CHAPTER 250 Fractionated Radiation Therapy for Malignant Brain Tumors
 Radiobiology
Radiobiology
All malignant neoplasms can potentially be eradicated with suitably high radiation doses. However, because the side effects from unrestricted delivery of radiation to large volumes of normal tissue would be catastrophic, a balance must be struck between treatment efficacy and toxicity, the so-called therapeutic window. Radiation doses are therefore restricted by the tolerance of surrounding tissues, including functional subunits, supporting cytoarchitecture, and vasculature. Radiation tolerance, in turn, is a function of multiple factors, including total dose, dose per fraction, frequency of administration, volume of tissue irradiated, anatomic site, tissue type, comorbid conditions such as hypertension and diabetes, preexisting functional deficits, and the underlying host genetic milieu. External beam RT is delivered through essentially two methods: fractionated external beam RT and stereotactic radiosurgery (SRS). SRS is discussed in detail elsewhere but seeks to limit radiation-related complications by precisely treating a finite volume of disease, usually rather small, with a single treatment. The “radioablative” effect is a combination of tumor cell killing and vascular obliteration engendered by the single high dose of radiation. Fractionation of the radiation dose, in contrast, provides a means of augmenting the dose while attempting to limit detrimental effects on adjacent normal tissue by taking advantage of inherent repair differences between normal and neoplastic tissue.1 Normal tissue exhibits improved repair of sublethal damage, thereby allowing increased cell survival between radiation fractions, which is not the case with most malignancies. The fractionated approach also allows potential reoxygenation of hypoxic regions of the tumor between fractions, thus providing improved radiation efficacy in future treatments through oxygen fixation of radiation damage. Another advantage demonstrated in in vitro systems is that the various phases of the cell cycle express differential radiosensitivity, and fractionated radiation prevents malignant cells from being treated only in a radioresistant portion of the cell cycle, as can happen with single-fraction treatments such as SRS. In the treatment of malignant central nervous system (CNS) entities, typical fraction sizes are between 1.8 and 2.0 Gy daily. A possible disadvantage of fractionation is reduced impact on the vasculature.
Radiation Delivery: Technical Issues
Various advances in radiation technique have improved the therapeutic ratio for fractionated RT by diminishing the volume of normal brain receiving significant radiation doses in patients with primary CNS neoplasms in whom partial brain irradiation is desired. The margin required for daily setup variability has been decreased by patient immobilization devices, such as a molded Aquaplast face mask; such devices enhance interfraction reliability in patient position and constrain intrafraction motion. Improvements in imaging with the advent and institution of cross-sectional imaging have allowed more precise delineation of targets, although a significant margin, particularly with gliomas, remains necessary to account for extension of subclinical disease.2 Most commonly, planning for management of CNS neoplasms is accomplished with the fused product of high-resolution diagnostic magnetic resonance imaging (MRI) and computed tomography (CT) performed in the treatment position (Fig. 250-E1). Further accuracy in localization of treatment is offered by image-guided RT, which permits pretreatment visualization of patient anatomy and tumor location, along with the ability to register this information with baseline readings. This approach allows daily adjustments to ensure accurate delivery of radiation and decreases the target volume by minimizing the range of setup error. Although frequently unnecessary in the treatment of CNS neoplasms, given the relatively static location of intracranial structures when the head is constrained by immobilization devices, daily image-guided RT using biplanar orthogonal x-ray imaging systems, cone beam CT, or megavoltage CT further improves positional reproducibility and permits decreased margins.
Three-Dimensional Planning and Treatment
As alluded to earlier, CT-based planning has catalyzed the evolution of three-dimensional conformal radiation therapy (3DCRT) in which non-coplanar treatment fields with unique entrance and exit pathways can be directed onto a patient-specific anatomic target. This allows the desired dose to be displayed in a volumetric distribution with modern treatment planning software. Avoidance of critical structures proximate to the treatment volume, such as the brainstem, optic apparatus, and spinal cord, can subsequently be enhanced by the precise delineation of volumetric distribution allowed by such planning. Early research confirmed the benefit of three-dimensional treatment planning in terms of the ability to reduce the volume of brain receiving full-dose treatment (95% isodose line) by 30% with nonaxial techniques when compared with conventional parallel opposed orientations.3 Intensity-modulated radiation therapy (IMRT) is a subset of 3DCRT that provides improved conformality through nonuniform delivery of doses by modulating the photon flux of specific beams during treatment. This approach results in highly shaped radiation dose distributions and is especially relevant when it is necessary to produce concave or convex distributions, which is of significance when tumors are in close proximity to the optic apparatus, vestibulocochlear structures, hypothalamic-pituitary axis, hippocampus, brainstem, and other structures. IMRT has generally been restricted in CNS applications because of the adequacy of 3DCRT plans in most situations. In addition, the tight conformality of doses within the high-dose regions provided by IMRT is typically accomplished through the delivery of lower doses of radiation to larger areas. Long-term data on the effect of normal regions of CNS receiving low doses of radiation are inadequate, although some dosimetric evaluations have countered this concern.4
Conventional External Beam Radiotherapy
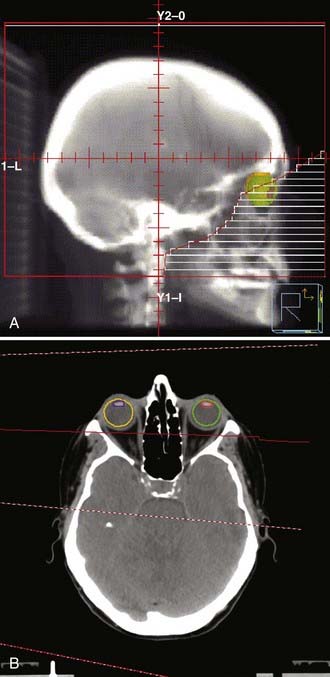
WBRT is most commonly delivered via parallel opposed lateral fields and 4- to 10-MV photons with an Aquaplast face mask or other custom immobilization (Fig. 250-E2A). Treatment simulations can be performed clinically on a kilovoltage x-ray machine or by CT with digitally reconstructed radiographic projections. To account for day-to-day intratreatment and intertreatment variability in setup and dosimetric factors such as the penumbra (a region of dose falloff at the margin of the radiation field where “cold spots” can occur), a margin of at least 1 cm is advocated. Bony landmarks for the intracranial contents include the calvaria, cribriform plate, and bases of the middle and posterior cranial fossae. Ideally, the lenses are spared from the radiation through appropriate field blocking and beam, couch, and collimator angling; this detail is important because delayed cataract formation can easily occur, even with very modest radiation delivered to the lens (Fig. 250-E2B). WBRT fields cover the brain to the bottom of the C1 vertebra, although additional extension to C2 is designed by convention for small cell lung cancer (SCLC), PCL, or overt disease in the posterior fossa. In addition, the WBRT field for CNS lymphoma, described as a German helmet, is extended anteriorly by marking the fleshy canthi in an attempt to cover the posterior half of the globe given the tendency of CNS lymphoma to involve the orbits. Inadequate attention to bony anatomic landmarks and appropriate margins as defined earlier can lead to regional underdosing, and isolated relapses within the inferior frontal lobes or posterior fossa have been reported. Cataract development and injury to the lacrimal gland appear to be minimized by appropriate design.
