Frostbite
Perspective
Unlike other animals that live outside the tropics, humans are susceptible to peripheral cold injuries. The highest homeostatic priority is to maintain the body’s core temperature. This is accomplished through peripheral vasoconstriction and shunting, which prevent adequate heat distribution to the extremities. As a result, failure to achieve adequate protection from the environment results in injuries that are usually preventable.1–3
Peripheral cold injuries include both freezing and nonfreezing syndromes, which may occur independently or in conjunction with systemic hypothermia.4 Frostbite is the most common freezing injury.5 Trench foot and immersion foot are nonfreezing injuries that result from exposure to wet cold.6 Nonfreezing injury that usually occurs after exposure to dry cold is termed chilblains (pernio).7
The incidence and severity of frostbite correlate with predisposing factors as well as with the degree of cold stress. Most cases of civilian frostbite result from exposure to cold by individuals who have not given due consideration to risk factors for cold injury.8,9 Well-equipped ascents of the world’s highest peaks have been completed without cold injury when appropriate steps have been taken to address these factors.10,11 An increase in outdoor recreational activities has increased the number of people exposed to severe cold conditions.12,13 Unsheltered homeless people are no longer the most likely group at risk in areas with moderate climates.14
Military history is replete with accounts of the effects of cold injury on combat troops.15,16 Amputations and time lost to local cold injuries in both world wars and the Korean conflict were extensive. Trench foot was common among Argentine and British forces in the Falkland Islands.4,17
Napoleon’s Surgeon General, Baron Larrey, first recorded the disastrous effects of the freeze-thaw-refreeze cycle.18 During the 1812 to 1813 Russian invasion and retreat, soldiers would acutely thaw frozen extremities directly over open fires. The subsequent refreeze further increased tissue destruction. Unfortunately, the resultant gangrene was misattributed to this rapid thawing of frostbite and trench foot injuries. Therefore, gradual thawing, often including friction massage with snow, remained the standard treatment regimen until the 1950s.19,20 In 1961, Mills ultimately popularized rapid warm immersion rewarming after extensive experience with severe Alaskan frostbite cases.20,21
Principles of Disease
In contrast to heat exposure, humans do not appear to display significant physiologic adaptation to the cold. Exposure of extremities to temperatures down to 15° C results in maximal peripheral vasoconstriction with minimal blood flow. Continued exposure to progressively colder temperatures down to 10° C produces the “hunting response,” which is cold-induced vasodilation.22 These periods of vasodilation, recurring in 5- to 10-minute cycles, interrupt vasoconstriction and serve to protect the extremity. Eskimos as well as Lapps and others of Nordic extraction are capable of stronger cold-induced vasodilation than that in individuals from tropical regions. Measurement of the speed of cold-induced vasodilation may help predict an individual’s risk for cold injury.23 There is evidence of adaptation rather than pure genetic control.24
Pathophysiology
The pathologic phases that occur with local cold injury often overlap and vary with the extent and rapidity of the cold response (Box 139-1). Frostbite occurs when the tissue supercools well below 0° C. The required temperature is at least −4° C and may be as low as −10° C.
There are two putative mechanisms of tissue injury: architectural cellular damage from ice crystal formation and microvascular thrombosis and stasis.25 In the prefreeze phase, tissue temperatures drop below 10° C and cutaneous sensation is lost. Before ice crystal formation, microvascular vasoconstriction occurs along with endothelial leakage of plasma into the interstitium. Radiation and conduction of heat from deeper tissues prevent crystallization until the skin temperature drops well below 0° C.26 In the freeze-thaw phase, the timing, location, and rate of ice crystal formation depend on the exposure circumstances. In addition to ambient temperatures, wind and moisture increase the freezing rate.
