include serotonin norepinephrine reuptake inhibitors (SNRIs) such as high-dose venlafaxine, desmethylvenlafaxine, duloxetine, and milnacipran, the latter two being U.S. Food and Drug Administration (FDA) approved for this condition. Standard doses of these medications usually suffice and can serve a dual purpose. These agents generally offer a more benign side-effect profile than the tricyclic antidepressants (TCAs), which have long been the mainstay for treatment for this condition. Cyclobenzaprine has also been shown to be useful for some patients.104 Pregabalin, in higher doses than used for neuropathic pain (225 to 300 mg per day usually divided into twice daily dosing) is effective, as well as gabapentin in standard doses.
Patients who are dependent on high-dose carisoprodol or short-acting BZDs can be converted to diazepam as per standard dosage converting tables and tapered using three-times-daily doses over 4 to 5 weeks. Patients taking BZDs in sizable doses over 6 months may be able to tolerate a 10% reduction every 1 to 2 weeks.105 For patients requiring muscle relaxants to tolerate functional restoration, we prefer antispasticity agents such as baclofen and tizanidine to centrally acting agents. The former agents may have analgesic properties as well.
Many spinal pain patients present with comorbid radiculopathy amenable to pharmacologic treatment. Again, the standard multipurpose pain agents such as low-dose TCAs, SNRIs, pregabalin, and gabapentin are useful for many patients. For second-line agents, we favor a neuromodulator such as low-dose oxycarbazepine, which may be given at bedtime, and topiramate, which has also been found to be effective. Some patients may require polypharmacy using agents with different pharmacologic mechanisms of action for treatment of chronic neuropathic pain. Sodium channel blockers such as mexiletine and lidocaine 5% patches can be effective in some patients, and a multitude of anticonvulsant medications such as lamotrigine and levetiracetam can occasionally be useful in patients who have failed first- and second-line therapies.106
Patients with more severe personality disorders, comorbid psychotic disorders, chemical dependency disorders, and treatment-resistant affective disorders may benefit from atypical neuroleptics. These agents may occasionally have analgesic effects in some patients.107
At PRIDE, we prefer using sublingual buprenorphine for substitution and tapering of opiate-dependent pain patients. Buprenorphine is an opiate receptor, partial µ-agonist/kappa antagonist. It is less potent than morphine but more tightly bound to the opiate receptor and dissociates more slowly from the receptor, thereby minimizing withdrawal symptoms in physically dependent patients. Buprenorphine is FDA approved in the United States in the sublingual form for the treatment of opiate dependence. It is combined with naloxone (Suboxone) to prevent intravenous abuse and is also available as buprenorphine alone as Subutex. It is FDA approved in intramuscular form as an analgesic agent (Buprenex). This agent is available in other countries in lower doses in sublingual tablet form and in a patch form as an analgesic agent.108
It is legal in all U.S. states to taper pain patients’ medications to avoid withdrawal symptoms. Unless a special certification is obtained, it is not legal to “detoxify” a substance abuser. In our experience patients treated with buprenorphine experience significantly reduced cognitive, sedative, and euphoriant effects and can undergo mood enhancement and a smooth, uncomplicated withdrawal process. This can allow them to safely participate in functional restoration physical training in a timely manner. Buprenorphine is a moderate analgesic that can enhance the patient’s ability to continue in treatment because it appears to be less associated with opioid-induced hyperalgesia than most other agents. When using buprenorphine, it cannot be given until the patient is in mild withdrawal because it preferentially displaces other opioids from the receptor site and precipitates a full and rapid withdrawal state.109
Staff and Duties
Key Points
1 Hashemi L, Webster BS, Clancy EA. Trends of disability duration and cost for workers’ compensation low back pain claims (1988-1996). J Occup Environ Med. 1998;40:1110-1119.
2 Cady LD, Bischoff D, O’Connell E, et al. Strength and fitness and subsequent back injuries in firefighters. J Occup Med. 1979;21:269-272.
3 Stover B, Wickizer TM, Zimmerman F, et al. Prognostic factors of long-term disability in a workers’ compensation system. J Occup Environ Med. 2007;49:31-40.
