Chapter 50 Fracture Healing: How Strong Is the Effect of Smoking on Bone Healing?
The negative sequelae of cigarette smoking on the musculoskeletal system and wound healing have become a popular topic of study.1–4 A variety of specialties and institutions (especially those responsible for healthcare costs) have focused on smoking as a potentially remediable risk factor for negative outcome after a variety of medical interventions. For example, epidemiologic and experimental studies in the plastic surgery literature identify nicotine as a deleterious constituent of cigarette smoke, leading to increased rates of wound complications and pedicle flap necrosis secondary to vasoconstriction and microthrombus formation.5–7
For years, orthopedic experts have anecdotally described the negative impact of cigarette smoking on the musculoskeletal system. More recently, this effect has been the subject of a number of reports: cigarette smoking leads to an increased incidence of pseudarthrosis after spinal fusion,8,9 increased time to union in tibial fractures10–13 lower bone mineral density,3 and poor outcome after treatment of chronic osteomyelitis.11,14 In addition, clinical and experimental evidence suggests that cigarette smoking leads to decreased mineralization and mechanical strength of regenerate bone during tibial lengthening.14–17
EVIDENCE
Basic Science Research
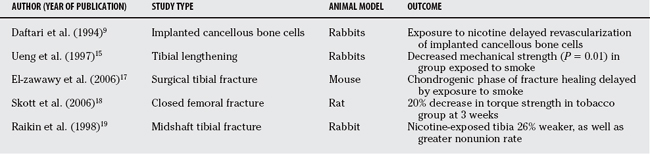
Regenerate bone is vulnerable to the vasoconstrictive and hypoxic effects of cigarette smoke and nicotine.14–17 In a model of tibial lengthening in rabbits, Ueng and colleagues15 demonstrate decreased production, maturation, and torsional strength of regenerate bone formation in subjects exposed to cigarette smoke. The same group demonstrated improved mechanical strength of regenerate bone in animals treated with hyperbaric oxygen, suggesting that local tissue oxygen tissue tension is of paramount importance.16 El-zawawy and coworkers17 show that the chondrogenic phase of tibial fracture healing in a mouse model was delayed by exposure to nicotine. Similarly, Skott and coauthors18 studied the effect of tobacco extract on the healing of a closed femoral fracture model in the rat. They found that the rats given tobacco extract had a 20% to 26% decrease in the mechanical strength of the femur 21 days after fracture. In a rabbit model, Raikin and colleagues19 conducted a study examining the effect of administration of nicotine on the mechanical strength of midshaft tibial osteotomies. They found a nearly identical 26% decrease in mechanical strength in the nicotine group with a corresponding decrease in callous size. In summary, abundant basic science evidence illustrates the negative effects of smoking in general and nicotine in particular on fracture healing (Table 50-1).
Clinical Research
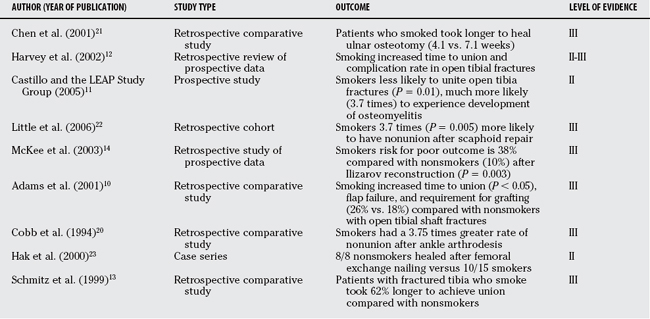
Numerous clinical studies describe the effects of smoking on various aspects of fracture healing, regenerate bone formation, spinal fusion, arthrodeses, or osteotomy healing.3,5,10–14,20, 21 Because of the nature of the condition, it is not possible to randomize patients into smoking and nonsmoking groups; therefore, most reports are Level III retrospective reviews, although there are some studies with prospectively gathered information in a comparative study (Level II).11,12, 14 However, even though it is of relatively low quality, the clinical information that is available is overwhelming in its description of the negative effects of smoking on bone healing (Table 50-2).
In the upper extremity, Chen and colleagues21 describe a 30% nonunion rate with a mean time to healing of 7.1 months in smokers versus a 0% nonunion rate and a mean healing time of 4.1 months in nonsmokers after ulnar shortening osteotomy. Similarly, in a series of 64 patients, Little and colleagues22 found that smokers were 3.7 times more likely (P = 0.005) than nonsmokers to have failure of their scaphoid nonunion repair.
In the lower extremity, Hak and coauthors23 found that all 8 smokers had a healed femoral nonunion after exchange reamed femoral nailing versus only 10 of 15 smokers. Folk, Starr, and Early5 found that smoking was a significant negative risk factor for wound complications in a series of 190 calcaneal fractures treated with surgery (relative risk, 1.2; P = 0.03). McKee and colleagues,14 in a series of 84 adult patients treated with Ilizarov reconstruction, found that smokers had a 38% chance of a poor outcome versus 10% in nonsmokers (P = 0.003). Significantly, all five amputations in their series were in smokers.