An additional insult, progressive dermal ischemia, is partially mediated by thromboxane.27 Fluid analysis of clear vesicles identifies prostaglandins. When subdermal vascular plexuses are injured, hemorrhagic blisters develop that also contain these prostanoids. The arachidonic acid breakdown products released from underlying damaged tissue into the blister fluid include both prostaglandins and thromboxane. These mediators produce platelet aggregation, vasoconstriction, and leukocyte immobilization.28
Edema progresses for 48 to 72 hours after tissue is thawed. Leukocyte infiltration, thrombosis, and early necrosis become apparent as this edema resolves. The dry gangrene carapace of frostbite is superficial in comparison to arteriosclerosis-induced, full-thickness gangrene. Although the historical surgical aphorism was “frostbite in January, amputate in July,” advances in imaging modalities can accelerate the identification of the demarcation between viable and nonviable tissue.21
Predisposing Factors
The extent of peripheral cold injury is determined by the type and duration of cold contact with the skin (Box 139-2).29 Predisposing risks include physiologic, mechanical, psychological, environmental, and cardiovascular factors.
Although air alone is a poor thermal conductor, associated cold and wind (wind chill index) markedly increase heat loss. Direct skin contact with good thermal conductors such as metal, water, and volatile liquids affects the extent and rapidity of tissue destruction. Commercial aerosol spray propellants, such as propane and butane, and carbon dioxide in fire extinguishers are potentially hazardous.30 Liquid oxygen and Freon can also cause frostbite.31,32 Overenthusiastic application of standard ice packs in the treatment of soft tissue injuries can also result in tissue loss.33 Cryotherapy is commonly prescribed in sports medicine. In addition to improper use of cold packs, vapor coolant sprays such as chloroethane can cause frostbite.34,35
Clinical Features
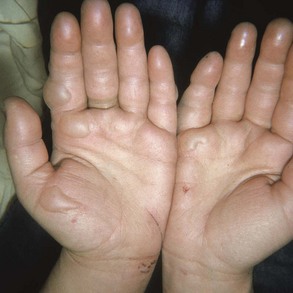
Classically, the initial presentation of frostbite is deceptively benign. Most patients do not arrive in the emergency department with frozen, insensate tissue. Frozen tissues often appear mottled or violaceous-white, waxy, or pale yellow. In severe cases, the examiner will not be able to roll the dermis over bone prominences. Rapid rewarming results in an initial hyperemia, even in severe cases. After thawing, partial return of sensation should be expected until blebs form.21
Classification by degrees is often incorrect in relation to the actual severity of the frostbite and thus therapeutically misleading. Mills suggests two simple retrospective classifications.20,21 Superficial or mild frostbite does not entail eventual tissue loss, whereas deep or severe frostbite does result in tissue loss. As a result, it is not feasible to predict, on presentation, the eventual tissue loss. Another classification attempts to establish severity on the basis of clinical features coupled with early bone scan results.17,36 Significant pain usually accompanies reestablishment of perfusion. With partial tissue destruction, intermittent pain may be noticed during ongoing exposure. The dull continuous ache evolves into a throbbing sensation in 48 to 72 hours. This often persists until tissue demarcation several weeks to months later. Nonfreezing cold injury occurs when tissue fluids have not frozen. Chilblain (pernio) is a mild form of cold injury that often follows repetitive exposure. These “cold sores” appear less than 24 hours after exposure and usually affect facial areas, the dorsa of the hands and feet, and the pretibial areas. Young women with a history of Raynaud’s phenomenon or systemic lupus erythematosus or with antiphospholipid antibodies are especially at risk. Persistent vasospasm and vasculitis result in burning, pruritus, erythema, and mild edema. Plaques, blue nodules, and ulcerations can develop and last 1 to 2 weeks.
The other common nonfreezing cold injury is trench foot (immersion foot). This remains a significant threat during recreational activities and military expeditions in cold, wet climates. Trench foot is produced by prolonged exposure to wet cold at temperatures above freezing.6 It usually develops slowly during several days and results in neurovascular damage in the absence of ice crystal formation. Immersion foot commonly develops while a person is wearing sweat-dampened or neoprene socks, vapor-barrier boots, or constrictive gaiters. Patients who soak their feet for hours each night in cool water for pain relief are also at risk.
