Functional Magnetic Resonance Imaging
Physiologic Basis of Functional Magnetic Resonance Imaging
Functional magnetic resonance imaging (fMRI) is based on the blood oxygen level–dependent (BOLD) contrast effect and neuronal activity–cerebrovascular flow coupling. Underlying the BOLD effect is the ability of MRI to differentiate magnetic properties of hemoglobin oxygenation states. Oxygenated blood (oxyhemoglobin) is diamagnetic, producing little susceptibility-related dephasing on MR signal. Deoxyhemoglobin is paramagnetic and elicits a more prominent effect on local field homogeneity and phase coherence, resulting in signal loss. Changes in the relative concentrations of oxyhemoglobin and deoxyhemoglobin in the vascular bed therefore result in changes in regional MR signal. The increase in cerebral blood flow that accompanies neuronal activity results in a relative increase in oxyhemoglobin concentration and a resultant localized increase in MR signal.1 Although seemingly straightforward, the actual physiology underlying the BOLD response is complex and is the subject of ongoing research.2 Many anatomic and physiologic changes occur during brain development that have the potential to alter the BOLD response in children compared with adults.3,4–6 Despite these physiologic differences, the basic BOLD response in children is generally similar to that in adults,3,7 albeit with some task-related differences.8 Neonates and infants may exhibit significantly different BOLD responses than do older children and adults, complicating interpretation in this age group.9–11
Technical Considerations for fMRI Performance in Children
The requirements for fMRI performance include an MRI scanner with gradient hardware capable of performing fMRI useable sequences, stimulation/presentation hardware and software linked to the scanner to allow for synchronization of stimuli and MR imaging, hardware and software for documenting patient responses, and postprocessing software for producing activation maps. The small BOLD effect changes in MR signal typically are detected by echo planar imaging (EPI) T2*-sensitive gradient recalled echo techniques. Because of increased signal to noise and sensitivity for BOLD contrast effects, 3T scanners are preferred for fMRI studies.12,13
Performance of useful clinical fMRI examinations in children requires specialized preparation and resources. Before the scheduled examination, the patient is assessed for underlying neurologic deficits, developmental level, and ability to complete the fMRI paradigms. Explanation of the MR procedure and fMRI paradigms to the patient and parents in a calm, child-centered environment is critical. Practicing the fMRI paradigms is important to maximize performance and to adapt the tasks for the patient’s clinical and developmental level. Video presentations and mock scanners are highly useful in preparing children for the fMRI environment.14,15 Patient comfort should be maximized.
Patient motion can influence fMRI performance in children.14,16 Despite some ability to retrospectively correct for head motion, gross head movement typically results in unusable fMRI data. Head coil bite bars, inflatable head cushions, and forehead and chin straps may be used but can be difficult to implement. Head motion is more pronounced in younger children and in boys.16 Older children and girls have a higher rate of successfully completed examinations.14 With care and adequate preparation, most children presenting for clinical fMRI studies can complete fMRI examinations with multiple administered paradigms. The routine application of real-time fMRI processing can reduce the number of inadequate studies.17
Repeated samplings of the brain (one brain volume scan during each repetition time period) while the subject alternates between active cognitive and control tasks typically is performed (the fMRI “paradigm”). Typical fMRI paradigms require 3 to 7 minutes of imaging time for acquisition of 100 or more image volumes during three to five cycles of alternating behavior. Although many approaches are possible, clinical fMRI is most commonly performed in a “blocked-periodic” design in which blocks of task and control (baseline) conditions are administered sequentially.18 The fMRI paradigm that is used ideally will result in activation of brain regions involved with the sensory, motor, or cognitive task presented, without activation in other regions. The proper choice of control and task conditions is important to allow this distinction and must be carefully matched to elicit detectable BOLD signal and isolate the function of interest.19,20 For successful performance of fMRI examinations in children, utilization of age and developmentally appropriate paradigms is mandatory.14,21
Imaging processing is required for fMRI and should involve the interpreting radiologist. After acquisition of images during the fMRI paradigm, the images typically are processed to diminish EPI artifacts, correct for susceptibility-related distortions, limit effects from patient movement during the paradigm, align and transform the T2* EPI images to a higher resolution anatomic dataset, and statistically analyze the images for BOLD signal changes between the task conditions on a voxel by voxel basis (the statistical map) (Fig. 27-1).22 Increasingly, streamlined, clinically oriented options are being offered on most clinical MR systems or by a growing number of third-party vendors. The most common statistical tests used for clinical fMRI are the general linear model21 and the cross-correlation method.18 Determination of the optimum statistical threshold for use in individual clinical patients is a complex issue.22,23 Evaluating fMRI studies at multiple different thresholds is important to maximize clinical effectiveness.