4 Mayer T, Polatin P, Smith B, et al. Secondary and tertiary nonoperative care. North American Spine Society Committee: Contemporary Concepts Review Committee. Spine J. 2003:28S-36S.
5 Mayer T, Gatchel R, Mayer H, et al. A prospective two-year study of functional restoration in industrial low back injury: an objective assessment procedure. JAMA. 1987;258:1763-1767.
6 Kidner C, Mayer T, Gatchel R. Higher opioid doses predict poorer functional outcome in chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg. 2009;91:919-927.
7 DeLorme TL. Restoration of muscle power by heavy-resistance exercises. J Bone Joint Surg. 1945;27:645-667.
8 Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541-546.
9 Kirsh K, Jass C, Bennett D, et al. Initial development of a survey tool to detect issues of chemical coping in chronic pain patients. Palliat Support Care. 2007;5:219-226.
10 Seidel S, Aigner M, Ossege M, et al: Antipsychotics for acute and chronic pain in adults. Cochrane Database Systematic Rev (4) CD004844, 2008.
1 Deyo R, Diehl A, Rosenthal M. How many days of bed rest for acute low back pain? N Engl J Med. 1986;315:1064-1070.
2 Quebec Taskforce on Spinal Disorders. Scientific approach to the assessment and management of activity-related spinal disorders. Diagnosis of the problem. Spine. 1987;12(suppl):S17-S21.
3 Coste J, Delecoeuillerie G, Cohen de Lara A, et al. Clinical course and prognostic factors in acute low back pain: an inception cohort study in primary care practice. Br Med J. 1994;308:577-580.
4 Webster BS, Snook SH. The cost of 1989 workers’ compensation low back pain claims. Spine. 1994;19:1111-1116.
5 Hashemi L, Webster BS, Clancy EA. Trends of disability duration and cost for workers’ compensation low back pain claims (1988-1996). J Occup Environ Med. 1998;40:1110-1119.
6 Weinstein JN, Lurie JD, Olson PR, et al. United States’ trends and regional variations in lumbar spine surgery: 1992-2003. Spine. 2006;31(23):2707-2714.
7 Rainville J, Pransky G, Indahl A, et al. The physician as disability advisor for patients with musculoskeletal complaints. Spine. 2005;30(22):2579-2584.
8 Modic M, Obuchowski N, Ross J, et al. Acute low back pain and radiculopathy: MR imaging findings and their prognostic role and effect on outcome. Radiology. 2005;237(2):597-604.
9 Deyo R, Ciol M, Cherkin D, et al. Lumbar spinal fusion. A cohort study of complications, reoperations, and resource use in the Medicare population. Spine. 1993;18(11):1463-1470.
10 Stover B, Wickizer TM, Zimmerman F, et al. Prognostic factors of long-term disability in a workers’ compensation system. J Occup Environ Med. 2007;49(1):31-40.
11 Mayer T, Polatin P, Smith B, et al. Spine Rehabilitation: Secondary and Tertiary Nonoperative Care. North American Spine Society Committee: Contemporary Concepts Review Committee. The Spine Journal. 2003:28S-36S.
12 Pain (Chronic) in Official Disability Guideline, Ed. Philip Denniston. Encinitas, CA, 2008, pp 1351-1632.
13 Chronic Pain. Chapter 6 in American College of Occupational and Environmental Medicine. Occupational Medicine Practice Guidelines, 2nd Ed. Kurt Hegmann. 2008. Revised
14 Cutler RB, Fishbain DA, Rosomoff HL, et al. Does nonsurgical pain center treatment of chronic pain return patients to work? A review and meta-analysis of the literature. Spine. 1994;19:643-652.
15 Hazard R. Spine Update: Functional Restoration. SPINE. 1995;20:2345-2348.
16 Mayer T, Polatin P, Smith B, et al. Contemporary concepts in spine care: spine rehabilitation: secondary and tertiary nonoperative care. Spine. 1995;18:2060-2066.