The clinical presentation varies. Most patients’ symptoms include cool, pale feet that are numb or tingle. Later the feet appear cyanotic, cold, and edematous. Often, numbness and leg cramping are present. The clinical hallmark is that after rewarming, the skin remains erythematous, dry, and very painful to touch. Rubor on dependency and pallor on elevation are caused by vasomotor paralysis. Infrared thermography in response to a cold stress may support the diagnosis and assess its severity.17
Diagnostic Strategies
Routine baseline radiographs should be obtained. Follow-up radiographs will begin to demonstrate specific frostbite abnormalities 4 to 10 weeks after injury. Intravenous isotope studies have had mixed success experimentally and clinically.37,38 In one study, triple-phase bone scans performed 2 days after cold injury demonstrated ischemic tissue at risk.39,40 Delayed bone scans in 7 to 10 days can image deep tissue and bone infarction. The absence of radionuclide uptake even after 10 days, however, does not reliably predict the eventual need for amputation. The patient should be advised that accurate prediction of eventual tissue loss is difficult. As an ancillary tool, scintigraphy predicts the eventual demarcation line better than thermography does.41 Scintigraphy as early as day 2 may predict tissue loss and monitor the efficacy of treatment.42
In clinical practice, magnetic resonance imaging and magnetic resonance angiography may be superior to technetium bone scanning. In one study, the clear-cut line of demarcation was noted before clinical demarcation. Magnetic resonance imaging of developing hyaline cartilage can demonstrate physeal injury, which has the largest impact on longitudinal growth.43
Management
Emergency Department
Pertinent history regarding the ambient temperature, wind velocity, and duration of exposure should be obtained. The type of apparel worn, the circumstances surrounding rescue, and the presence of preexisting cardiovascular or neurologic diseases that could affect tissue loss should be noted.44
Thaw
Frozen or partially thawed tissue should be rapidly and actively rewarmed by immersion in gently circulating water that is carefully maintained at a temperature of 37 to 39° C by thermometer measurement.20 Marginal tissue can suffer thermal injury when the water temperature exceeds 42° C. Although a circulating tank is ideal for arms or legs, a large container suffices for the hands or feet. Water warmer than 39° C is less well tolerated and causes more pain. Care should be taken to prevent the frostbitten area from bumping or rubbing against the side of the container.
Patients with completely frozen extremities are usually hypothermic and at risk for significant fluid and electrolyte fluxes during rewarming. The acute thawing of large amounts of distal musculature extinguishes peripheral vasoconstriction. This results in the sudden return of cold, hyperkalemic, acidotic blood to the central circulation. This produces “core temperature afterdrop,” which is dysrhythmogenic. In the most severe cases, extracorporeal rewarming should be considered for management of these massive metabolic and electrolyte derangements (Box 139-3).
Post-thaw
The clinical role of thromboxane inhibition in frostbite seems to be limited. Thromboxane inhibition does not appear to result in additional clinically significant tissue salvage. In one experimental model, methimazole did not improve tissue survival even when therapy was initiated immediately.45 Progressive secondary dermal ischemia is addressed by attempts to limit the accumulation of the products of arachidonic acid breakdown. Topical aloe vera (Dermaide) every 6 hours is a specific thromboxane inhibitor when it is applied directly to frostbitten areas but has not definitively been proven to salvage tissue.28 Other alternatives include topical antibiotic ointment. Theoretically, oral ibuprofen appears preferable to oral aspirin. Although both agents inhibit the arachidonic acid cascade, ibuprofen also produces fibrinolysis. Parenteral ketorolac can also be considered.
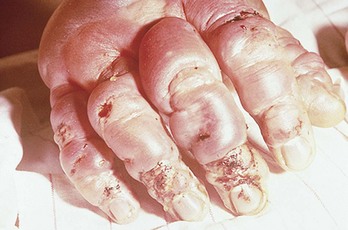
Frostbite blister management varies widely. Recommended options for large clear blisters include leaving them intact, débridement, and aspiration (Fig. 139-1). Although most clinicians débride broken blisters, many prefer to aspirate intact clear blisters rather than to débride them. In contrast, if hemorrhagic blisters are débrided, secondary desiccation of deep dermal layers appears to extend the injury (Fig. 139-2). In this case, aspiration is preferable to débridement.