Fractionated Stereotactic Radiotherapy
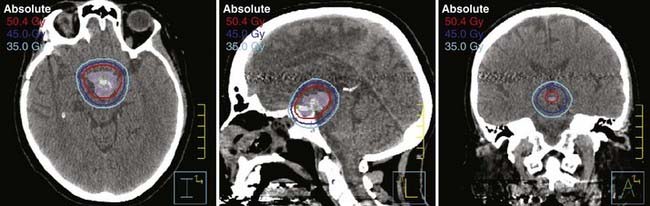
Fractionated stereotactic radiotherapy (FSRT) is a hybrid technique that incorporates the precision of radiosurgery and the improved therapeutic ratio of conventional, fractionated RT. FSRT commonly uses multiple non-coplanar fixed fields and precision planning facilitated by improved patient tracking or immobilization. The advent of robotic devices and mini-multileaf collimators permits very precise and conformal dose delivery with this method, also similar to that offered by SRS (Fig. 250-E3). An additional advantage in the cranium, because of its spheroidal geometry, is access to numerous beam entry points, thereby further improving dose conformality. Various FSRT systems with reported precision between 1 and 3 mm have been developed.5,6 FSRT is used for lesions requiring high precision that cannot be appropriately treated with SRS. Common disqualifications for SRS include a maximum dimension greater than 4.0 cm and location in a critical anatomic region that is radiation sensitive and cannot be avoided by the high-dose region of the SRS dose distribution.
Charged particle radiation is a specific type of external beam radiation that delivers charged particles (most commonly protons) rather than photons. Charged particles have the inherent advantage of deposition of the majority of the dose at a depth that depends on the initial energy, thus avoiding the typical exit dose present in photon therapy. Avoidance of the exit dose can theoretically decrease the integral radiation dose significantly. The region of maximum dose deposition is known as the Bragg peak. Narrow Bragg peaks can be achieved with pencil scanning proton beams, but coverage of larger volumes typically requires the proton beams to be modified by passive range modulators to disperse the Bragg peak and broaden deposition of the dose. Although proton beam therapy can be used in any CNS location, it has been applied more commonly for tumors of the skull base, such as chordomas and chondrosarcomas; these tumors require high doses for durable local control and are surrounded by essential radiosensitive structures, which limits dose escalation when using non-IMRT photon fields.7,8 Proton beam therapy has also been explicitly advocated for the treatment of childhood tumors because of the decreased integral radiation dose.9 There are in fact several limitations with this modality. The number of proton therapy facilities worldwide has been limited because of the immense expense of construction and operation, usually 10- to 100-fold greater than the cost for a conventional facility. Proton therapy facilities are, however, anticipated to become somewhat more common, with several facilities recently being commissioned or in the planning or construction phases. The vast majority of these facilities are built with older cyclotron-based technologies, the inherent limitations of which include significant expense, space needs, technology support, neutron contamination (with the associated concern of increased second malignancies),10 and lack of intensity modulation, which implies that the overall dosimetric advantages over sophisticated IMRT plans are indeed limited.11 Several modern technologic advances will make it possible to eliminate the neutron contamination, miniaturize the equipment so that it can fit in standard hospital facilities, reduce cost by fivefold or greater, and incorporate proton intensity modulation, which will provide genuine dosimetric advantages. There are clearly concerns that as these facilities proliferate, overuse might become commonplace.12 Overuse is especially worrisome because no level 1 evidence has demonstrated the superiority of protons over photons in any clinical setting.
Brain Metastases
The exact incidence of brain metastases remains unknown because brain metastasis as an event is not coded in cancer databases. Estimated rates depend on whether the incidence is calculated from autopsy data, clinical studies, tumor registries, hospital records, or other sources.13 If the rate of brain metastases is calculated from autopsy data, the annual estimated incidence in the United States is as high as 170,000 cases per year.14 Of the patients in whom brain metastases develop, the vast majority die within a few months of diagnosis, thus making brain metastasis one of the most common immediate causes of death in the United States. Lung cancer accounts for approximately half of all secondary tumors to the brain; other primary tumors commonly causing brain metastases include breast cancer and melanoma. Less commonly, primary tumors of the gastrointestinal tract and genitourinary system, lymphomas, sarcomas, and prostate cancer also metastasize to the brain. In terms of disease-specific risk, melanoma has the greatest likelihood of metastasizing to the brain. The overall incidence of brain metastases is probably increasing because of the combination of better diagnostic techniques and small gains in systemic therapy. Improved systemic therapeutic options have altered the conventional disease course such that patients with primary cancers live longer. With longer survival, asymptomatic micrometastatic disease in the brain is more likely to become overt, thereby inflating the incidence of the problem. Furthermore, the brain has traditionally been thought to represent a sanctuary site by not permitting penetration of most cytotoxic chemotherapeutic agents when the blood-brain barrier is intact. Effective eradication of systemic micrometastatic disease therefore introduces the possibility of having brain-only metastatic disease remaining, which is then expressed clinically over time.
Older data from the 1970s suggest that the median survival time of untreated patients with brain metastases is approximately 1 month.15,16 The principal cause of this rapid mortality is uncontrolled peritumoral vasogenic edema leading to herniation and rapid death. As a consequence, measures to decrease vasogenic edema are commonly used in the initial management of patients with metastatic brain tumors, and they provide relief of symptoms in the majority of patients.16 In particular, glucocorticoids have been shown to control vasogenic edema and result in minimal prolongation of median survival to approximately 2 months.15,17
Fractionation Trials
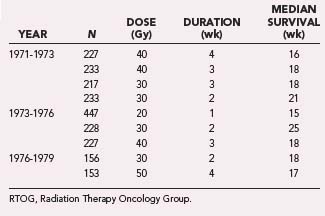
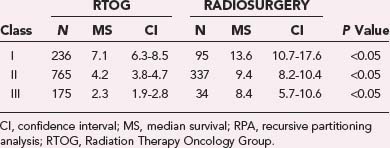
Chao and colleagues first reported the value of external beam RT for brain metastases in 1954.18 Subsequently, the Radiation Therapy Oncology Group (RTOG) conducted a series of studies exploring a variety of fractionation schedules and evaluated their impact on outcome in patients with brain metastases. These trials were primarily conducted in the 1970s and focused on identifying the appropriate schedule and dose.19,20 Table 250-1 summarizes the outcome data from the first three RTOG brain metastasis fractionation trials.