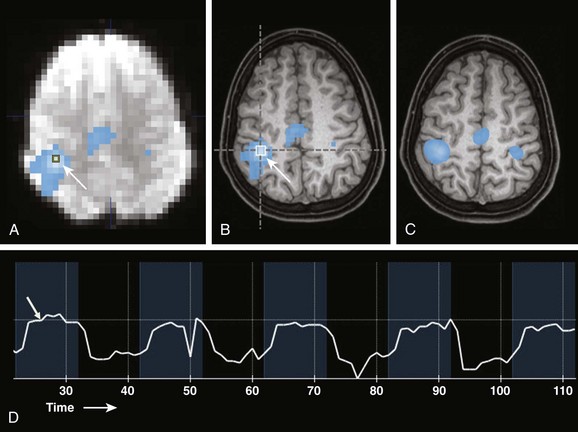
Figure 27-1 Example of bilateral finger tapping functional magnetic resonance imaging (fMRI) and the effect of smoothing on the appearance of fMRI statistical maps.
A, A source echo planar image with an overlayed nonsmoothed statistical activation map. A single voxel region of interest (arrow) is located in the region of maximal statistical significance. B, A nonsmoothed fMRI statistical activation map overlayed onto a 1 mm isotropic T1-weighted image shows activation overlying the right central sulcus in the hand motor region (arrow). Overlay onto anatomic MRI images allows the spatial relationships of the fMRI activation areas to be appreciated and related to anatomy and pathology. C, An fMRI statistical activation map overlayed on a 1 mm isotropic T1-weighted image with smoothing applied to fMRI data. D, Actual signal intensity within a voxel of interest (arrow in A) during the fMRI paradigm. Blue time periods are during finger tapping. Black periods are times of rest. Note the delay in the signal increase (hemodynamic response, arrow) after task initiation.
Clinical Applications of fMRI in Children
Limitations
Some fundamental concepts must be kept in mind when performing and interpreting fMRI studies in clinical patients.24 For example, fMRI activation regions are not functionally specific, and lack of activation in a brain region does not indicate lack of critical brain function. The fMRI procedure is an indirect evaluation of neuronal function and relies on statistical mapping techniques that are not clinically standardized. The BOLD effect can be directly altered by pathologic states with changes in cerebrovascular autoregulation and neurovascular coupling,25 including vascular steno-occlusion, tumors with high vascularity, and arteriovenous malformations.25–27 Artifacts from regions of susceptibility effect (e.g., skull base, sinuses, hemorrhage, and prior surgery) may limit fMRI sensitivity. Sedation also can alter the BOLD response significantly.28
Sensorimotor System Evaluation
The most common and reproducible application of fMRI in clinical patients is assessment of the sensorimotor system (Fig. 27-2).24,29 The fMRI activation areas are somatotopically arranged along the central sulcus. Secondary regions including the supplementary motor area and premotor cortex often commonly are identified. Validation with direct electrocortical simulation (ECoS), the surgical gold standard, generally has been excellent,30–33 with recent studies demonstrating nearly 100% concordance.29 Sensorimotor fMRI evaluation is most useful when the normal anatomic relationships relating to the central sulcus are distorted by adjacent mass lesions; fMRI can help guide operative approaches in these cases (Fig. 27-3). High success rates of motor fMRI (93%) in children undergoing surgery for epilepsy have been documented.34
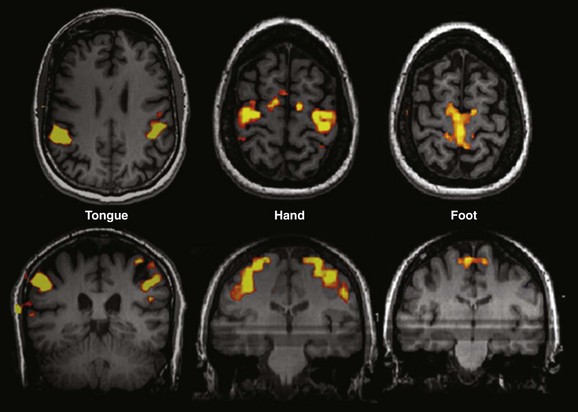
Figure 27-2 Examples of different motor paradigms useful for clinical functional magnetic resonance imaging.
Tongue: Sequential tongue movement while the mouth is closed. Hand: Sequential bilateral finger tapping. Foot: Sequential bilateral foot flexion and extension. Typically performed clinical paradigms include sequential finger thumb opposition, hand grasping, wrist flexion and extension, foot flexion and extension, lip puckering, and tongue movement for motor strip assessment and tactile stimulation with brushes or air puffs for sensory component evaluation.
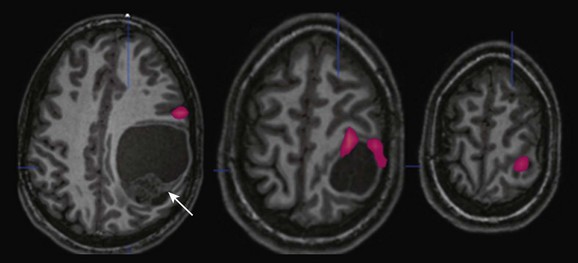
Figure 27-3 A 15-year-old boy with progressive right-sided motor weakness.