Management of the chilblains syndrome is usually supportive. Nifedipine (20-60 mg daily) is an effective treatment of refractory perniosis.46,47 Topical or systemic corticosteroids have also been useful. Other options include oral pentoxifylline and limaprost, a prostaglandin E1 analogue.
Adjunctive Treatment
Numerous ancillary modalities have been suggested for frostbite.48 Capillary flow ceases early after cold injury, whereas thrombosis proceeds.10,40 This observation has led to multiple experimental antithrombotic and vasodilation treatment regimens, although most lack adequate controls. Many of these studies were conducted before the elucidation of some of the pathophysiologic consequences of frostbite. Triple-phase bone scans have demonstrated that thrombolytic agents may restore some flow to severely frostbitten limbs.
Thrombolytic Therapy
Thrombolytic therapy may address the primary residual pathophysiologic changes if the cumulative cold-ischemia time (frozen time) and warm-ischemia time (thawed and unperfused) is not excessive. In one retrospective study, intravenous tissue plasminogen activator and heparin reduced predicted digit amputations in severe frostbite.42 Nonresponders had more than 24 hours of exposure, more than 6 hours of warm ischemia, or multiple freeze-thaw cycles. In another study, intra-arterial tissue plasminogen activator decreased the incidence of amputations when it was administered within 24 hours.49
A screening and treatment tool is proposed for thrombolysis.50,51 If the flow is absent after thawing and both the cold-exposure and warm-ischemia times are each less than 24 hours, angiography is performed with intra-arterial vasodilators. Nitroglycerin and papaverine have both been used successfully. If flow is not reestablished, continuously infuse intra-arterial catheter-directed tissue plasminogen activator to a maximum dose of 1 mg/hr. Heparin is administered concurrently and continued for 72 to 96 hours.51,52 A second alternative approach combines systemic intravenous thrombolytic therapy with subsequent vascular evaluation by technetium scanning.
Low-molecular-weight dextran may inhibit intravascular cellular aggregation. Animal models suggest that low-molecular-weight dextran is not harmful. Pentoxifylline, a phosphodiesterase inhibitor, may decrease blood viscosity and increase tissue oxygenation.53 Its ability to increase red blood cell flexibility facilitates revascularization and may enhance tissue survival. The suggested dosage is 400 mg three times daily for 2 to 6 weeks.54
Various anti-inflammatory drugs and other agents have not been conclusively evaluated. These include steroids, nonsteroidal anti-inflammatory drugs, dipyridamole, dimethyl sulfoxide, nonionic detergents, and calcium channel blockers.55,56 A long-acting alpha-blocker, phenoxybenzamine, may decrease vasospasm while increasing peripheral blood flow. The dosage starts with 10 mg/day to a maximum of 60 mg/day. With this agent, adequate hydration is necessary to prevent orthostatic hypotension.
Hyperbaric oxygen produces vasoconstriction and subsequently reduces cutaneous blood flow. A small number of patients report a temporary flush and increased limb motion, but this appears to depend on the elapsed time interval after injury. Hyperbaric oxygen has the potential to accelerate demarcation. There are insufficient data to assess the potential value of hyperbaric oxygen therapy for tissue salvage in severe frostbite.57,58
Sympathectomy
Iloprost, a prostacyclin analogue, also has vasodilatory properties that mimic a chemical sympathectomy. The risk of amputation is significantly lower in a controlled trial of patients with severe frostbite who received intravenous iloprost plus aspirin after thawing.59 Selected patients in this series with severe frostbite were also treated with recombinant tissue plasminogen activator. Forearm nerve blocks also produce in effect a chemical sympathectomy that increases finger skin temperature.60
Early results with surgical sympathectomy were encouraging. Bouwman and colleagues performed unilateral surgical sympathectomy on 10 patients with bilateral matched frostbite injuries.61 Delayed protection against reinjury was one direct benefit. Ultimately, however, there was no increased tissue salvage. Mills observed that surgical sympathectomy produces a smoother initial clinical course but no long-term benefits, with the possible exception of decreased causalgia.20,21