17 Fore L, Keeley J, Mayer T. Functional Capacity Evaluation for Patients with Chronic Pain. In: Genovese E, Galper G, editors. Chapter 15 in Guide to the Evaluation of Functional Ability. Chicago, IL: AMA Press; 2009:291-312.
18 Mayer T, Gatchel R, Kishino N, et al. Objective assessment of spine function following industrial injury: a prospective study with comparison group and one-year follow-up. 1985 Volvo Award in Clinical Sciences. Spine. 1985;10:482-493.
19 Mayer T, Gatchel R, Mayer H, et al. A prospective two-year study of functional restoration in industrial low back injury: an objective assessment procedure. JAMA. 1987;258:1763-1767.
20 Garcy P, Mayer T, Gatchel R. Recurrent or new injury outcomes after return to work in chronic disabling spinal disorders: tertiary prevention efficacy of functional restoration treatment. Spine. 1996;21:952-959.
21 Jordan K, Mayer T, Gatchel R. Should extended disability be an exclusion criterion for tertiary rehabilitation? Socioeconomic outcomes of early vs. late functional restoration in compensation spinal disorders. Spine. 1998;23:2110-2117.
22 Mayer T, Gatchel R, Evans T. Effect of age on outcomes of tertiary rehabilitation for chronic disabling spinal disorders. Spine. 2001;26:1378-1384.
23 Gatchel R, Mayer T, Kidner C, McGeary D. Are gender, marital status or parenthood risk factors for outcome of treatment for chronic disabling spinal disorders? JOR. 2005;15:191-201.
24 Wright A, Mayer T, Gatchel R, Outcomes of disabling cervical spine disorders in compensation injuries. a prospective comparison to tertiary rehabilitation response for chronic lumbar spinal disorders. Spine. 1999;24:178-183.
25 Mayer T, Anagnostis C, Gatchel R, Evans T. Impact of functional restoration after anterior cervical fusion on chronic disability in work-related neck pain. Spine J. 2002;2:267-273.
26 Mayer T, Gatchel R, Polatin P, Evans T. Outcomes comparison of treatment for chronic disabling work-related upper extremity disorders and spinal disorders. J Occup Environ Med. 1999;41:761-770.
27 Mayer T, McMahon M, Gatchel R, et al. Socioeconomic outcomes of combined spine surgery and functional restoration in workers compensation spinal disorders with matched controls. Spine. 1998;23:598-606.
28 Dersh J, Mayer T, Theodore B, et al. Do psychiatric problems first appear preinjury or postinjury in chronic disabling occupational spinal disorders? Spine. 2007;32:1045-1051.
29 Dersh J, Mayer T, Gatchel R, et al. Prescription opioid dependence is associated with poorer outcomes in disabling spinal disorders. Spine. 2008;33:2219-2227.
30 Gatchel R, Mayer T, Eddington A. MMPI disability profile: the least known, most useful screen for psychopathology in chronic occupational spinal disorders. Spine. 2006;31:2973-2978.
31 Kidner C, Mayer T, Gatchel R. Higher opioid doses predict poorer functional outcome in chronic disabling occupational musculoskeletal disorders. J Bone Joint Surg. 2009;91:919-927.
32 Proctor T, Mayer T, Theodore B, Gatchel R. Failure to complete a functional restoration program for chronic musculoskeletal disorders: a prospective 1-year outcome study. Arch Phys Med Rehabil. 2005;86:1509-1515.
33 McGeary D, Mayer T, Gatchel R. High pain ratings predict treatment failure in chronic occupational musculoskeletal disorders. J Bone Joint Surg. 2006;88-A:317-325.
34 Proctor T, Mayer T, Gatchel R, McGeary D. Unremitting health-care-utilization outcomes of tertiary rehabilitation of chronic musculoskeletal disorders. J Bone Joint Surg. 2004;86:62-69.
35 Anagnostis C, Mayer T, Gatchel R, Proctor T. The million visual analog scale: its utility for predicting tertiary rehabilitation outcomes. Spine. 2003;28:1051-1060.