Perhaps the most closely studied aspect of orthopedic trauma with respect to smoking is that of the fracture that continues to plague orthopedic surgeons with poor outcomes, nonunion, infection, and limb loss: the open tibia fracture. In retrospective reviews, Harvey and investigators,12 Schmitz and researchers,13 and Adams, Keating, and Court-Brown10 all describe consistently longer times to union, greater complication rates, and greater rates of secondary intervention in smokers. In a prospective (Level II) report, the LEAP (Lower Extremity Assessment Project) Study group found that both current and former smokers had increased rates of nonunion (current smokers: 37%; P = 0.01; former smokers: 32%; P = 0.04) and osteomyelitis (current smokers: relative risk, 3.7; P = 0.01; former smokers: relative risk, 2.8; P = 0.07) compared with patients who had never smoked.10–14
AREAS OF UNCERTAINTY
Deleterious Physiologic Effects of Smoking as It Relates to Bone Healing
Basic science research shows that nicotine has a vasoconstrictive effect that inhibits tissue differentiation and the angiogenic response necessary in the early stages of fracture healing.9 Chronically, this (reversible) effect can lead to accelerated atherosclerosis, arterial narrowing, platelet thrombi, and irreversible ischemic changes. At the cellular level, nicotine (among other components of cigarette smoke) is a toxin that also interferes with osteoblast function and alters skeletal metabolism.17–19 Nicotine has also been shown to have an immunosuppressive effect, which may explain the greater rates of infection and delayed union described in some series.4,11, 14 Used even in isolation, orally or parenterally, it still demonstrates this effect.18,19 What is unclear is the point at which reversible changes become irreversible, the effects of one type of tobacco product versus another, and why there appears to be considerable interpatient variability.
Definition of “Smoker”
The definition of “smoker” in the majority of available studies remains problematic.1,2 Because most studies of this nature are retrospective, there is rarely an accurate history of the type of smoking material or the amount smoked. For most studies, a smoker is typically defined as a patient who was actively smoking cigarettes during the treatment period. Conversely, nonsmokers are defined as patients who were not actively smoking during the study period.10–14 The deficiencies of such a definition are obvious: A person who smokes one or two cigarettes a day is not equivalent to one who smokes two or three packs a day, yet both are included as “smokers.” Also, the inclusion of patients who use other tobacco products such as pipes, cigars, or chewing tobacco is not consistent: Because they are usually excluded from the “smoking” group, their inclusion as “nonsmokers” is problematic. As a result of poor quality retrospective data, no correlation can be established between quantity and duration of smoking and treatment outcomes; it may well be that such a relationship exists.
Smoking Cessation
Although there are some general data in the medical literature regarding when the medical risks associated with smoking return to a nonsmoking baseline level after cessation of smoking, there is little such information with regards to fracture healing.11 Thus, the effect on bone healing in a heavy smoker who quits smoking 6 months before being included in a study is unknown. As stated, most reports include only patients actively smoking as “smokers,” and this distinction may be artificial. Although active smoking is probably the least favorable environment, the chronic negative effects of long-standing smoking (i.e., vascular occlusion and ischemia) linger long past the point of smoking cessation. One prospective study divided patients with open tibial fractures into three groups: active smokers, former smokers, and nonsmokers. They found that former smokers had risks of delayed union and infection greater than nonsmokers but less than current smokers. This is the best evidence available that smoking cessation can decrease risk, although not to that of nonsmoking control subjects.11
A number of effective cessation programs are available including nicotine replacement therapy, psychotropic medications, education and motivational counseling, and skills training.24,25 The reported smoking cessation rates after intervention vary from 20% to 60% at 1 year, with increased success rates in programs utilizing a focus on immediate complications. Moller and colleagues,26 in a randomized clinical trial, described a prearthroplasty smoking cessation program and were able to get the majority of patients in the treatment group to stop or decrease their cigarette consumption. They also showed a significantly decreased postoperative complication rate compared with control subjects (18% vs. 52%; P = 0.001). Thus, the setting of an acute fracture may be an ideal opportunity for positive intervention in a patient’s life with a greater long-term significance than simple fracture union. Ideally, in the elective setting (i.e., before osteotomy or arthrodesis), smoking should be considered a medical condition with a significant negative effect on outcome (not unlike diabetes) that can and should be “optimized” before surgery. It is rare for an orthopedic surgeon to have the time or skill for this intervention; it requires a multidisciplinary approach that typically involves the patient’s primary care physician.
Confounding Variables
It can be stated with some certainty that smokers represent a unique group in society, in whom other confounding variables usually exist. For example, a prospective study at our institution found that 45% of patients admitted with an open tibial fracture were smokers, compared with 19% of the general Canadian population.1 Similarly, a study on the effects of smoking on Ilizarov reconstruction revealed that alcoholism, drug addiction, and narcotic dependency were seen almost exclusively in the smoking group.14 Therefore, even though a study may purport to compare “smokers” versus “nonsmokers,” there are other variables (alcohol consumption, risk-taking behavior, drug dependency) that may be overrepresented in the “smoker” group that can confound results.
