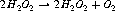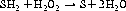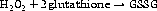Chapter 7 Free radicals
How are Free Radicals Formed?
Electrons like to exist in pairs, so when atoms are chemically separated from their covalent bonds (see Chapter 3 ‘Bonds found in biological chemistry’ p. 13) they do not normally split to leave a single unpaired electron. When this does happen, a free radical is formed.
The ‘attacked’ molecule loses its electron and in turn becomes a free radical. This occurs quickly – in much less than a second – and the process rapidly starts a chain reaction that, once started, can cascade and ultimately result in the disruption of a cell.
What Causes Free Radicals?
How do Free Radicals Cause Damage?
Free radicals cause damage in four ways:
Normal Occurrences of Free Radicals
The body needs free radicals for certain functions:
In a healthy individual, the free radicals that are formed by the body are dealt with via a variety of radical ‘quenching’ systems. However, if too many free radicals form then there is a problem.
Reactive Oxygen Species
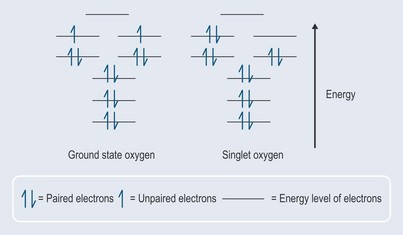
When an oxygen molecule interacts with other molecules to form a covalent bond, it needs to accept two electrons that are spinning in the opposite direction to its own electrons in the outermost orbital or they will not fit (it would be rather like trying to put two like poles of a magnet together, which results in repulsion). In the presence of energy, one of the electrons can change its direction of spin and pairs up with the other one to create singlet oxygen (activated oxygen) (Figure 7.1). This is not as stable as the ground state oxygen and will change back fairly quickly (less than 0.04 microseconds) but in that time it will have affected its surrounding environment.
Singlet oxygen interacts with other molecules in two ways:
Thus, oxygen is activated by two different mechanisms.
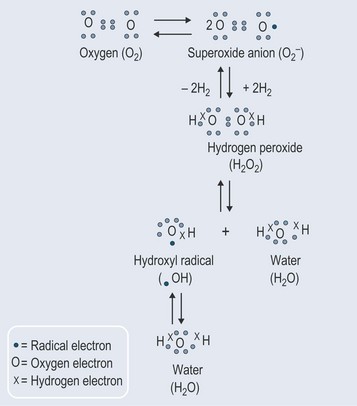
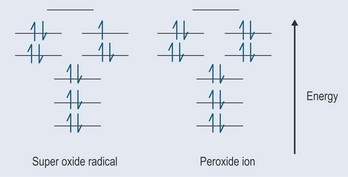
Superoxides
The most important source of the superoxide radical is the electron transport chains in the mitochondria and endoplasmic reticulum (where the cytochrome P450 is stored and works in the liver). The electrons should pass from one component of these chains to another, but the relay system is not perfect and there is thought to be some ‘leakage’.
Effects of Free Radical Damage
Damage to Lipids
Oils go rancid as a result of oxidation (see Chapter 4 ‘Bonds continued’, p. 27). In the late 1800s, it was observed that – when exposed to air – a layer of walnut oil on water would absorb three times its own volume of air in 10 days. For this reason, margarine manufactures must slow or prevent this oxidation process.
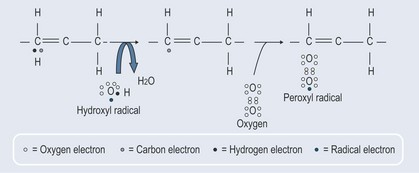
Cells walls are comprised of lipids and, as hydrogen peroxide so easily permeates these membranes, peroxidation of the fatty acid part of the phospholipids in the cell wall is relatively easy, occurring in the area between the double bonds (Figure 7.4).
Damage to Proteins
Generally, protein is less susceptible to free radical damage than lipids. The extent to which proteins are affected by free radicals very much depends on their amino acid make up. Sulphur-containing amino acids are the most susceptible to free radical damage (or certainly attack from an activated oxygen molecule). Proteins associated with metals, e.g. haem compounds found in enzymes, are also vulnerable to free radical damage (see Chapter 37 ‘Metabolic disorders’, p. 296).