Anatomic magnetic resonance imaging revealed a large cystic and solid mass within the left parietal lobe (arrow) that was markedly distorting normal anatomy; functional magnetic resonance imaging (fMRI) was requested to outline the location of the motor strip more definitively. An fMRI image obtained during right-sided sequential finger tapping demonstrates activation along the superior and anterior aspects of the mass. A posterior surgical approach was performed with gross total resection and no new deficit. Pathology revealed that the mass was an anaplastic ependymoma.
Language Evaluation
Multiple clinical and fMRI studies have established that a left hemispheric dominance exists for semantic and phonological language functions in most persons. Most fMRI studies of language lateralization in children have shown similar patterns of activation compared with adults, supporting the theory that language networks are established by early childhood. However, multiple cross-sectional and longitudinal fMRI studies have demonstrated changes in BOLD localization20,35–39 with multiple language paradigms during development. In general, greater and more widespread activation is present in children compared with adults, which likely is related to differential brain maturation. Hemispheric language dominance is related to handedness, with approximately 95% of right-handed subjects being left hemispheric dominant for language, whereas approximately 20% of non–right-handed subjects (those who are ambidextrous or left handed) exhibit atypical (nonlateralizing or right-sided) hemispheric language dominance.40 These findings should be kept in mind when interpreting language fMRI examinations in children.
Paradigms for Language Assessment
Multiple fMRI paradigms have been created to assess different aspects of language function (Fig. 27-4 and e-Fig. 27-5). It is important to utilize multiple language tasks in clinical patients to more fully define language processing.20,41–43 The use of multiple tasks reduces the likelihood of nondiagnostic findings, improves inter-rater reliability, and helps in the confirmation of language laterality.43 Three of the most studied paradigms in children are verb generation, semantic decision, and story processing (Fig. 27-2).
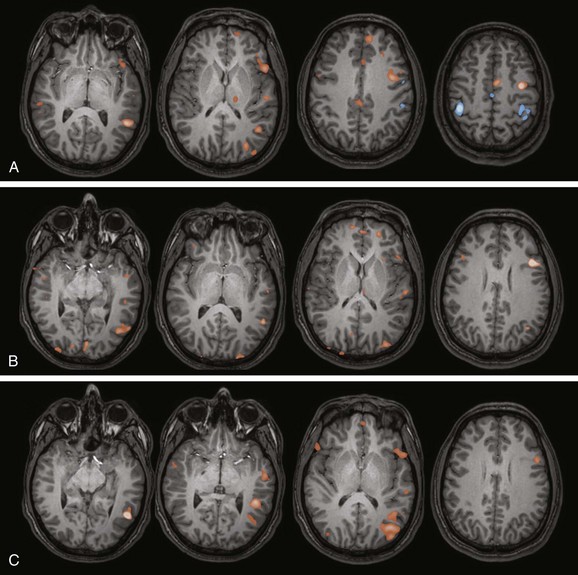
Figure 27-4 Examples of language functional magnetic resonance imaging (fMRI) paradigms in a 12-year-old right-handed girl with intractable epilepsy.
A, Verb generation (control task: finger tapping). A statistical map of fMRI activation during verb generation (orange areas) demonstrates left lateralization, particularly in the left inferior frontal lobe and left temporal lobe, which is typical for this paradigm. Activation during finger tapping is noted along both central sulci in the hand motor regions, as well as the putative supplementary motor area in the midline. B, Semantic decision (control task: tone discrimination). A similar left lateralizing pattern is noted with robust inferior frontal and temporal parietal activation, which is typical for this paradigm. The semantic decision task provides an additional assessment of frontal and temporal language areas and has the added capability of allowing direct assessment of patient performance. C, Story processing (control task: backwards speech). Clear left lateralizing activation is noted in the temporal lobe. Left lateralizing inferior frontal activation also is noted. These paradigms together indicate typical left lateralization of language in this patient.
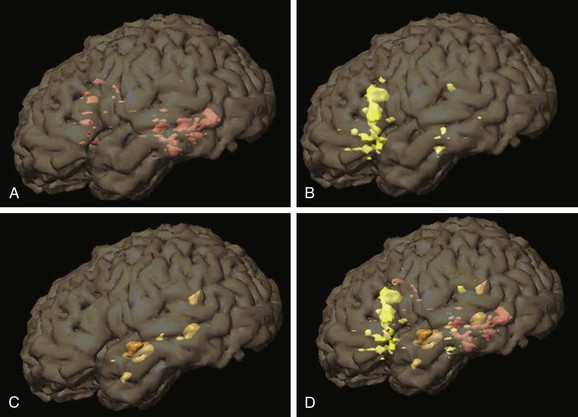
e-Figure 27-5 Left hemispheric activation areas from language tasks in Figure 27-4 are superimposed on three-dimensional (3D) segmented brain images, a very useful tool for presurgical mapping and understanding the 3D relationships between functional magnetic resonance imaging activation regions. A, Verb generation. B, Semantic decision. C, Story processing (temporal lobe regions). D, Combined activation regions.