Based on more than 2000 patients enrolled in these three RTOG trials, it was apparent that no specific fractionation schedule was markedly superior. The dose range that was explored was 20 to 50 Gy, with median survival ranging from 15 to 21 weeks. Response rates after WBRT varied, but complete or partial responses were documented in approximately 60% of patients, with the caveat that the imaging was suboptimal by modern standards and the response rates are most likely overestimated.21 The best median survival was seen in a group of 233 patients treated with 30-Gy WBRT delivered over a period of 2 weeks. This cohort of patients exhibited a median survival of 21 weeks. As a consequence, this schedule has become the most commonly used fractionation scheme for patients with brain metastases treated in the United States over the past 2 decades.22 Although hypofractionated schedules are infrequently used in the United States, WBRT delivered over a single week (20 Gy in five fractions) is not uncommon in Europe.23
Dose-Response Relationship
There appears to be a dose-response relationship between radiation dose and local control. This issue is of critical significance in that in the older RTOG studies 50% or more of patients died of neurological deterioration/progression, and it would be logical to assume that if such deterioration could be controlled, survival may be enhanced or at least the quality of survivorship might be improved. To test this hypothesis, the RTOG conducted study 8528 to evaluate the role of dose escalation for solitary brain metastases via accelerated hyperfractionation (1.6 Gy twice daily up to 32 Gy to the whole brain followed by a tumor boost to total doses ranging from 48 to 70.4 Gy). This trial demonstrated a significant advantage in survival and neurological improvement with higher doses, thus suggesting that intracranial disease control is related to dose and that such control actually translates into neurological improvement and a survival advantage.24 However, the confirmatory trial, RTOG 9104, failed to validate this observation.25
The relationship between local control and total dose has been further inferred by case-control analysis. Nieder and colleagues treated a group of 164 patients with the standard WBRT regimen of 30 Gy in 10 fractions, monitored all patients by serial CT imaging, and correlated local control and survival with various prognostic factors.26 Subsequently, 39 patients were treated to a total dose of 40 to 60 Gy and compared with the cohort of 164 patients treated with a total dose of 30 Gy by using the matched-cohort analysis method. The matching procedure produced equivalent groups of patients and showed a significant dose effect, with 30 Gy resulting in a local response rate of 50% versus 77% for doses in the 40- to 60-Gy range. In this analysis, although local control improved from 50% to 77% by escalating the radiation dose (P = .05), survival was not significantly altered. The lack of a distinct survival advantage demonstrated the competing causes of mortality in patients with brain metastases and confirmed that local control will improve survival only in patients who are not experiencing simultaneous systemic failure. In a subsequent analysis from the same group, the CT scans of 322 patients were analyzed to specifically evaluate the impact of dose (in the 25- to 50-Gy range) on local control. The total dose was recalculated with the linear quadratic model and expressed as the biologically effective dose for tumor (BED10). BED10 values ranged from 37.5 to 72 Gy, and the best local control was found to be a function of a higher BED, the number of brain metastases, and the histology of the primary tumor. An increasing BED resulted in a significant decline in 1-year failure rates, with low BED values resulting in failure rates of 44% as opposed to 31% for high BED values. Once again, overall survival was not dependent on the total dose.27 In a large analysis, Lagerwaard and coworkers identified prognostic factors in a group of almost 1300 patients treated for brain metastases.28 In this multivariate analysis the single most important independent prognostic factor predicting improved survival was treatment modality, with more aggressive therapy having the potential to improve survival.
How does one interpret these conflicting data? It is quite clear that a significant proportion of patients with brain metastases succumb to systemic disease, and therefore enhancing control of intracranial diseases is unlikely to provide a survival benefit to this group of patients. In clinical trials, whether prospective or retrospective, in which a significant majority of patients harbor considerable systemic disease that will dictate the outcome, improvement in survival from more aggressive intracranial local control is unlikely to be demonstrated. However, in clinical situations in which patient selection identifies individuals who are less likely to rapidly succumb to systemic progression and patients are at risk for dying of intracranial disease, local control of brain metastases becomes critical. This has best been demonstrated in surgical trials comparing resection and WBRT with WBRT alone. Two such randomized trials by Vecht and associates29,30 and Patchell and colleagues31 have validated this paradigm by showing improved survival in the surgical arms. A third study, by Mintz and coworkers, did not find an improvement in overall survival when surgery was added to WBRT.32 A slightly higher percentage of patients had disseminated systemic disease at the time of enrollment than in the previous two studies, again emphasizing the difficulty of teasing out survival benefit from improved local control of CNS metastases when other risk factors for disease-specific mortality are in play.
Prognostic Factor Analysis
The role of WBRT and, more specifically, escalated delivery of radiation to brain metastases has at times been obscured by the difficulty in identifying patients most likely to succumb to their intracranial disease versus systemic progression. Among the heterogeneous population of patients with brain metastases, those with limited life expectancy because of a heavy systemic burden of disease would obviously derive less benefit from aggressive intervention than would those with prolonged anticipated overall survival. Some of the seminal work in this area of subgroup stratification was done by the RTOG, which identified a set of prognostic criteria predictive of survival. Such criteria included age younger than 60 years, a Karnofsky Performance Scale (KPS) score higher than 70, controlled primary tumor, absence of extracranial metastases, and three or fewer lesions.19,33,34 In a seminal analysis, 1200 patients from three consecutive RTOG trials conducted from 1979 to 1993 were amalgamated into a single database, and a statistical methodology known as recursive partitioning analysis (RPA) was used to identify subsets of patients based on evaluation of prognostic factors. This analysis suggested that patients with brain metastases can be broadly categorized into three classes with different outcomes (Table 250-2). The most favorable group, class I, includes patients with a KPS score of 70 or higher, age younger than 65 years, controlled primary, and absence of extracranial metastases. These patients have a median survival of 7.1 months. Patients in the worst prognostic group, class III, were characterized by a KPS score of less than 70, and their median survival was 2.3 months. The intermediate group, described as class II, included all patients not specifically within class I or III and had a median survival of 4.2 months.35 The discrepancy in anticipated overall survival between RPA classes emphasizes the importance of appropriate patient selection in studies evaluating the impact of aggressive interventions.
Although the RTOG RPA classification has been useful in trial design, additional factors appear to play a role in determining overall survival. Despite not being found to be significant in the RTOG RPA, the number/volume of brain metastases probably has some impact. Patients with four or more brain metastases appear to have a poorer prognosis, but the RPA classification does not categorically identify this factor, in part because the database contained patients imaged with both CT and MRI whereas current practice relies primarily on MRI.36 Similarly, the distinction of single versus multiple brain metastases was not found to be statistically significant in the RTOG RPA system, but analysis of a study by Patchell’s group,37 which evaluated postoperative RT for single, resected brain metastases, showed no difference in overall survival regardless of patient class (I versus II).38 A large, retrospective study of 916 patients treated from 1985 to 2000 within a single institution showed improvement in overall survival in both class I and II when patients with single brain metastases were compared with those with multiple metastases within the same class, again inferring relevance to the number of brain metastases.39 A prognostic score index for radiosurgery in patients with brain metastases (SIR) has also been developed; scores between 0 and 10 are assigned on the basis of lesion number and the largest lesion volume, as well as age, KPS score, and extent of extracranial disease.40 This index has been validated in another smaller patient cohort.41 Finally, primary tumor type probably also has some role in predicting survival.