Disposition
Whirlpool hydrotherapy with an antiseptic should be performed two or three times daily for 20 to 30 minutes to débride dead tissue and to decrease the amount of bacterial colonization. Range-of-motion exercises should be encouraged during immersion. Severe cases may require position-of-function splinting. Hydrotherapy is continued as the eschar sloughs. During hospitalization, all vasoconstrictive agents, including nicotine, should be avoided. Vacuum-assisted closure therapy attracts wound edges centripetally, reduces tissue edema, and promotes angiogenesis in burns and complex wounds. With frostbite, it could prove beneficial to prevent grafting or amputation.62
Sequelae
Direct neuronal damage and residual abnormalities in sympathetic tone are responsible for most of the common symptomatic sequelae of frostbite. In a series of military patients with documented frostbite, 65% had long-term residual symptoms. Vasospasm with secondary cold intolerance is the other major sequela.63
Delayed cutaneous findings include nail deformities and pigmentation changes. Squamous and epidermoid cell carcinoma can occur. Osseous reabsorption and subchondral lytic defects develop months after the cold insult. In pediatric patients, concerns include premature fusion, destruction, and fragmentation of epiphyses. Shortening of the distal phalanges is common.63 Frostbite arthritis also occurs, commonly 3 to 10 years later. Thumb sparing is a characteristic idiosyncrasy. Clenching of the fists can spare both thumbs and metacarpophalangeal joints. Identification of subchondral cysts after bone infarction differentiates frostbite arthritis from osteoarthritis.64 In severe cases involving extremity muscle compartments, rhabdomyolysis and subsequent renal failure are a concern. Continuous monitoring of serum muscle enzymes and urinalyses is warranted.
Surgical decisions regarding amputation are complex.63,65 The amount of tissue eventually salvaged often exceeds even optimistic initial estimates. Historically, the natural progression of demarcation, mummification, and eventual sloughing was allowed to occur. Advances in radiologic assessment of tissue viability are facilitating earlier surgical intervention. Free flap tissue transfer to salvage function after earlier débridement of soft tissues should be a consideration.65,66 Compared with grafts, flaps provide their own vascularity and are less dependent on the recipient bed.67
Various neuropathic, musculoskeletal, and dermatologic sequelae of frostbite are listed in Box 139-4.68
References
1. Hamlet, MP. Prevention and treatment of cold injury. Int J Circumpolar Health. 2000;59:108.
2. Long, WB, et al. Cold injuries. J Long Term Eff Med Implants. 2005;15:67.
3. Angert, D, Schaff, EA. Preventing injuries and illnesses in the wilderness. Pediatr Clin North Am. 2010;57:683.
4. Thomas, JR, Oakley, HN, Nonfreezing cold injury, Textbook of Military Medicine: Medical Aspects of Harsh Environments. Pandolf, KB, Burr, RE, eds., Textbook of Military Medicine: Medical Aspects of Harsh Environments. Washington, DC:Office of the Surgeon General, Borden Institute; 2002;vol 1:467.
5. Koljonen, V, et al. Frostbite injuries treated in the Helsinki area from 1995 to 2002. J Trauma. 2004;57:1315.
6. Atenstaedt, RL. Trench foot: The medical response in the first World War 1914-1918. Wilderness Environ Med. 2006;17:282.
7. Makinen, TM, et al. Occurrence of frostbite in the general population—work-related and individual factors. Scand J Work Environ Health. 2009;35:384.
8. Kroeger, K, Janssen, S, Niebel, W. Frostbite in a mountaineer. Vasa. 2004;33:173.
9. Ervasti, O, et al. The occurrence of frostbite and its risk factors in young men. Int J Circumpolar Health. 2004;63:71.
10. Harirchi, I, et al. Frostbite: Incidence and predisposing factors in mountaineers. Br J Sports Med. 2005;39:898.
11. Subedi, BH, et al. Frostbite in a Sherpa. Wilderness Environ Med. 2010;21:127.
12. McCauley, RL, et al. Frostbite. In: Auerbach PS, ed. Wilderness Medicine. 5th ed. St. Louis: Mosby/Elsevier; 2007:195.
13. Castellani, JW, et al. American College of Sports Medicine position stand: Prevention of cold injuries during exercise. Med Sci Sports Exerc. 2006;38:2012.