The verb generation task35 (Fig. 27-4, A) involves the auditory presentation of a series of concrete nouns. The patient covertly (silently) generates as many verbs associated with the noun as possible. Control tasks include rest or bilateral sequential finger tapping.35,36,44 The semantic decision task has been modified for children and involves the auditory presentation of single words (animal names)41,45 (Fig. 27-4, B). The child makes a button press if the animal fits a target semantic property (e.g., “Does the animal walk on four legs?”). In the control condition, the patient listens to a series of tones for a specific tonal sequence. The story processing task41,46,47 typically involves the auditory presentation of simple stories, each composed of sentences with specifically formulated complex syntactic constructions (Fig. 27-4, C). The control tasks are listening to various tonal sequences20 or to identical periods of temporally reversed speech.47 Other tasks such as linguistic prosody, read response naming, and auditory and reading sentence comprehension also have been used successfully with children.41,48,49
Clinical Examination Interpretation
Interpretation of language fMRI in children can be difficult. Descriptions of language laterality and hemispheric dominance in clinical and research contexts typically have been described on the basis of a region of interest (ROI) “laterality” or “asymmetry” index (LI).20,24 LI calculations are dependent on the statistical technique and threshold used for calculating activated voxels, ROI, and fMRI task.50,51 They are more robust when ROIs targeted to typical language areas are used.52,53 Use of multiple thresholds and fMRI tasks in individual patients to more completely assess language laterality is recommended.50 Interestingly, visual assessment of lateralization in individual patients actually may be as good as ROI-based LI calculations.49,54 Typically, fMRI examinations are interpreted in clinical patients as left hemispheric dominant (typical activation pattern), right hemispheric dominant, and bilateral or nonlateralizing language representation. Bilateral activation patterns can be problematic in evaluation of individual clinical patients because this fMRI pattern often cannot be conclusively interpreted.50 Epilepsy,55 perinatal stroke,56 and a prenatally or perinatally acquired lesion57 can effect language development and lead to altered lateralization of language function (e-Fig. 27-6).
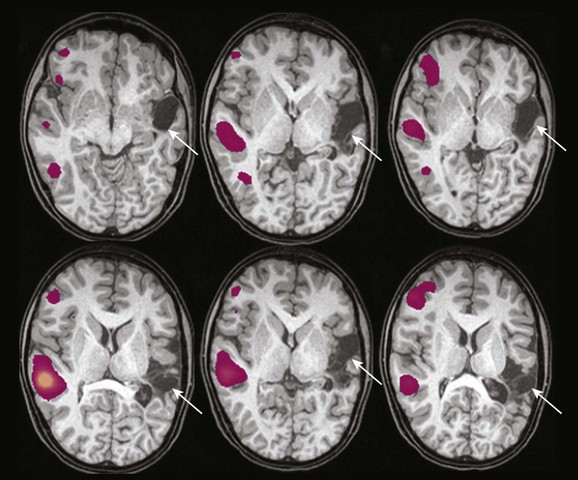
e-Figure 27-6 An 11-year-old boy with intractable epilepsy and a history of perinatal left middle cerebral artery infarct.
Large areas of encephalomalacia are identified in the left hemisphere (arrows). Statistical activation maps demonstrate clear right lateralization of activation with verb generation in right hemispheric homologous regions. Right hemispheric dominance for language is confirmed by an intraarterial amobarbital procedure.
Validation of Language fMRI
The traditional method for determining language dominance before surgery has been the Wada or intracarotid amobarbital procedure (IAP). Another method of language mapping is direct ECoS either during surgery with the patient awake or with use of subdural grid electrodes.58 The IAP is expensive, invasive, and more difficult to perform in children.58,59 It carries with it a small but definite risk of complications,59–61 and its invasive nature limits repeated assessments.62 Although it provides lateralization information, the IAP cannot spatially localize language functions in the brain, which is an advantage for fMRI.
Correlation studies between fMRI and IAP for language lateralization consistently have shown an 85% to 90% concordance rate,19,60,61,63,64 with the use of multiple tasks increasing the degree of concordance.52,53 Discrepancies are most common in patients who exhibit atypical language lateralization, particularly patients with bilateral, nonlateralized representation.62,63,65 Discordance between fMRI and the IAP also is more common in patients with neocortical epilepsy (a more common scenario in pediatric patients than in the adult epilepsy population) and left temporal lobe seizure origin.54 The few studies that have specifically compared language-based fMRI with the IAP in children have demonstrated concordance in 80% to 100% of patients.62,66 Although fMRI may not be able to replace the IAP in all circumstances, routine fMRI can diminish IAP utilization significantly.