Two main reactions occur as a result of free radical damage to proteins:
Body’s Protection Against Free Radicals
Tissues are full of enzymes designed to protect against free radical damage.
Superoxide Dismutases (SOD)
Superoxide dismutases (SOD) can contain zinc and copper (Cu-Zn-SOD) and also manganese or iron. SOD converts superoxide to hydrogen peroxide and oxygen (Figure 7.5), and minimizes production of the hydroxyl radical that causes so much damage (it is the most potent of the oxygen free radicals). This system is nearly always the antioxidant defence in cells exposed to oxygen. It is extremely quick and can more than match the production of superoxide when working properly.
There are three forms of SOD in the human body:
Mutations in SOD1 have been linked to familial amyotrophic lateral sclerosis (ALS).
Catalases and Peroxidases
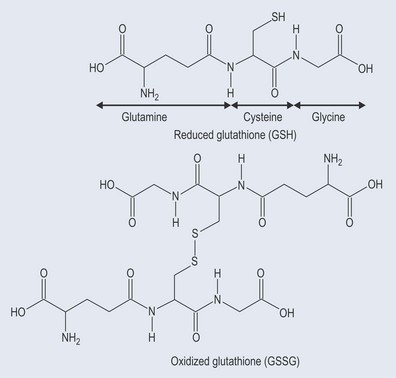
Peroxidases react differently, using a sulphur-containing compound called glutathione (see Figure 7.7):
Catalyse is present in all the major body organs. It is concentrated in the liver and erythrocytes but there is very little in the brain, heart and skeletal muscle.
Glutathione peroxidase is found largely in the liver and – to a much lesser extent – in the heart, lungs and brain, with very little activity in muscle. It contains selenium (see Chapter 13 ‘Vitamins and minerals’, p. 100), a dietary source of which is required for its activity.
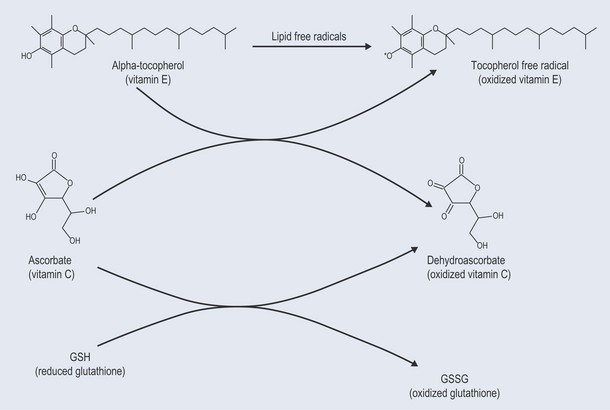
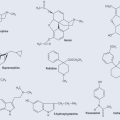
Antioxidants
• Ascorbic acid
• Flavonoids
•Glutathione
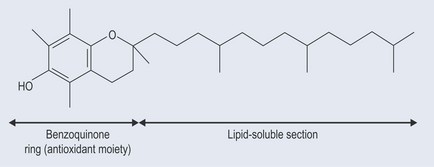
• Tocopherols
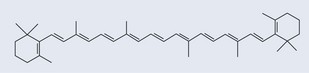
• Carotenoids
Beta-carotene (Figure 7.10) is a more efficient scavenger of singlet oxygen than tocopherol. Not only does it have an aromatic part, it also possesses a polyunsaturated section (see Figure 10.1, p. 74). The mechanism of its antioxidant action is not properly understood. It has been demonstrated to quench singlet oxygen, scavenge peroxyl radicals and inhibit lipid peroxidation. There does not appear to be a mechanism to recycle beta-carotene.
Useful Free Radicals
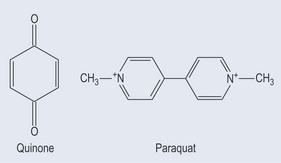
Poisoning with Herbicides
A major class of herbicides works by interrupting photosynthetic electron flow in plants. They can do this because their structure (which is similar to that of the quinines) enables them to intercept electrons intended for flow down the electron chain (Figure 7.13; see Figure 21.7, p. 153). As human metabolism also uses quinines, it is possible that an accumulation of this type of pesticide will produce free radicals.