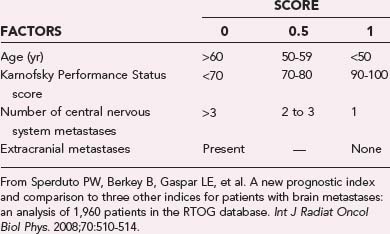
Most recently, a new prognostic index described as the Grade Prognostic Index (GPA) was derived from an RTOG database of 1960 patients with brain metastases, including the recently reported RTOG 9508 (Table 250-3).42 The four components included as part of the GPA are age, KPS score, extracranial metastases (none and present), and number of metastases (one, two to three, more than three). Each category receives a score of 0, 0.5, or 1, with a cumulative range between 0 and 4. This index overcomes some of the drawbacks of the RPA by incorporating the number of metastases and removing the frequently ambiguous descriptor of a controlled primary site, which could vary depending on the type, timing, and technique of restaging studies. The GPA does not require that treatment planning information, such as the tumor volume required by SIR, be available, thus permitting its use in guiding the choice of treatment. Based on the GPA, median survival times were 2.6 months for a GPA of 0 to 1, 3.8 months for a GPA of 1.5 to 2.5, 6.9 months for a GPA of 3, and 11.0 months for a GPA of 3.5 to 4.0, it and was strongly significant (P < .0001).
Role of Adjuvant Whole-Brain Radiotherapy
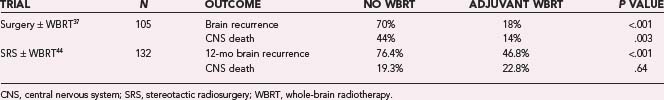
Level 1 evidence has shown an improvement in overall survival with surgery for single metastases31 and SRS for solitary metastases and less than level 1 evidence (subset analysis of a prospective randomized trial) for one to three metastases with age younger than 50 years, non–small cell lung carcinoma (NSCLC) primary, and RPA class I.43 With the emergence and increased use of these local control modalities for brain metastases, the role of adjuvant WBRT has been questioned. WBRT has been used in this clinical situation under the rationale that micrometastatic disease in the brain needs to be controlled. A multi-institutional, prospective, randomized trial evaluated the value of postoperative WBRT in patients managed by surgical resection with or without postoperative WBRT (Table 250-4).37,44 Ninety-five patients with single brain metastases were treated by complete resection as verified by postoperative MRI and subsequently randomized to postoperative WBRT, 50.4 Gy in 28 fractions of 1.8 Gy each, or no postoperative WBRT. Forty-nine patients were assigned to the WBRT group and 46 to the observation group. Recurrence of tumor in the brain was significantly less frequent in the RT group (18%) than in the observation group (70%). Patients in the RT group (14%) were also less likely to die of neurological causes than those in the observation group (44%). There was, however, no significant difference in overall survival between the two groups, even though the study was not powered to detect a survival difference (Table 250-4). The lack of impact by WBRT on overall survival may be partially attributable to the high proportion of patients (61%) in the surgery-alone arm who received WBRT at the time of recurrence.45 Similar results were found when evaluating SRS with or without adjuvant WBRT. Aoyama and coauthors published a prospective phase III trial that randomized 132 patients (mostly lung cancer) with a KPS score of 70 or greater and four or fewer metastases to SRS with or without WBRT.44 No improvement in overall survival was observed; median survival time and the 1-year survival rate were 7.5 months and 38.5% in the group treated with WBRT plus SRS and 8.0 months and 28.4% for SRS alone (P = .42). Parenthetically, this study was also not adequately powered to detect improvement in survival. A significant increase in freedom from new brain metastases was identified in patients receiving WBRT (76.4% versus 46.8%, P < .001). These two studies demonstrate that postoperative WBRT significantly diminishes intracranial relapse and neurological death in well-selected patients with single brain metastases undergoing either SRS or resection without having an impact on overall survival.
Those who argue against the use of adjuvant WBRT cite the lack of overall survival benefit with adjuvant WBRT, the possibility of using WBRT or repeat SRS for recurrence, and potential late neurocognitive side effects from WBRT.21 Although no survival benefit was demonstrated in either the Aoyama or Patchell studies, neither was adequately powered for this end point, and in both, a significant proportion of patients underwent salvage WBRT. A retrospective study from the Mayo Clinic examined patients with single brain metastases treated by surgical excision and further stratified by the extent of resection, the presence of extracranial disease, and the use of WBRT. The 46 patients with complete resection and no systemic disease treated with WBRT were found to have improved overall survival (median, 1.3 years; 2-year survival rate, 41%; 5-year survival rate, 21%) when compared with the 75 patients who did not receive adjuvant WBRT (median, 0.7 year; 2-year survival rate, 19%; 5-year survival rate, 4%).46 In a recent retrospective review, local control was found to be enhanced in patients who underwent surgical excision followed by WBRT and a tumor boost (RR 2.31) or complete surgical resection (RR 3.79).47 This improved local control parlayed into an improvement in overall survival.
 Radiation Sensitizers
Radiation Sensitizers
Because of the persistence of local failure despite escalating doses from 20 to 50 Gy, the RTOG strategy in the 1980s focused on exploring the use of radiation sensitizers to improve outcome in this group of patients. Two clinical trials using significantly different sensitizers, misonidazole, a hypoxic cell sensitizer, and bromodeoxyuridine (BUdR), a halogenated pyrimidine and an S-phase sensitizer, were conducted. In a four-arm trial evaluating misonidazole, no significant survival advantage was noted. Similarly, the BUdR trial failed to show a significant survival advantage.48 Recently, the RTOG evaluated the antiangiogenic agent thalidomide49 and the chronomodulator melatonin50 as putative radiosensitizers in the treatment of brain metastases, and neither showed a survival benefit.