The fMRI procedure also has been used in an attempt to guide resections near eloquent language regions. Because fMRI will demonstrate areas of the brain that are associated with, but not necessarily essential to, a particular task, fMRI will always exhibit a lack of specificity for language mapping in this scenario. Few direct comparisons between language fMRI and ECoS for regional language mapping have been performed, and the results have been variable. The sensitivity of fMRI in identifying critical language areas as established by ECoS varies between 22% to 100%, with specificities of between 61% and 100%.67 A recent detailed study in adult patients with a brain tumor found an overall fMRI sensitivity of 80% and a specificity of 78% for critical language areas compared with the results of ECoS.68 Despite the variable reported correlations, fMRI may be very useful to guide intraoperative ECoS procedures and for counseling parents and children regarding surgery in eloquent areas.17,69
Other Indications
The fMRI procedure has been used to investigate a wide variety of clinical conditions in children, including the evaluation of reading and dyslexia, attention deficit–hyperactivity disorder, autism, and hearing loss,70–72 to name a few. Clinical applications in these conditions are evolving, and at present, should be regarded as research tools. Assessment of memory by fMRI is possible and has begun to be assessed in clinical patients. Clinical research studies have been performed that correlate fMRI activation patterns with the IAP73,74 and postoperative memory after temporal lobe resection in adults,74 with encouraging results; however, evaluation of the application in pediatric patients is incomplete. The fMRI procedure, often in combination with other functional modalities (e.g., diffusion tensor imaging and magnetoencephalography), may allow for guidance during surgical access and resection using modern neuronavigation systems (functional neuronavigation).75 Use of fMRI in conjunction with ECoS and frameless stereotaxy has been found to help facilitate tumor resection in children with a wide variety of lesions near eloquent cortical regions (e-Figs. 27-7 and 27-8).76
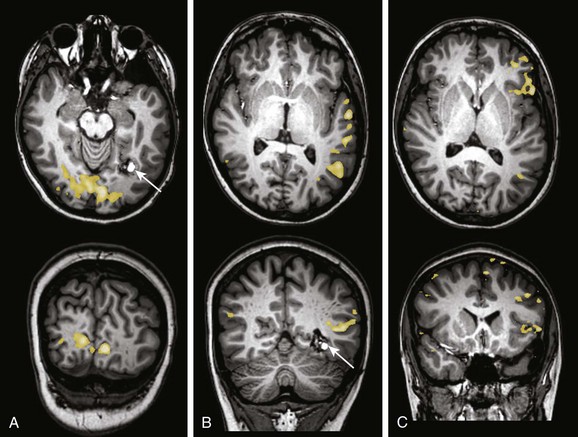
e-Figure 27-7 A 15-year-old boy with seizures and headache.
A cavernous malformation (arrows) is identified in the left inferior medial occipital lobe along the temporal occipital junction. The patient was referred for functional magnetic resonance imaging and diffusion tensor imaging–tractography to aid in operative planning. A, A visual stimulation paradigm demonstrates activation along the calcarine fissure medial to the lesion. B, A story processing task demonstrates activation along the left temporal lobe very asymmetric to the right, lateral, and slightly superior to the plane of the lesion. C, A verb generation task demonstrates clear left lateralization of activation in the frontal and temporal parietal regions. Verb generation and story processing tasks were consistent with left hemispheric lateralization of language.
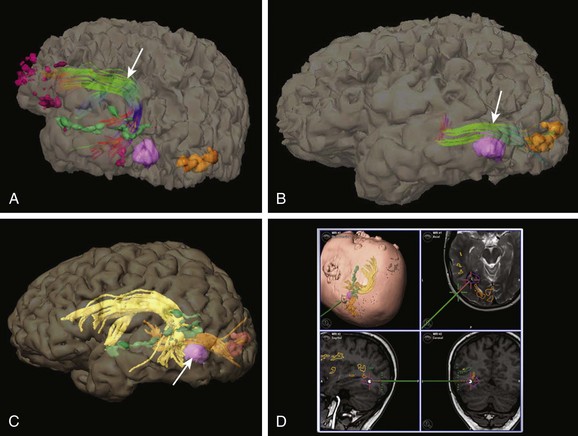
e-Figure 27-8 Utility of combined functional magnetic resonance imaging (fMRI) and tractography for operative planning (same patient shown in Fig. 27-6).
A, Tractography is performed to outline the arcuate fasciculus orientation (arrow) using fMRI activation regions to guide seed points. Dark purple areas: fMRI activation regions from verb generation. Green areas: fMRI activation areas from a story processing task. Light purple area: cavernous malformation (surgical target). Orange areas: fMRI activation regions from visual stimulation. The posterior arcuate fasciculus is anterior to the lesion. B, Tractography is performed to outline the geniculocalcarine tract (arrow). The geniculocalcarine tract lies directly above and touches the superior aspect of the lesion. C, Because of the close proximity of the lesion (arrow) to functionally important regions, integration of the tractography and fMRI data with anatomic images was performed. Objects were created from the lesion, temporal lobe language areas as demonstrated by a story processing task, arcuate fasciculus, and geniculocalcarine tract. D, Images captured during surgery demonstrating the operative approach, which was guided by anatomic and functional imaging. Complete resection was performed with no neurologic deficit. (Intraoperative images courtesy Francesco Mangano, DO.)