14. Jurkovich, GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247.
15. DeGroot, D, et al. Epidemiology of U.S. Army cold weather injuries, 1980-1999. Aviat Space Environ Med. 2003;74:564.
16. Moran, D, et al. Hypothermia and local cold injuries in combat and non-combat situations: The Israeli experience. Aviat Space Environ Med. 2003;74:281.
17. Imray, C, et al. Cold damage to the extremities: Frostbite and non-freezing cold injuries. Postgrad Med J. 2009;85:481.
18. Paton, BC. “From Larrey to Mills”: The road to rapid rewarming—a commentary. Wilderness Environ Med. 1998;9:223.
19. Paton, BC. A history of frostbite treatment. Int J Circumpolar Health. 2000;59:99.
20. Mills, WJ, Jr., Whaley, R, Fish, W. Frostbite: Experience with rapid rewarming and ultrasonic therapy: Part III, 1961. Alaska Med. 1993;35:19.
21. Mills, WJ, Jr. Summary of treatment of the cold injured patient, 1980. Alaska Med. 1993;35:50.
22. Brajkovic, D, Ducharme, MB. Facial cold-induced vasodilatation and skin temperature during exposure to cold wind. Eur J Appl Physiol. 2006;96:711.
23. Daanen, H, Van Der Struijs, NR. Resistance index of frostbite as a predictor of cold injury in arctic operations. Aviat Space Environ Med. 2005;76:1119.
24. Keramidas, ME, et al. Enhancement of the finger cold-induced vasodilation response with exercise training. Eur J Appl Physiol. 2010;109:133.
25. Golant, A, et al. Cold exposure injuries to the extremities. J Am Acad Orthop Surg. 2008;16:704.
26. Lee, CK, Hansen, SL. Management of acute wounds. Surg Clin North Am. 2009;89:659.
27. Ozyazgan, I, et al. Eicosanoids and inflammatory cells in frostbitten tissue: Prostacyclin, thromboxane, polymorphonuclear leukocytes, and mast cells. Plast Reconstr Surg. 1998;101:1881.
28. Heggers, JP, et al. Frostbite: Experimental and clinical evaluations of treatment. J Wilderness Med. 1990;1:27.
29. Geng, Q, et al. Temperature limit values for touching cold surfaces with the fingertip. Ann Occup Hyg. 2006;8:851.
30. Sever, C, et al. Frostbite injury of the foot from portable fire extinguisher. Dermatol Online J. 2009;15:10.
31. Uygur, F, Sever, C, Noyan, N. Frostbite burns caused by liquid oxygen. J Burn Care Res. 2009;30:358.
32. Sever, C, et al. Hand burn caused by Freon gas. Burns. 2008;34:1210.
33. Keskin, M, et al. Frostbite injury due to improper usage of an ice pack. Ann Plast Surg. 2005;55:437.
34. Soyuncu, S, Yigit, O, Eken, C. Frostbite injury related to chlorethane application. Wilderness Environ Med. 2009;20:103.
35. Gamble, WB, Bonnecarre, ER. Coffee, tea, or frostbite? A case report of inflight freezing hazard from dry ice. Aviat Space Environ Med. 1993;67:880.
36. Cauchy, E, et al. Retrospective study of 70 cases of severe frostbite lesions: A proposed new classification scheme. Wilderness Environ Med. 2001;12:248.
37. Kenney, A, 3rd., Vyas, P. Frostbite injury: Appearance on three-phase bone scan. Clin Nucl Med. 1998;23:188.
38. Greenwald, D, Cooper, B, Gottlieb, L. An algorithm for early aggressive treatment of frostbite with limb salvage directed by triple-phase scanning. Plast Reconstr Surg. 1998;102:1069.
39. Aygit, AC, Sarikaya, A. Imaging of frostbite injury by technetium-99m-sestamibi scintigraphy: A case report. Foot Ankle Int. 2002;23:56.
40. Cauchy, E, et al. The value of technetium 99 scintigraphy in the prognosis of amputation in severe frostbite injuries of the extremities: A retrospective study of 92 severe frostbite injuries. J Hand Surg Am. 2000;25:969.