More recently, motexafin gadolinium (MGd), a metalloporphyrin redox modulator that has demonstrated selective tumor localization with potentiation of reactive oxygen species and depletion of thiol-based free radical scavengers, was tested in a randomized fashion as an adjunct to RT. No difference in overall survival (5.2 months for MGd versus 4.9 months for WBRT alone, P = .48) or time to neurological progression (9.5 months for MGd versus 8.3 months for WBRT alone, P = .95) was identified, although patients with NSCLC, who represented greater than 50% of the population, had a significant improvement in time to neurological progression (median, not reached for MGd versus 7.4 months for WBRT; P = .048).51 A follow-up phase III trial randomized 554 patients with NSCLC to WBRT with or without MGd. The primary outcome was neurological progression, and a trend toward improvement was found with MGd (15 versus 10 months, P = .12). The number of patients requiring retreatment of intracranial disease also decreased when MGd was administered (P < .001). In a preplanned partition by geographic region, North American patients, in whom treatment was more prompt, were found to have a statistically significant prolongation of time to neurological progression from 8.8 months for WBRT to 24.2 months for WBRT plus MGd (P = .004; hazard ratio [HR], 0.53).51a
Similarly, a phase III trial evaluating WBRT with or without efaproxiral, a synthetic small molecule that alters the oxygen-hemoglobin dissociation curve and thereby increases capillary PO2 and presumably tumor oxygenation, showed no difference in median survival. In the subgroup with NSCLC or breast cancer, median survival trended toward improvement, 6.0 and 4.4 months, respectively (HR, 0.82; P = .07), thus suggesting a potential survival benefit, with most of the benefit being derived from breast cancer patients.52 A phase III trial examining the potential benefit in women with primary breast cancer, the ENRICH trial, recently closed and the results are pending.53
Side Effects of Whole-Brain Radiotherapy
Modern, appropriately fractionated WBRT is generally well tolerated; acute side effects are typically limited and can include fatigue, hair loss, particularly along the midline and vertex, erythema, and otitis. Impairment of neurocognitive function months to years after WBRT is the most significant potential late side effect of radiation and is the most common reason cited for not using RT in the adjuvant setting. DeAngelis and coauthors reviewed the Memorial Sloan-Kettering Cancer Center’s patients from 1978 to 1985 treated postoperatively with WBRT (79 patients) and no RT (19 patients).54 In 4 (11%) of the WBRT patients who survived longer than 1 year, neurotoxicity with progressive dementia, ataxia, and urinary incontinence presumed to be secondary to the radiation developed. All these patients had received at least part of their radiation course at greater than 3 Gy per fraction, a hypofractionated approach that would not fall under the current standard of care in North America. A similar series involving an overlapping patient cohort from the same institution identified a rate of radiation-induced dementia of 1.7% and 5.1% for those receiving WBRT alone or after surgery, respectively.55 Again, the majority of these patients (75%, 9 of 12) had their WBRT delivered at greater than 3 Gy per fraction.
Prospective data evaluating neurocognitive function, however, have shown a more complex picture, with baseline detriment in neurocognitive function created from a confluence of factors, including the presence of brain metastases, neurosurgical interventions, chemotherapy, and other neurotoxic therapies such as steroids and anticonvulsants.56 In a phase III trial evaluating WBRT with or without MGd, 90.5% of patients exhibited impairment in one or more neurocognitive tests at baseline.57 Test scores of memory, fine motor speed, executive function, and global neurocognitive impairment at baseline correlated with brain tumor volume and were predictive of survival. Neurocognitive deterioration was correlated with poor response to WBRT and disease progression.58 In a correlative study involving SRS with or without WBRT, mini-mental status examination (MMSE) scores correlated with KPS score, tumor volume, extent of tumor edema, and age.59 Control of the brain tumor was concluded to be the most important factor in stabilizing neurocognitive function. In an attempt to isolate the effects of WBRT from the presence and potential progression of macroscopic brain metastases, 96 patients with SCLC treated by prophylactic cranial irradiation (PCI) were evaluated by formal neurocognitive testing.60 Again, a large number of patients (47%) had impaired neurocognitive function before WBRT. Sixty-nine patients underwent PCI and were compared with those who deferred PCI in this nonrandomized study. Although significant transient impairment was identified in executive function and language at early time points in patients who underwent PCI, these deficits were not sustained, and no difference was detected between the two groups at late time points. Similar cognitive deficits at baseline but no ultimate detriment in neurocognitive function attributable to PCI have been found by other groups despite the inclusion of large dose fractions of radiation.61
 Prophylactic Cranial Irradiation
Prophylactic Cranial Irradiation
Several diseases have a natural history that involves frequent metastases to the brain. Given the relatively impervious nature of an intact blood-brain barrier to chemotherapeutics when compared with other body compartments, some patients have had systemic eradication of their disease with a combination of surgery, RT, or chemotherapy only to ultimately have fatal brain parenchymal metastases subsequently develop. PCI was instituted in such populations in an attempt to improve overall survival by eradication of disease from this potential sanctuary site and has been most classically used for limited-stage SCLC. A phase III study evaluating 300 patients with SCLC who had a complete response to systemic treatment and were subsequently randomized to PCI or observation showed a decrease in the brain as a first site of relapse (45% versus 19%, P = 10−6) and in the 2-year rate of development of brain metastases (67% to 40%, P = 10−13), but a nonstatistical difference in overall survival at 2 years (21.5% versus 29%, P = .14).62 No significant difference between the groups was found in terms of neuropsychological function. A subsequent meta-analysis found an improvement in overall survival rates with PCI at 3 years from 15.3% to 20.7%.63 PCI for locally advanced (stage III) NSCLC has also been advocated and studied, albeit less extensively than for SCLC. Unfortunately, a large, randomized trial evaluating PCI for stage III NSCLC closed early because of slow accrual. PCI has classically been confined to limited-stage SCLC, but a recent European Organization for Research and Treatment of Cancer (EORTC) study found a survival advantage in patients with extensive-stage SCLC.64
Reirradiation of Brain Metastases
The use of relatively moderate doses of WBRT frequently results in a clinical situation in which patients have progressive brain metastases but their performance status or size and number of metastases do not qualify them for either resection or SRS. This is a very frustrating experience because adequate doses of radiation cannot be delivered without significant risk to these patients. Several single-institution reports that used doses in the range of 20 to 30 Gy for the management of these patients are available in the literature, and in the majority of these studies, patients derived some clinical benefit but overall outcome remained poor.65,66 It is unclear whether reirradiation provided substantial benefit for these patients, and therefore when opting for reirradiation in such patients, significant attention must be paid to the individual patient’s history and clinical findings to determine whether there is any role for reirradiation. The decision of whether to repeat WBRT or use limited-field RT must also be made. Aggressive, primary treatment interventions to provide durable local control is obviously preferable to being backed into this unfortunate situation of recurrent intracranial disease with limited treatment options.
Primary Central Nervous Neoplasms
In 2008 there were an estimated 21,810 new cases of CNS malignancies in the United States and 13,070 deaths.67 The incidence of CNS and primary malignant brain tumors, excluding lymphoma, leukemia, pituitary, and olfactory tumors, is 6.4 cases per 100,000 person-years and is slightly higher in males.68 Meningiomas represent the only CNS tumor seen more commonly in females. Given the infiltrative nature of many CNS neoplasms and the essential and nonredundant nature of the majority of CNS structures restricting surgical excision, external beam RT has long held a role in the treatment of CNS malignancies.