Future Techniques
Newer techniques of fMRI acquisition and processing hold promise to expand the utility of fMRI in children. These techniques include advanced connectivity analyses of fMRI data and fMRI performed in the so-called resting state.77 Resting state fMRI analyses have begun to be used in evaluating the functional networks in the developing brain78 and may have particular clinical use in identifying functionally important cortex and networks in patients in which task-based fMRI examinations are not feasible (e.g., in neonates, infants, and sedated patients). Although currently it is a research tool, initial clinical applications have been described in adults and children.79,80
Full references for this chapter can be found on www.expertconsult.com.
Holland, SK, Vannest, J, Mecoli, M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–551.
Kotsoni, E, Byrd, D, Casey, BJ. Special considerations for functional magnetic resonance imaging of pediatric populations. J Magn Reson Imaging. 2006;23:877–886.
Leach, JL, Holland, SK. Functional MRI in children: clinical and research applications. Pediatr Radiol. 2010;40:31–49.
O’Shaughnessy, E, Berl, M, Moore, E, et al. Pediatric functional MRI: issues and applications. J Child Neurol. 2008;23(7):791–801.
Wilke, M, Holland, SK, Myseros, JS, et al. Functional magnetic resonance imaging in pediatrics. Neuropediatrics. 2005;34(5):225–233.
References
1. Huettel, SA, Song, AW, McCarthy, G. Spatial and temporal properties of fMRI. In: Huettel SA, Song AW, McCarthy G, eds. Functional magnetic resonance imaging. Sunderland, MA: Sinauer Associates, 1992.
2. Logothetis, NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23:3963–3971.
3. Gaillard, WD, Grandin, CB, Xu, B. Developmental aspects of pediatric fMRI: considerations for image acquisition, analysis, and interpretation. Neuroimage. 2001;13:239–249.
4. Chugani, HT, Phelps, ME, Mazziotta, JC. Positron emission tomography study of human brain functional development. Ann Neurol. 1987;22:487–497.
5. Levin, JM, Frederick, B, Ross, MH, et al. Influence of baseline hematocrit and hemodilution on BOLD fMRI activation. Magn Reson Imaging. 2001;19:1055–1062.
6. Chiang, LK, Dunn, AE. Cardiology. In: Siberry GK, Iannone R, eds. The Harriet Lane handbook: a manual for pediatric house officers. St. Louis: Mosby, 2001.
7. Schlaggar, BL, Brown, TT, Lugar, HM, et al. Functional neuroanatomical differences between adults and school aged children in the processing of single words. Science. 2002;296:1476–1479.
8. Brauer, J, Neumann, J, Friederici, AD. Temporal dynamics of perisylvian activation during language processing in children and adults. Neuroimage. 2008;41:1484–1492.
9. Heep, A, Scheef, L, Janowski, J, et al. Functional magnetic resonance imaging of the sensorimotor system in preterm infants. Pediatrics. 2009;123:294–300.
10. Morita, T, Kochiyama, T, Yamada, H, et al. Difference in the metabolic response of the lateral geniculate nucleus and the primary visual cortex of infants: a fMRI study. Neurosci Res. 2000;38:63–70.
11. Born, AO, Rostrup, E, Miranda, MJ, et al. Visual cortex reactivity is sedated children examined with perfusion MRI (FAIR). Magn Reson Imaging. 2002;20:199–205.
12. Kruger, G, Kastrup, A, Glover, GH. Neuroimaging at 1.5 T and 3.0 T: comparison of oxygenation-sensitive magnetic resonance imaging. Magn Reson Med. 2001;45:595–604.
13. Voss, HU, Zevin, JD, McCandliss, BD. Functional MR imaging at 3.0T versus 1.5T: a practical review. Neuroimag Clin North Am. 2006;16(2):285–297.
14. Byars, AW, Holland, SK, Strawsburg, RH, et al. Practical aspects of conducting large scale functional magnetic resonance image studies in children. J Child Neurol. 2002;17:885–889.
15. Altman, NR, Bernal, B. Pediatric applications of fMRI. In: Faro S, Mohamed FB, eds. Functional MRI. Basic principles and clinical applications. New York: Springer, 2006.
16. Yuan, W, Altaye, M, Ret, J, et al. Quantification of head motion in children during various fMRI language tasks. Hum Brain Mapp. 2009;30(5):1481–1489.
17. Kesavadas, C, Thomas, B, Sujesh, S, et al. Real-time functional MRI (fMRI) for presurgical evaluation of pediatric epilepsy. Pediatr Radiol. 2007;37:964–974.
18. Bandettini, P, Jesmanowicz, E, Wong, E, et al. Processing strategies for time course data sets in functional MRI of the human brain. Magn Reson Med. 1993;30:161–173.
19. Binder, JR, Swanson, SJ, Hammeke, TA, et al. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49(12):1980–1997.
20. Holland, SK, Vannest, J, Mecoli, M, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46:533–551.
21. Friston, K, Jezzard, P, Turner, R. Analysis of functional MRI time series. Hum Brain Mapp. 1998;6:283–300.
22. Goebel, R. Localization of brain activity using functional magnetic resonance imaging. In Stippich C, ed.: Clinical functional MRI. Presurgical functional neuroimaging, 1st ed, Heidelberg: Springer-Verlag, 2007.