In summary, grade B evidence exists that smoking has a consistent deleterious effect on bone healing (including fracture, osteotomy, and arthrodesis union rates and regenerate bone formation). Grade A evidence exists that smoking cessation programs have a success rate in the 50% range, and that success increases if there is a focus on an acute medical event (i.e., fracture or operation). Emerging prospective evidence reports that smoking cessation may lower complication rates after orthopedic procedures. Table 50-3 provides a summary of recommendations.
| RECOMMENDATIONS | LEVEL OF EVIDENCE/GRADE OF RECOMMENDATION |
|---|---|
1 Statistics Canada. Smoker’s Report. Ottawa, Canada: Government of Canada Publication, 1999.
2 Gilpin EA, Pierce JP, Cavin S, et al. Estimates of population smoking prevalence: Self vs proxy reports of smoking status. Am J Public Health. 1994;89:1576-1579.
3 Porter SE, Hanley ENJr. The musculoskeletal effects of smoking. J Am Acad Orthop Surg. 2001;9:9-17.
4 Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372-377.
5 Folk JW, Starr AJ, Early JS. Early wound complications of operative treatment of calcaneal fractures: analysis of 190 fractures. J Orthop Trauma. 1999;13:369-374.
6 Kaufman T, Eichenlaub EH, Levin M, et al. Tobacco smoking: Impairment of experimental flap survival. Ann Plast Surg. 1984;13:468-472.
7 Nolan J, Jenkins RA, Schultz RC, et al. The acute effects of cigarette smoke exposure on experimental skin flaps. Plast Reconstr Surg. 1985;75:544-549.
8 Glassman SD, Anagnost SC, Parker A, et al. The effect of cigarette smoking and smoking cessation on spinal fusion. Spine. 2000;25:2608-2615.
9 Daftari TK, Whitesides TE, Hellen JG, et al. Nicotine on the revascularization of bone graft: An experimental study in rabbits. Spine. 1994;19:904-911.
10 Adams CI, Keating JF, Court-Brown CM. Cigarette smoking and open tibial fractures. Injury. 2001;32:61-65.
11 Castillo RC, Bosse MJ, MacKenzie Ej, Patterson BM, LEAP Study Group. Impact of smoking on fracture healing and risk of complications in limb-threatening open tibia fractures. J Orthop Trauma. 2005;19:151-157.
12 Harvey EJ, Agel J, Selznick HS, et al. Deleterious effect of smoking on healing of open tibia-shaft fractures. Am J Orthop. 2002;31:518-521.
13 Schmitz M, Finnegan M, Champine J. The effect of smoking on the clinical healing of tibial shaft fracture healing. Clin Orthop Relat Res. 1999;365:184-200.
14 McKee MD, Dipasquale D, Wild LM, et al. The effect of smoking on clinical outcome and complication rates following Ilizarov reconstruction. J Orthop Trauma. 2003;17:663-667.
15 Ueng SWN, Lee M, Li AFY, et al. Effect of intermittent cigarette smoke inhalation on tibial lengthening: Experimental study on rabbits. J Trauma Inj Infect Crit Care. 1997;42:231-238.
16 Ueng SWN, Lin SS, Wang CR, et al. Bone healing of tibial lengthening is delayed by cigarette smoking: Study of bone mineral density and torsional strength on rabbits. J Trauma. 1999;46:110-115.
17 El-zawawy HB, Gill CS, Wright RW, Sandell LJ. Smoking delays chondrogenesis in a mouse model of closed tibial fracture healing. J Orthop Res. 2006;249:2150-2158.
18 Skott M, Andreassen TT, Ulrich-Vinther M, et al. Tobacco extract but not nicotine impairs the mechanical strength of fracture healing in rats. J Orthop Trauma. 2006;24:1472-1479.
19 Raikin SM, Landsman JC, Alexander VA, et al. Effect of nicotine on the rate and strength of long bone fracture healing. Clin Orthop Relat Res.; 353; 1998; 231-237.
20 Cobb TK, Gabrielsen TA, Campbell DC, et al. Cigarette smoking and nonunion after ankle arthrodesis. Foot Ankle. 1994;15:64-67.
21 Chen F, Osterman AL, Mahony K. Smoking and bony union after ulnar-shortening osteotomy. Am J Orthop. 2001;30:486-489.
22 Little CP, Burston BJ, Hopkinson-Woolley J, Burge P. Failure of surgery for scaphoid non-union is associated with smoking. J Hand Surg [B]. 2006;3193:252-255.
23 Hak DJ, Lee SS, Goulet JA. Success of exchange reamed intramedullary nailing for femoral shaft nonunion or delayed union. J Orthop Trauma. 2000;14:178-182.
24 Cinciripini PM, Lapitsky L, Seay S, et al. A placebo controlled evaluation of the effects of busipirone on smoking cessation. J Clin Psychopharmacol. 1995;15:182-191.
25 Fiore MC, Smith SS, Jorenby DE, et al. The effectiveness of the nicotine patch for smoking cessation. J Am Med Assoc. 1995;74:1347-1352.
26 Moller AM, Villebro N, Pedersen T, Tonnesen H. Effect of preoperative smoking intervention on postoperative complications: A randomized clinical trial. Lancet. 2002;359:114-117.