Malignant Glioma
Malignant gliomas are relatively uncommon neoplasms that account for only about 40% of the approximately 21,810 new annual cases of CNS tumors in the United States in 2008, but they represent 80% of malignant CNS tumors.67 Their clinical impact continues to far outweigh their incidence, principally because of their high fatality rate. Malignant or high-grade gliomas are rapidly growing tumors that tend to diffusely infiltrate the brain parenchyma, thus typically preventing complete microscopic surgical excision. These tumors can develop at any age but are most commonly seen in late adulthood. Malignant gliomas are usually divided into grade III and grade IV gliomas, which have distinct molecular features, responses to treatment, and prognoses.69
Glioblastoma
Glioblastoma, or grade IV astrocytoma, accounts for about 80% of all malignant gliomas, and 1- and 5-year survival rates were only 29.3% and 3.3%, respectively, in an analysis of the Surveillance, Epidemiology, and End Results (SEER) database from 1973 to 2002.68 Average survival has improved from 12 to 14 months with a combination of RT and adjuvant TMZ chemotherapy, but long-term survivorship remains low. Malignant gliomas such as glioblastoma rarely metastasize, and mortality is primarily attributed to nearly universal local failure, which leads to early institution of RT as an adjunct to surgery in an attempt to improve local control.
Utility of Radiation Therapy
Although retrospective analysis demonstrates that nearly gross total resection (>98%) of glioblastoma multiforme (GBM) leads to an improvement in overall survival,70 the infiltrative nature of GBM prohibits complete surgical eradication of disease without unacceptable neurological compromise. This limitation has led to the establishment of RT in the adjuvant setting as a cornerstone of treatment. An institutional review by Bouchard and coauthors in the late 1960s of patients given higher doses of radiation (50 to 60 Gy) found a survival rate of 44% at 1 year in those treated with a combination of surgical resection and adjuvant RT,71 which compared favorably with other institutions in which RT was not used. Intra-institutional comparisons in Sweden72 and at the University of California at San Francisco,73 University of California at Los Angeles,74 and the Mayo Clinic75 retrospectively identified improved survival when adjuvant RT was administered.
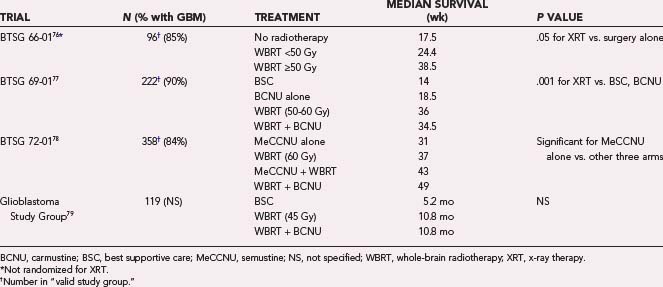
These favorable institutional data led to trials examining the adjuvant use of RT after surgical resection by the Brain Tumor Study Group (BTSG; later renamed the Brain Tumor Cooperative Groups [BTCG]), although the trials had patients with “malignant gliomas,” including anaplastic astrocytomas. The first randomized trial (BTSG 66-01) was actually designed to compare the activity of mithramycin postoperatively in the experimental arm versus surgery alone (Table 250-5).76–79 Although no difference based on the administration of mithramycin was identified, 55% of the patients received adjuvant RT at the discretion of the treating physician. Median survival was 36 weeks for those who received any dose of radiation and 15 weeks for those without. Patients receiving 50 Gy or greater had a median survival of 38.5 weeks; if less than 50 Gy was delivered, overall survival appeared to have been 33 weeks.
The role of RT was explicitly evaluated in BTSG 69-01, a four-arm trial that examined postoperative adjuvant carmustine (BCNU) alone, WBRT alone, WBRT and BCNU, or best supportive care.77 The radiation was delivered to the whole brain via opposed lateral fields to a dose of 50 to 60 Gy. WBRT led to improved overall survival (P = .001), and BCNU was found to provide minor prolongation in survival when compared with best supportive care (25 versus 17 weeks). No difference in median overall survival was identified when BCNU was added to WBRT (34.5 versus 36.0 weeks), but more patients remained alive at 12 and 18 months, thus suggesting a benefit.
BTSG 72-01 built on 69-01 and offered a four-arm comparison of the then new chemotherapeutic agent semustine (MeCCNU) used adjuvantly with or without RT and the previous regimens of WBRT alone or with BCNU.78 The WBRT-plus-BCNU arm appeared to have the lengthiest median survival at 49 weeks, but this was not significant in comparison to RT alone when either the valid study group or total randomized cohort was analyzed statistically. The benefit of adjuvant WBRT was confirmed, and only the semustine-alone arm was deemed to be statistically inferior.
Andersen and colleagues published a study from Denmark that randomized patients with grade IV gliomas based on birth date (odd versus even) to surgical resection with or without RT.80 When patients who survived less than 2 months were excluded, RT was found to offer a greater probability of survivorship at 6 months (54% versus 28%) and 1 year (19% versus 0%). A trial of the Scandinavian Glioblastoma Study Group compared best supportive care with RT delivered to the supratentorial intracranial contents at a dose of 45 Gy with or without BCNU postoperatively.79 Median survival with RT was 10.8 months and was unaffected by the administration of BCNU. Median survival with best supportive care was 5.2 months, and these patients deteriorated more quickly. Sandberg-Wollheim and associates found improvement with RT and procarbazine, CCNU (lomustine), and vincristine (PCV) chemotherapy versus PCV chemotherapy alone.81 These multiple positive studies led to the adoption of postresection RT as a standard of care given the clear improvement in survival.
Some have argued that elderly patients or those with poor performance status do not gain significant benefit from adjuvant RT and should instead receive supportive care alone. Keime-Guibert and associates randomized patients older than 70 years with a KPS score of 70 or higher to supportive care versus 50 Gy of radiation in 1.8-Gy fractions.82 Median survival was 29.1 weeks with RT and supportive care versus 16.9 weeks with supportive care alone (HR, 0.47; P = .002). No detriment in quality of life with RT was identified. The combination of adjuvant RT and TMZ was also found to be well tolerated, safe, and effective in a cohort older than 70 years.83 Although survival expectations are lower in patients with advanced age, benefit still seems to be derived by aggressive treatment in patients with adequate performance status. Age alone should not be seen as a contraindication to postresection RT with or without TMZ.