36 Anagnostis C, Gatchel R, Mayer T. The pain disability questionnaire: a new psychometrically sound measure for chronic musculoskeletal disorders. Spine. 2004;29:2290-2302.
37 Gatchel R, Mayer T, Theodore R. The pain disability questionnaire: relationship to one-year functional and psychosocial rehabilitation outcomes. JOR. 2006;16:75-94.
38 Mayer T, Kondrakse G, Beals S, Gatchel R. Spinal range of motion: accuracy and sources of error with inclinometric measurement. Spine. 1997;22:1976-1984.
39 Brady S, Mayer T, Gatchel R. Physical progress and residual impairment quantification after functional restoration, part II: isokinetic trunk strength. Spine. 1994;18:395-400.
40 Curtis L, Mayer T, Gatchel R. Physical progress and residual impairment after functional restoration, part III: isokinetic and isoinertial lifting capacity. Spine. 1994;18:401-405.
41 Mayer T, Brady S, Bovasso E, et al. Noninvasive measurement of cervical tri-planar motion in normal subjects. Spine. 1993;18:2191-2195.
42 Mayer T, Gatchel R, Keeley J, et al. A Male Incumbent Worker Industrial Database, Part I: Lumbar Spinal Physical Capacity. Spine. 1994;19:755-761.
43 Mayer T, Gatchel R, Keeley J, et al. A Male Incumbent Worker Industrial Database, Part II: Cervical Spinal Physical Capacity. Spine. 1994;19:762-764.
44 Mayer T, Gatchel R, Keeley J, et al. A Male Incumbent Worker Industrial Database, Part III: Lumbar/Cervical Functional Testing. Spine. 1994;19:765-770.
45 Mayer T, Pope P, Tabor J, et al. Physical progress and residual impairment quantification after functional restoration, part I: lumbar mobility. Spine. 1994;18:389-394.
46 Galper J, Reese D, Shea R, Mayer T. Use of computerized extremity and trunk tests in functional capacity evaluation. In: Genovese E, Galper G, editors. Chapter 7 in Guide to the Evaluation of Functional Ability. Chicago, IL: AMA Press; 2009:117-129.
47 DeLorme TL. Restoration of muscle power by heavy-resistance exercises. J Bone Joint Surg. 1945;27:645-667.
48 DeLorme T, Watkins A. Progressive Resistance Exercise: Technic Medical Application. New York: Appleton-Century-Crofts, Inc.; 1951.
49 Keeley J, Mayer T, Cox R, et al. Quantification of lumbar function part 5: reliability range of motion measures in the sagittal plane and in vivo torso rotation measurement technique. Spine. 1986;11:31-35.
50 Mayer T, Tencer A, Kristoferson S, Mooney V. Use of noninvasive techniques for quantification of spinal range of motion in normal subjects and chronic low-back dysfunctional patients. Spine. 1984;9:588-595.
51 Loebl W. Measurement of spinal posture and range in spinal movements. Ann Phys Med. 1967;9:103.
52 Mayer T, Gatchel R. Functional Restoration for Spinal Disorders: The Sports Medicine Approach. Philadelphia, PA: Lea & Febiger; 1988.
53 Engelberg A, editor. American Medical Association Guides to the Evaluation of Permanent Impairment, 3rd Edition, Chicago, IL: AMA Press, 1988.
54 Kohles S, Barnes D, Gatchel R, Mayer T. Improved physical performance outcomes following functional restoration treatment in patient with chronic low back pain: early versus recent training results. Spine. 1990;15:1321-1324.
55 Mayer T, Gatchel R, Betancur J, Bovasso E. Trunk muscle endurance measurement: isometric contrasted to isokinetic testing in normal subjects. Spine. 1995;20:920-927.
56 Mayer T, Gatchel R, Keeley J, Mayer H. Optimal spinal strength normalization factors among male railroad workers. Spine. 1993;18:239-244.
57 Mayer T, Smith S, Keeley J, Mooney V. Quantification of lumbar function part 2: sagittal plane trunk strength in chronic low back pain patients. Spine. 1985;10:765-772.