41. Bhatnagar, A, et al. Diagnosis, characterization and evaluation of treatment response of frostbite using pertechnetate scintigraphy: A prospective study. Eur J Nucl Med. 2002;29:170.
42. Twomey, JA, et al. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350.
43. Khanna, PC, Thapa, MM. The growing skeleton: MR imaging appearances of developing cartilage. Radiol Clin North Am. 2009;47:899.
44. Biem, J, et al. Out of the cold: Management of hypothermia and frostbite. CMAJ. 2003;168:305.
45. Goldberg, BD, Robinson, WA, Watson, WA. Impact of delayed presentation on the efficacy of thromboxane inhibition in the treatment of frostbite. J Wilderness Med. 1994;5:325.
46. Parlette, EC, Parlette, HL, III. Erythrocyanotic discoloration of the toes. Cutis. 2000;65:223.
47. Simon, TD, Soep, JB, Hollister, JR. Pernio in pediatrics. Pediatrics. 2005;116:e472.
48. Petrone, P, Kuncir, EJ, Asensio, JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165.
49. Bruen, KJ, et al. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546.
50. Sheridan, RL, et al. Case 41-2009: A 16-year-old boy with hypothermia and frostbite. N Engl J Med. 2009;361:2654.
51. Saemi, AM, Johnson, JM, Morris, CS. Treatment of bilateral hand frostbite using transcatheter arterial thrombolysis after papaverine infusion. Cardiovasc Intervent Radiol. 2009;32:1280.
52. Bruen, KJ, Gowski, WF. Treatment of digital frostbite: Current concepts. J Hand Surg Am. 2009;34:553.
53. Purkayastha, SS, et al. Immediate treatment of frostbite using rapid rewarming in tea decoction followed by combined therapy of pentoxifylline, aspirin and vitamin C. Indian J Med Res. 2002;116:29.
54. Hayes, DW, Jr., et al. Pentoxifylline: Adjunctive therapy in the treatment of pedal frostbite. Clin Podiatr Med Surg. 2000;17:715.
55. Muelleman, RL, Grandstaff, PM, Robinson, WA. The use of pegorgotein in the treatment of frostbite. Wilderness Environ Med. 1997;8:17.
56. Teixeira, F, et al. Use of antioxidants for the prophylaxis of cold-induced peripheral nerve injury. Mil Med. 2002;167:753.
57. Finderle, Z, Cankar, K. Delayed treatment of frostbite injury with hyperbaric oxygen therapy: A case report. Aviat Space Environ Med. 2002;73:392.
58. McCrary, BF, Hursh, TA. Hyperbaric oxygen therapy for delayed frostbite injury. Wounds. 2005;17:327.
59. Cauchy, E, Cheguillaume, B, Chetaille, E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. N Engl J Med. 2011;364:189.
60. Chandran, GJ, et al. The hyperthermic effect of a distal volar forearm nerve block: A possible treatment of acute digital frostbite injuries? Plast Reconstr Surg. 2010;126:946.
61. Bouwman, DL, et al. Early sympathetic blockade for frostbite: Is it of value? J Trauma. 1980;20:744.
62. Poulakidas, S, Cologne, K, Kowal-Vern, A. Treatment of frostbite with subatomospheric pressure therapy. J Burn Care Res. 2008;29:1012.
63. Su, CW, Lohman, R, Gottlieb, LJ. Frostbite of the upper extremity. Hand Clin. 2000;16:235.
64. Kahn, JE, Lidove, O, Laredo, JD, Blétry, O. Frostbite arthritis. Ann Rheum Dis. 2005;64:966.
65. Leonard, LG, et al. Salvage of a vascular bone from frostbite with free tissue transfer. Ann Plast Surg. 2001;46:431.
66. Classen, DA. Free flap coverage of bilateral frostbite of the feet. Plast Reconstr Surg. 2000;106:1316.
67. Delgado-Martinez, J, et al. Skin coverage in frostbite injuries: Experimental study. J Plast Resconstr Aesthet Surg. 2010;63:713.
68. Oumlish, OY, Parish, LC. Marching in the army: Common cutaneous disorders of the feet. Clin Dermatol. 2002;20:445.