Radiation Target Volume
Delineation of the location and extent of GBMs in planning for RT has been and remains a challenge. Before cross-sectional imaging, clinicians relied on more limited neuroradiologic techniques such as pneumoencephalograms and angiographic studies for tumor visualization. These early neuroradiologic techniques were incapable of consistently defining either the gross or microscopic extent of the glioblastoma. Defining a precise location required additional caution, as well as knowledge of the natural history and spread patterns of GBMs and the belief that they were frequently multifocal in nature. Even after hemispherectomy, gliomas were known to recur in the contralateral hemisphere by spread along white matter tracts.84 An early radiographic-pathologic correlation study performed on patients in a Medical Research Council (MRC) study who died shortly after being admitted for RT showed that autopsy demarcation of the tumor indicated that planned smaller RT fields would have missed the tumor in 8 of 21 patients and that coverage in an additional 11 patients was questionable. Larger fields would have covered the tumors in at least three quarters of the patients.85 The authors concluded that “with our present methods of localization and knowledge of spread of gliomata there is no place for small or medium volume radiation therapy in the treatment of the more highly malignant gliomata.” Todd reviewed 23 patients with supratentorial GBM and found that patients treated with larger volumes (500 cc or an 8 × 8 × 8-cm field) did better than those treated with more limited volumes, regardless of total dose.86 The concern of missing tumor volume underlined by these studies led to the adoption of WBRT in the BTSG trials and by other groups, thereby avoiding regional undertreatment at the expense of having the entire intracranial contents exposed to high doses of radiation with the possibility of significant late neurotoxicity and radiation necrosis.87
The use of WBRT for GBM was never universal, and some centers reported equivalent outcomes with WBRT and involved-field radiation therapy (IFRT).88 Ramsey and Brand, in comparing patients treated with WBRT (44 Gy) and IFRT (53 Gy), found improved survival with more limited fields receiving higher dosage, although all volumes were still larger than 1000 cc.89 After the advent of CT, Hochberg and Pruitt correlated autopsy findings with findings on CT performed within 2 months before death and also examined serial CT scans.90 When compared with autopsy data, CT showed the tumor margin to be within 2 cm of the autopsy-determined tumor extent in 29 of the 35 patients. On serial CT scans, 90% of the patients had an element of local recurrence within 2 cm of the primary site. Finally, true multifocality was found in only 3% of untreated and 6% of treated gliomas; tumors that were thought to be multicentric frequently had gross or microscopic evidence of contiguity on autopsy examination. The relative concordance of CT and pathologic findings, the propensity for predominantly local recurrence, and the rarity of true multicentricity lent credence to a more focal approach to cranial RT.
The advent of MRI provided further tumor detail. Kelly and colleagues compared 195 stereotactic biopsy specimens from 40 patients with gliomas evaluated by CT and MRI. Histologic examination of the biopsy samples correlated the regions of contrast uptake with regions of neoplastic glial cells without intervening normal parenchyma.91 The T2 abnormalities were more extensive than the areas of hypodensity on CT and were infiltrated by isolated tumor cells that extended to at least the edge of the T2 abnormalities. Distinguishing between peritumoral edema and tumor infiltration was not feasible with conventional-sequence MRI, thus implying that all abnormal T2 regions should be covered by the full-dose RT volume. Additional support for partial brain irradiation arose from analysis of the radiation portion of a multi-arm BTCG trial. WBRT to 60.2 Gy was compared with WBRT to 43 Gy followed by a 17.2-Gy boost based on CT volumes expanded by 2 cm.92 No difference in survival was identified, which led the authors to endorse an approach to RT that involved less than WBRT. One small study found improvement in performance status and overall survival when partial-brain RT was used for the entire 60 Gy versus 40-Gy WBRT followed by a 20-Gy, localized boost.93 Treating limited volumes has led to the distinct combined advantage of maximizing intensification of the dose to the neoplasm and margin while potentially limiting normal tissue toxicity. Based on these studies showing equivalent, if not superior outcomes with IFRT, IFRT became the standard of care for the treatment of GBM.
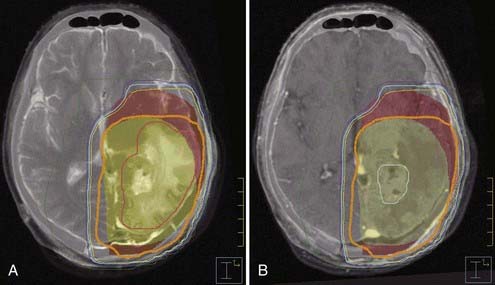
Current practice calls for the inclusion of all radiographic evidence of tumor and associated edema with generous margins. The RTOG bases treatment volumes on postoperative CT or MRI studies (MRI preferred), with substitution allowed for preoperative CT or MRI if needed. The initial treatment volume includes the contrast-enhancing lesion, resection cavity, and surrounding edema if present (best seen on T2-weighted images), followed by a 2.0-cm expansion, and is named the planned target volume 1 (PTV1) (![]() see Fig. 250-E1A). After 46 Gy of radiation, a coned-down volume consisting of the contrast-enhancing lesion with a 2.5-cm expansion is treated to create PTV2 (
see Fig. 250-E1A). After 46 Gy of radiation, a coned-down volume consisting of the contrast-enhancing lesion with a 2.5-cm expansion is treated to create PTV2 (![]() see Fig. 250-E2B). PTV2 volume is not to exceed the volume of PTV1 at any point. Clinical judgment can be used in avoiding full doses to areas of high toxicity such as the chiasm or in excluding areas that have natural anatomic barriers to spread such as the cerebellum (tentorium), contralateral hemisphere (falx), or ventricles. Although the RTOG guidelines are followed by most radiation oncology practices, some have advocated not including the peritumoral edema/T2 abnormalities within the clinical target volume before expansion and instead placing a 2.0-cm margin around the region of contrast enhancement.94 This mildly decreases the percent volume of the brain receiving 30, 46, and 50 Gy while leaving the central pattern of failure unaltered. This variation in gross tumor volume (GTV), clinical tumor volume (CTV), and subsequent PTV contouring underlines the past and continued difficulties in delineation of GBM. In particular, it is unclear whether the region of T2 abnormality accurately reflects microscopic disease extent. Magnetic resonance spectroscopy has demonstrated tumor volume involving approximately 50% of the T2 changes but simultaneously identified frequent extension beyond the T2 abnormalities.95 Further advances in MRI and functional imaging may ultimately alter treatment planning volumes.
see Fig. 250-E2B). PTV2 volume is not to exceed the volume of PTV1 at any point. Clinical judgment can be used in avoiding full doses to areas of high toxicity such as the chiasm or in excluding areas that have natural anatomic barriers to spread such as the cerebellum (tentorium), contralateral hemisphere (falx), or ventricles. Although the RTOG guidelines are followed by most radiation oncology practices, some have advocated not including the peritumoral edema/T2 abnormalities within the clinical target volume before expansion and instead placing a 2.0-cm margin around the region of contrast enhancement.94 This mildly decreases the percent volume of the brain receiving 30, 46, and 50 Gy while leaving the central pattern of failure unaltered. This variation in gross tumor volume (GTV), clinical tumor volume (CTV), and subsequent PTV contouring underlines the past and continued difficulties in delineation of GBM. In particular, it is unclear whether the region of T2 abnormality accurately reflects microscopic disease extent. Magnetic resonance spectroscopy has demonstrated tumor volume involving approximately 50% of the T2 changes but simultaneously identified frequent extension beyond the T2 abnormalities.95 Further advances in MRI and functional imaging may ultimately alter treatment planning volumes.
Recurrence Patterns
Despite the recognized benefit of adjuvant RT in improving the durability of local control, the vast majority of patients with GBM ultimately experience local recurrence. Hochberg and Pruitt demonstrated that more than 80% of failures occur within 2 cm of the primary tumor, with only 1 in 35 (3%) patients in their series having an isolated recurrence outside this 2-cm risk zone.90 In an analysis of 34 patients treated with three-dimensional, CT-based treatment plans, 33 of the 34 recurrences were completely situated within the original 90% isodose line.96 In this report, the recurrent tumor that surpassed the outside surface of the PTV was still predominantly centered within the tumor bed. An examination of patients who received high-dose RT (70 to 80 Gy) for GBM similarly showed only 1 of 36 patients having a recurrence, with 50% by volume located outside the high-dose region and the remainder considered to be “central” or “in-field.”97 The relentless ability of GBM to recur locally provides strong rationale for the investigation of aggressive modalities of local control in an attempt to improve overall outcome in patients with malignant glioma.