23. Peck, KK. Methods of analysis. In: Holodny A, ed. Functional neuroimaging. A clinical approach. New York: Informa Healthcare, 2008.
24. Stippich, C, Blatow, M, Krakow, K. Presurgical functional MRI in patients with brain tumors. In: Stippich C, ed. Clinical functional MRI. Presurgical functional neuroimaging. Heidelberg: Springer-Verlag, 2007.
25. Ulmer, JL, Hacein-Bev, L, Matthews, VP, et al. Lesion induced pseudo-dominance at functional magnetic resonance imaging: implications for preoperative assessments. Neurosurgery. 2004;55(3):569–579.
26. Rossini, PM, Altamura, C, Ferretti, A, et al. Does cerebrovascular disease affect the coupling between neuronal activity and local haemodynamics? Brain. 2004;127:99–110.
27. Kamba, M, Sung, Y-W, Ogawa, S. Alteration of blood oxygen level-dependent signaling by local circulatory condition. J Magn Reson Imaging. 2007;26:1506–1513.
28. Qiu, M, Ramani, R, Swetye, M, et al. Anesthetic effects on regional CBF, BOLD, and the coupling between task-induced changes in CBF and BOLD: an fMRI study in normal human subjects. Magn Reson Med. 2008;60(4):987–996.
29. Roessler, K, Donat, M, Lanzenberger, R. Evaluation of preoperative high magnetic field motor functional MRI (3 Tesla) in glioma patients by navigated electrocortical stimulation and postoperative outcome. J Neurol Neurosurg Psychiatry. 2005;76:1152–1157.
30. Yetkin, FZ, Mueller, WM, Morris, GL, et al. Functional MR activation correlated with intraoperative cortical mapping. AJNR Am J Neuroradiol. 1997;18:1311–1315.
31. Lehericy, S, Duffau, H, Cornu, P, et al. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J Neurosurg. 2000;92:589–598.
32. Shinoura, N, Yamada, R, Kodama, T, et al. Intraoperative cortical mapping has low sensitivity for the detection of motor function in the proximity to a tumor in the primary motor area. Sterotact Funct Neurosurg. 2005;83:135–141.
33. Krishnan, R, Raabe, A, Hattingen, E, et al. Functional magnetic resonance imaging-integrated neuronavigation: correlation between lesion to motor cortex distance and outcome. Neurosurgery. 2004;55:904–915.
34. De Tiège, X, Connelly, A, Liégeois, F, et al. Influence of motor functional magnetic resonance imaging on the surgical management of children and adolescents with symptomatic focal epilepsy. Neurosurgery. 2009;64(5):856–864.
35. Holland, SK, Plante, E, Byars, AW, et al. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843.
36. Schapiro, MB, Schmithorst, VJ, Wilke, M, et al. BOLD-fMRI signal increases with age in selected brain regions in children. Neuroreport. 2004;15(17):2575–2578.
37. Brown, TT, Lugar, HM, Coalson, RS, et al. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15:275–290.
38. Gaillard, WD, Hertz-Pannier, L, Mott, SH, et al. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54:180–185.
39. Gaillard, WD, Balsamo, LM, Ibrahim, Z, et al. fMRI identifies regional specialization of neural networks for reading in young children. Neurology. 2003;60:94–100.
40. Szaflarski, JP, Binder, JR, Possing, ET. Language lateralization in left handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244.
41. Vannest, J, Karunanayaka, PR, Schmithorst, VJ, et al. Language networks in children: evidence from functional MRI studies. AJR Am J Roentgenol. 2009;192:1190–1196.
42. Wilke, M, Lidzba, K, Staudt, M, et al. An fMRI task battery for assessing hemispheric language dominance in children. Neuroimage. 2006;32:400–410.
43. Gaillard, WD, Balsamo, L, Xu, B, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408.
44. Szaflarski, JP, Holland, SK, Schmithorst, VJ, et al. An fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27(3):202–212.
45. Binder, JR, Frost, JA, Hammeke, TA, et al. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17:353–362.
46. Karunanayaka, PR, Holland, SK, Schmithorst, VJ, et al. Age related connectivity changes in fMRI data from children listening to stories. Neuroimage. 2007;34:349–360.
47. Ahmad, Z, Balsamo, LM, Sachs, BC, et al. Auditory comprehension of language in young children. Neural networks identified with fMRI. Neurology. 2003;60:1598–1605.
48. Gaillard, WD, Pugilese, M, Grandin, CB, et al. Cortical localization of reading in normal children. An fMRI language study. Neurology. 2001;57:47–54.
49. Gaillard, WD, Balsamo, MA, Xu, B, et al. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265.
50. Wellmer, J, Weber, B, Weis, S, et al. Strongly lateralized activation in language fMRI of atypical dominant patients—implications for presurgical work-up. Epilepsy Res. 2008;80:67–76.
51. Ruff, IM, Petrovich Brennan, NM, Peck, KK, et al. Assessment of the language laterality index in patients with brain tumor using functional MR imaging: effects of thresholding, task selection, and prior surgery. AJNR Am J Neuroradiol. 2008;29:528–535.
