Chapter 27 Fragile X Syndrome
PATHOPHYSIOLOGY
Mutations in the fragile X mental retardation (FMR1) gene are associated with fragile X syndrome, fragile X–associated tremor/ataxia syndrome (FXTAS), and FMR1-related premature ovarian failure. Individuals with fragile X syndrome have an intellectual disability; individuals with FXTAS and FMR1-related premature ovarian failure usually do not have an intellectual disability, but they are at high risk for having children or grandchildren with fragile X syndrome.
The FMR1 gene is located on the X chromosome; thus women inherit two copies (alleles) of the FMR1 gene and men inherit one allele. A particular region on the FMR1 gene (often referred to as the fragile site) has three DNA bases (cytocine [C], guanine [G], guanine) that are repeated (trinucleotide repeat) many times. There are essentially four allelic categories that define repeat length.
A normal allele is characterized by 5 to 40 CGG repeats. An intermediate (gray zone) allele has 41 to 58 repeats. A premutation allele has 59 to 200 CGG repeats. A premutation allele is not associated with intellectual disability, but does convey an increased risk for FXTAS and FMR1-related premature ovarian failure. Women who have 59 to 200 CGG repeats are considered to be at risk for having children affected with fragile X syndrome. Individuals who have more than 200 hypermethylated CGG repeats (usually several hundred to several thousand) have full mutations; they have fragile X syndrome. The number of repeats is unstable from generation to generation, making the pattern of inheritance difficult to predict.
The FMR1 gene codes for a protein called the FMR protein, made in many tissues and having a high concentration in the brain and testes. The FMR protein plays a role in the development of synapses between nerve cells in the brain where cell to cell communication occurs. The connection between nerve cells can change and adapt over time in response to experience (synaptic plasticity). The FMR protein is thought to help regulate synaptic plasticity, which is important for learning and memory. Consequently, an individual born with a full mutation in his or her FMR1 gene suffers from cognitive and neuropsychologic problems.
The more profound intellectual disability (formerly known as mental retardation) seen in males can be explained by X inactivation that occurs during embryogenesis. A female embryo inherits two X chromosomes; two active X chromosomes are not compatible with life, and thus one X chromosome in every cell has to be inactivated. The inactivation process is random; in a female embryo, it does not automatically inactivate the X chromosome that has a defective FMR1 gene.
For example, if a female inherits a defective FMR1 gene from her father, it could work out that 80% of his X chromosomes will be inactivated. That, in turn, means that 80% of her cells will be making adequate FMR1 protein; she will probably not have a severe intellectual disability, and perhaps not any. On the other hand, if 80% of her mother’s X chromosomes were inactivated, the child would most likely have an intellectual disability.
INCIDENCE
1. Fragile X syndrome is the number one cause of inherited intellectual disability.
2. Fragile X affects 1 in 4000 males and 1 in 8000 females.
3. The prevalence is similar in most ethnic and racial groups.
4. High repeat numbers (41 to 199 CGG repeats) occur in 4% of all males and 8% of all females; they are considered carriers.
5. Approximately 15% of women with premature ovarian failure have 35 to 54 CGG repeats.
6. Approximately 3% of men over age 50 years with unexplained ataxia have 83 to 109 CGG repeats.
CLINICAL MANIFESTATIONS
2. Developmental characteristics
LABORATORY AND DIAGNOSTIC TESTS
Refer to Appendix D, Laboratory Values, for normal values and ranges of laboratory and diagnostic tests.
2. Prenatal genetic testing is conducted when a mother is a known carrier.
3. Preimplantation genetic testing (see Box 27-1 for CLIA-approved clinics in the United States)
MEDICAL MANAGEMENT
Medical management is directed to providing care that addresses primary care needs and provides supports for long-term management. For additional information on the long-term management of the child with fragile X syndrome, refer to Chapter 44. The child with fragile X syndrome will require long-term monitoring to detect secondary conditions that may develop. Medical treatment includes pharmacologic management for hyperactivity, depression, sleep disorders, and seizures. Methylphenidate and dextroamphetamine are prescribed to treat hyperactivity, and antidepressants (selective serotonin reuptake inhibitors [SSRIs]) for depression; traxodone and melatonin are used to treat sleep disorders. Carbamazepine is commonly prescribed, and it may help with behavior problems. Carbamazepine is altered by macrolide antibiotics, cimetidine, propoxyphene, and isoniazid. Estimated average lifetime cost of care for a patient with fragile X syndrome is $957,000.