58 Smith S, Mayer T, Gatchel R, Becker T. Quantification of lumbar function part 1: isometric and multispeed isokinetic trunk strength measures in sagittal and axial planes in normal subject patients. Spine. 1985;10:757-764.
59 Gracovetsky S, Farfan H. The optimum spine. Spine. 1986;11:543-573.
60 Chaffin D. Human Strength capability and low-back pain. J Occup Med. 1974;16:248-254.
61 Kishino N, Mayer T, Gatchel R, et al. Quantification of lumbar function part 4: isometric and isokinetic lifting simulation in normal subjects and low back dysfunction patients. Spine. 1985;10:921-927.
62 Kroemer K. Human strength. Terminology, measurement and interpretation of data. Human Factors. 1970;12:297-313.
63 Mayer T, Barnes D, Kishino N, et al. Progressive isoinertial lifting evaluation part 1: a standardized protocol and normative database. Spine. 1988;13:993-997.
64 Mayer T, Barnes D, Nichols G, et al. Progressive isoinertial lifting evaluation part 2: a comparison with isokinetic lifting in a disabled chronic low-back pain industrial population. Spine. 1988;13:998-1002.
65 Mayer T, Gatchel R, Barnes D, et al. Progressive isoinertial lifting evaluation: erratum notice. Spine. 1990;15:5.
66 Gatchel R, Polatin P, Mayer T, Garcy P. Psychopathology and the rehabilitation of patients with chronic low back pain disability. Arch Phys Med Rehabil. 1994;75:666-670.
67 Kinney R, Gatchel R, Polatin P, et al. Prevalence of psychopathology in acute and chronic low back pain patients. J Occup Rehab. 1993;3:95-103.
68 Polatin P, Kinney R, Gatchel R, et al. Psychiatric illness and chronic low back pain: the mind and the spine–which goes first? Spine. 1993;18:66-71.
69 Mooney V, Cairns D, Robertson J. A system for evaluating and treating chronic back disability. West J Med. 1976;124:370-376.
70 Capra P, Mayer T, Gatchel R. Adding psychological scales to your back pain assessment. J Musc Med. 1985;2:41-52.
71 Fairbank J, Cooper J, Davies J, Obrien J. The Oswestry low back pain disability questionnaire. Physioth. 1980;66:271-273.
72 Jaeschke R, Singer JE, Gordon GH. Measurement of health status. Controlled Clinical Trials. 1989;10:407-415.
73 Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56:395-407.
74 Copay AG, Subach BR, Glassman SD, et al. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541-546.
75 Spratt KF. Minimal clinically important difference based on clinical judgment and minimally detectable measurement difference: a rationale for the SF-36 physical function scale in the SPOT interventional disc herniation cohort. Spine (Phila Pa 1976). 2009;34:1722-1731.
76 Wilson H, Mayer T, Gatchel J. The association between self-reported minimum clinically important differences (MCID) and objective outcomes in a chronic disabling occupational spinal disorder population. Spine. 2009. Submitted for publication
77 Gatchel RJ, Mayer TG. Testing minimal clinically important difference: additional comments and scientific reality testing. Spine J. 2010;10:330-332.
78 Million R, Haavik K, Jayson MIV, et al. Evaluation of low back pain and assessment of lumbar corsets with and without back supports. Ann Rheumat Dis. 1981;40:449-454.
79 Rondinelli R, editor. American Medical Association Guides to the Evaluation of Permanent Impairment, 6th Edition, Chicago, IL: AMA Press, 2008.
80 Beck A. Depression: Clinical, Experimental and Theoretical Aspects. New York: Harper & Row; 1967.
81 Zung WW. A self rating depression scale. Archives of General Psychiatry. 1995;12:63-70.
82 Rush AJ, Giles DE, Schlesser MA, et al. The inventory of depressive symptomatology (IDS): Preliminary findings. Psychiatric Research. 1986;18:65-87.
83 Rush AJ, Gullion CM, Basco MR, et al. The inventory of depressive symptomatology (IDS): psychometric properties. Psychological Medicine. 1996;26:477-486.
84 Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56-62.