52. Rutten, GJ, Ramsey, NF, van Rijen, PC, et al. Reproducibility of fMRI-determined language lateralization in individual subjects. Brain Lang. 2002;80:421–437.
53. Arora, J, Pugh, K, Westerveld, M, et al. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225–2241.
54. Woerman, FG, Joeckert, H, Luerding, R, et al. Language lateralization by the Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61(5):699–701.
55. Yuan, W, Szaflarski, JP, Schmithorst, VJ, et al. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47(3):593–600.
56. Tillema, J-M, Byars, AW, Jacola, LM, et al. Cortical reorganization of language functioning following perinatal left MCA stroke. Brain Lang. 2008;105:99–111.
57. Staudt, M, Grodd, W, Niemann, G, et al. Early left periventricular brain lesions induce right hemispheric organization of speech. Neurology. 2001;57:122–125.
58. Schevon, CA, Carlson, C, Zaroff, CM, et al. Pediatric language mapping: sensitivity of neurostimulation and Wada testing in epilepsy surgery. Epilepsia. 2007;48(3):539–545.
59. Baxendale, SA, Thompson, PJ, Duncan, JS. Evidence-based practice: a reevaluation of the intracarotid amobarbital procedure (Wada test). Arch Neurol. 2008;65(6):841–845.
60. Abou-Khalil, B. An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer non-invasive alternatives. Epilepsia. 2007;48(3):442–455.
61. Kloppel, S, Buchel, C. Alternatives to the Wada test: a critical view of functional magnetic resonance imaging in preoperative use. Curr Opin Neurol. 2005;18:418–423.
62. Anderson, DP, Harvey, SA, Saling, MM, et al. fMRI lateralization of expressive language in children with cerebral lesions. Epilepsia. 2006;47(6):988–1008.
63. Lee, D, Swanson, SJ, Sabsevitz, DS, et al. Functional MRI and Wada studies in patients with interhemispheric dissociation of language functions. Epilepsy Behav. 2008;13:350–356.
64. Sabsevitz, DS, Swanson, SJ, Hammeke, TA, et al. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792.
65. Benke, T, Koylu, B, Visani, P, et al. Language lateralization in temporal lobe epilepsy: a comparison between fMRI and the Wada test. Epilepsia. 2006;47(8):1308–1319.
66. Liegois, F, Cross, HJ, Gadian, DG, et al. Role of fMRI in the decision making process: epilepsy surgery for children. J Magn Reson. 2006;23:933–940.
67. Smits, M, Visch-Brink, E, Schraa-Tam, C, et al. Functional MR imaging of language processing: an overview of easy-to-implement paradigms for patient care and clinical research. Radiographics. 2006;26:S145–S158.
68. Bizzi, A, Blasi, V, Falini, A, et al. Presurgical functional MRI of language and motor functions: validation with intraoperative electrocortical mapping. Radiology. 2008;248(2):579–589.
69. Ojemann, JG, Ojemann, GA, Lettich, E. Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. J Neurosurg. 2002;97:33–38.
70. Patel, AM, Cahill, LD, Ret, J, et al. Functional magnetic resonance imaging of hearing impaired children under sedation before cochlear implantation. Arch Otolaryngol Head Neck Surg. 2007;133(7):677–683.
71. Seyffert, M, Castellanos, FX. Functional MRI in pediatric neurobehavioral disorders. Int Rev Neurobiol. 2005;67:239–284.
72. Kelly, AMC, Margulies, DS, Castellanos, FX. Recent advances in structural and functional brain imaging studies of attention–deficit/hyperactivity disorder. Curr Psych Report. 2007;9:401–407.
73. Szfalrski, JP, Holland, SK, Schmithorst, VJ, et al. High resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004;5:244–252.
74. Rabin, ML, Narayan, VM, Kimberg, DY. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004;127:2286–2298.
75. Nimsky, C, Ganslandt, O, Buchfelder, M, et al. Intraoperative visualization for resection of gliomas: the role of functional neuronavigation and intraoperative 1.5T MRI. Neurol Res. 2006;28:482–487.
76. Stapleton, SR, Kiriakopoulos, E, Mikulis, D, et al. Combined utility of functional MRI, cortical mapping, and frameless stereotaxy in resection of lesions in eloquent areas of brain in children. Pediatr Neurosurg. 1997;26:68–82.
77. Fox, MD, Greicius, M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4(19):1–13.
78. Smyser, CD, Snyder, AZ, Neil, JJ. Functional connectivity MRI in infants: exploration of the functional organization of the developing brain. Neuroimage. 2011;56(3):1437–1452.
79. Shimony, JS, Zhang, D, Johnston, JM, et al. Resting-state spontaneous fluctuations in brain activity: a new paradigm for presurgical planning using fMRI. Acad Radiol. 2009;16(5):578–583.
80. Böttger, J, Margulies, DS, Horn, P, et al. A software tool for interactive exploration of intrinsic functional connectivity opens new perspectives for brain surgery. Acta Neurochir (Wien). 2011;153(8):1561–1572.