NURSING ASSESSMENT
1. Accurate assessment of the family’s cognitive development and disease conditions is imperative.
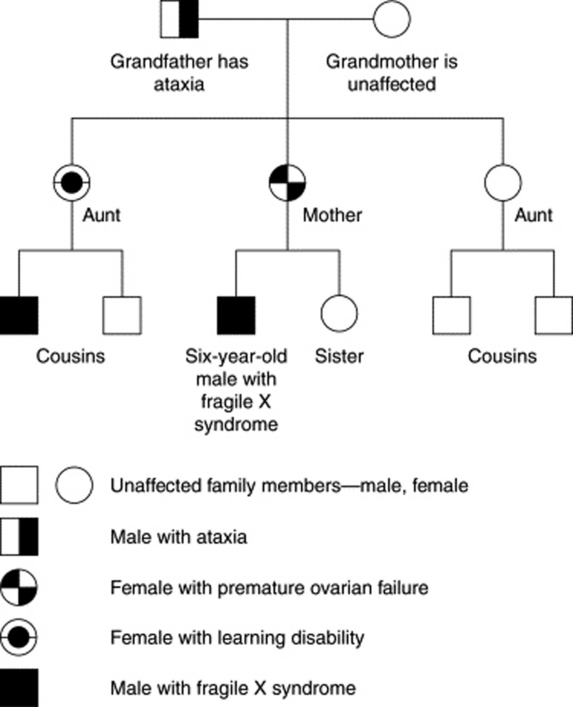
2. Construct a four-generation pedigree to elucidate suspicious diseases (Figure 27-1).
3. Ask focused questions about parents, children, siblings, aunts, uncles, and grandparents (Box 27-2).
4. If answers to questions raise suspicion for fragile X–related disorders, refer to genetic specialist for risk assessment (Box 27-3) (cost of antenatal screening per carrier detected is estimated to be $46,400).
5. Assess for ethical, legal, and social implications (ELSI).
Box 27-2 Focused Family Nursing Assessment
Questions for Pregnant Couples, Parents, and Adults Who Are Concerned about Fragile X–Related Disorders
1. Did you ever receive special education services?
2. Did any of your family members receive special education services?
3. Is this family member still menstruating?
4. Does this family member suffer from tremors, rigidity, slow movements, or balance problems?
Box 27-3 Risk Assessment: Patients Who May Benefit from Genetic Counseling and/or Testing
A Family/Medical History Risk Assessment
a. Patients of either sex with intellectual disability, developmental delay, or autism
b. Family history of fragile X syndrome
c. Patient or family member has undiagnosed intellectual disability or tremor/ataxia syndrome
d. Individuals seeking reproductive counseling who have a family history of intellectual disability
e. Fetuses of known carrier mothers
f. Women under forty who have elevated follicle-stimulating hormone (FSH) levels
B Genetic Patterns of Inheritance Risk Assessment
E Risk Assessment for Family Premutation
a. Male who is a premutation carrier—all his daughters will be premutation carriers.
b. Male who is a premutation carrier—None of his sons inherit the premutation because they inherit his Y chromosome.
Box 27-4 Nondiscrimination Genetics Laws
1. Americans with Disabilities Act of 1990—protects individuals with genetic disabilities.
2. Health Insurance Portability and Accountability Act of 1996 (HIPPA)—applies to employer-based group health insurance. It prohibits employers from using genetics information to exclude anyone or charge more for the same services; a genetic abnormality without illness is not a preexisting condition.
3. HIPPA National Standards to Protect Patients’ Personal Medical Records (December 2002)—Improper use or disclosure of health information has criminal and civil penalties.
Visit www.hhs.gov/ocr/hipaa/ for more information.
NURSING DIAGNOSES
• Knowledge of fragile X–related disorders, Readiness for enhanced
• Conflict, decisional, secondary to proceeding with DNA testing for fragile X mutation
• Conflict, decisional, secondary to sharing testing results with other family members
• Conflict, decisional, secondary to terminating pregnancy
• Family processes, Interrupted
• Self-care deficit, bathing/hygiene
• Self-care deficit, dressing/grooming
NURSING INTERVENTIONS
1. Instruct family and reinforce information on knowledge of fragile X–related disorders.
2. Answer family concerns regarding the decision to have DNA testing for fragile X mutation.
3. Address family concerns regarding the decision to share testing results with other family members.
4. Address parental concerns about the decision to terminate a fragile X–affected fetus.
Discharge Planning and Home Care
1. Refer family to community resources for support and assistance.
2. Promote the infant’s, child’s, or youth’s development (see Chapter 44).
3. Serve as health care resource and/or consultant to child’s disability service coordinator and/or care manager.
CLIENT OUTCOMES
1. Family will be educated about fragile X–related disorders.
2. Family will seek genetic counseling and, if appropriate, genetic testing.
3. Family will attend family counseling therapy.
4. Family will understand inheritance patterns of fragile X–related disorders.
5. Family will be adept in accessing community resources.
6. Family will be adept and adjust to the long-term management needs of child.
7. Child or youth will acquire developmental competencies appropriate for age and level of cognitive and adaptive functioning.
8. Child or youth will demonstrate autonomy, self-determination, and self-advocacy behaviors.
9. Youth will graduate from high school or obtain high school certificate.
10. Youth will have acquired school-based work experience as precursor for adult employment.
11. Child or youth will have participated in inclusive school and community-based activities.
12. Child or youth will have age-appropriate social relationships.
Biancalana V, et al. FMR1 premutations associated with fragile X-associated atrophy. Arch Neurol. 2005;62(6):962.
Ensenauer RE, Michels VV, Reinke SS. Genetic testing: Practical, ethical, and counseling consideration. Mayo Clin Proc. 2005;80(1):63–73.
Hagerman RJ. Fragile X syndrome. Jackson P, Vessey J. Primary care of the child with a chronic health condition, ed 4, St. Louis: Mosby, 2004. editors
[Human Genome Project Information: Ethical, legal and social issues (website):] www.ornl.gov/sci/techresources/Human_Genome/elsi/legistat.shtml, January 16 2006. Accessed
[National Human Genome Research Institute, National Institutes of Health: Genetic discrimination in health insurance (website):] www.genome.gov/10002328, January 16, 2006. Accessed
Sherman R, Pletcher BA, Driscoll DA. Fragile X syndrome: Diagnostic and carrier testing. Genet Med. 2005;7(8):584.