85 Ward N. Tricyclic Antidepressant for chronic low back pain: mechanism of action and predictors of response. Spine. 1986;11:661-665.
86 McCracken L, Zayfert C, Gross R. The pain anxiety symptom scale: development and validation of a scale to measure fear of pain. Pain. 1992;50:67-73.
87 Waddell G, Newton M, Henderson I, et al. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance in chronic low back pain and disability. Pain. 1993;52:152-168.
88 Ware J, Sherbourne C. The MOS 36-item short-form health survey (SF-36). Med Care. 1992;30:473-483.
89 Barnett J: The Million Behavioral Health Inventory and the Minnesota Multiphasic Personality Inventory Compared as Predictors of Treatment Outcome in a Rehabilitation Program for Chronic Low Back Pain. PhD dissertation, Division of Psychology, University of Texas Southwestern Medical Center, 1986.
90 First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York State Psychiatric Institute: Biometric Research Department; 1995.
91 Spitzer R, Kroenke K, Williams J. Patient Health Questionnaire Primary Care Study Group, validation and utility of a self-report version of Prime-MD: The PHQ Primary Care Study. JAMA. 1999;292:1737-1744.
92 Cailliet R. The Illustrated Guide to Functional Anatomy of the Musculoskeletal System. Chicago: AMA-Press; 2004.
93 Akeson W, Amiel D, Woo S. Immobility effects on synovial joints. The pathomechanics of joint contracture. Biorheology. 1980;17:95-100.
94 Montgomery J, Steadman J. Rehabilitation of the injured knee. Clin Sports Med. 1985;4:333-343.
95 Salter R, Field P. The effects of continuous compression on living articular cartilage. An experimental study. J Bone J Surg. 1961;43B:376-386.
96 Ruben C: Osteoregulatory Mechanisms…Kappa Delta Award in Transactions of Annual Meeting, American Academy of Orthopedic Surgeons, New Orleans, 1985.
97 Hoshizaki T, Massey B. Relationships of muscular endurance among specific muscle groups for continuous and intermittent static contractions. Res Quart for Exercise and Sport. 1986;57:229-235.
98 Haggmark T. Comparison of Isometric Muscle Training and Electrical Stimulation Supplementing Isometric Muscle Training in the Recovery After Major Knee Ligament Surgery. Am J Sports Med. 1979;7:169-171.
99 Eriksson E. Sports Injuries of Knee Ligaments. Their Diagnosis, Treatment, Rehabilitation and Prevention. Med Sci Sports. 1976;8:133-144.
100 Hagglund M, Walden M, Ekstrand J. Lower reinjury rate with a coach-controlled rehabilitation program in amateur male soccer: a randomized controlled trial. American Journal of Sports Medicine. 2007;35:1433-1442.
101 Askling C, Karlsson J, Thorstensson A. Hamstring injury occurrence in elite soccer players after preseason strength training with eccentric overload. Scandinavian Journal of Medicine & Science in Sports. 2003;13:244-250.
102 Kirsh K, Jass C, Bennett D, et al. Initial development of a survey tool to detect issues of chemical coping in chronic pain patients. Palliat Support Care. 2007;5:219-226.
103 Mayer T, Towns B, Neblett R, et al. Chronic widespread pain in patients with occupational spinal disorders: prevalence, psychiatric comorbidity, and association with outcomes. Spine. 2008;33:1889-1897.
104 Verdu B, Decoslerd I, Buclin T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611-2632.
105 Smith D, Wesson D, Sabnani S. Benzodiasepine and other sedative hypnotic dependence. In: Gabbar G, editor. Gabbards treatment of psychiatric disorders. 4th edition. Arlington, VA: American Psychiatric Publishing; 2007:207-216.
106 Jackson K. Pharmacotherapy for neuropathic pain. Pain Pract. 2006;6:27-33.
107 Seidel S, Aigner M, Ossege M, et al: Antipsychotics for acute and chronic pain in adults. Cochrane Database Syste Rev (4) CD004844, 2008.
108 Johnson R, Strain E, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:559-577.
109 Koppert W, Ihmsen H, Körber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain. 2005;118:15-22.