Dose Escalation
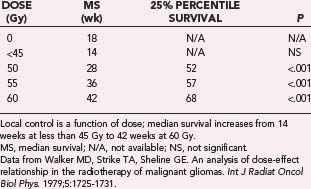
As described previously, the clinical trials conducted by the BTSG in the 1970s found local control and survival to be improved in patients with malignant glioma treated with postoperative RT versus those undergoing surgical resection only. In addition, subsequent analysis of their serial trials demonstrated a possible dose-response survival relationship over a range of RT doses from 45 to 62 Gy.98 Median survival improved from 14 weeks with less than 45 Gy to 42 weeks with 60 Gy. These data are presented in Table 250-6. Further confirmation of a dose-benefit relationship was identified from a randomized MRC trial of two RT doses for the treatment of malignant glioma.99 Patients receiving 60 Gy had a 12-month median survival, whereas survival in those receiving 45 Gy was just 9 months (P = .007). These data established the standard postoperative radiation dose at 60 Gy.
There is compelling evidence from in vitro studies that a dose-response relationship for malignant gliomas exists above 60 Gy.100 Unfortunately, our ability to escalate the external beam RT dose is severely limited by the accompanying increase in neurotoxicity. For example, Marks and coworkers demonstrated that risk for brain necrosis increases substantially with doses higher than 60 to 70 Gy.87 Significant clinical evidence indicating improved survival with doses greater than 60 Gy is lacking. In an analysis of more than 600 patients treated in an intergroup RTOG/Eastern Cooperative Oncology Group (ECOG) study, 70 Gy did not result in increased survival compared to 60 Gy.101 RTOG 83-02, a randomized phase I dose escalation trial of twice-daily RT, accrued more than 700 patients treated with a dose ranging from 64.8 to 81 Gy; although the 72-Gy arm had the best survival, toxicity beyond this dose resulted in worse survival.102 In a prospective phase I/II dose escalation study by Urtasun’s group, external beam doses of up to 80 Gy in a hyperfractionated regimen of 1 Gy three times daily were achieved without substantial toxicity or evidence of improved local control or survival.103
To determine whether higher doses affect quality of life, 786 patients with malignant glioma enrolled in the RTOG phase I/II study 83-02 were analyzed with a modified quality-adjusted survival model. This model allowed the inclusion of both improvement and decline in neurological functional status, and patients were scored by the presence or absence of 15 neurological signs and symptoms during the study and at every follow-up. Within each category were included gradations of severity, and quality survival time was adjusted according to any changes in these neurological findings. The summation of all changes in signs and symptoms was weighted and incorporated into the model. Overall, the average quality-adjusted survival time was 18.5 months for the study, with the 72-Gy arm having the best result of 20.8 months, which was significantly longer than that of all other groups.104 Based on such data, accelerated hyperfractionation was pursued in the successor randomized trial RTOG 94-11, which placed patients in 64-Gy or 70.4-Gy groups based on size (≤20 cm2 or >20 cm2).105 Unfortunately, this trial demonstrated no statistically significant survival advantage with dose escalation. Additional randomized studies have not found an improvement in survival with dose escalation to 66 Gy (1.1 Gy twice daily),106 70 Gy (2 Gy daily),107 or 70.4 Gy (1.6 Gy twice daily)108 when compared with standard doses (59.8 or 60 Gy).
The University of Michigan dose-escalated 34 patients with high-grade supratentorial gliomas to 90 Gy delivered via a 3DCRT technique.109 Although this trial demonstrated the feasibility of such an approach with no significant toxicities observed, the clinical result was a modest median survival of 11.7 months. Despite the aggressive dose escalation to 90 Gy, the pattern of failure was not significantly altered, with all patients having central, in-field, or marginal recurrences. Dose escalation by interstitial brachytherapy and colloid, balloon-based treatments has also been attempted and is discussed elsewhere.110,111 The failure to obtain easily identifiable benefit from such significantly elevated external beam RT doses has pushed clinical investigation away from dose escalation alone and more toward the concurrent use of compounds, both chemotherapeutic and otherwise, that serve as radiosensitizers.
 Chemotherapy
Chemotherapy
The use of chemotherapy for GBM has a long history full of peaks and valleys with squandered enthusiasm for multiple agents that held initial promise but were ultimately revealed to have limited clinical utility. The use of chemotherapy for GBM is discussed extensively elsewhere, but the utility of adjuvant chemotherapy in conjunction with RT was revolutionized by Stupp and coauthors, who reported an improvement in median survival from 12.1 to 14.6 months with concurrent and adjuvant administration of TMZ with RT.112 The addition of TMZ also significantly increased the number of patients alive and without disease progression at 2 years. Much of the current clinical research emphasis on GBM involves the dose and timing of TMZ, as well as evaluation of the addition of other agents to TMZ and RT in the primary setting.
Sensitizer Trials
One of the identifying pathologic features of GBM is necrosis. Viable cells exist between the necrotic, typically central, regions of tumor and the proliferating periphery. The oxygen enhancement ratio was developed from laboratory studies that found improved radiation susceptibility when cells were exposed to at least low levels of oxygen (tumor susceptibility at oxygen concentrations of 0.5% was midway between that of hypoxic and fully oxygenated conditions).1 Studies attempting to overcome the innate tumor hypoxia in malignant gliomas through the use of radiation modifiers in conjunction with RT have consistently shown disappointing results. The use of hyperbaric oxygen and RT in 38 patients versus RT alone in 42 patients increased median survival from 31 to 38 weeks and 18-month survival rates from 10% to 28%.113 The expense and inconvenience of such an approach are not insignificant, however, and facilities that administer hyperbaric oxygen are not widely available. In an attempt to provide the same theoretical benefit of decreased tumor hypoxia, various agents have been investigated to improve hypoxic cell sensitivity to radiation. Nitroimidazole compounds such as metronidazole and misonidazole are hypoxic cell sensitizers that are orally available and able to penetrate the blood-brain barrier. Interest in using metronidazole to make the hypoxic cells of GBM more radiosensitive was stoked by a small randomized trial that found a delay in time from progression to death in those who had received metronidazole.114 In the control arm of an RTOG study to confirm this benefit, patients received 60-Gy RT plus BCNU chemotherapy. In the experimental group, misonidazole was given once a week for 6 weeks, and the radiation schedule was adjusted to take into account the small number of fractions given concurrently with misonidazole. No significant benefit from misonidazole was observed.115 Median patient survival with RT and BCNU was 55 weeks and that with misonidazole, RT, and BCNU was 46 weeks. The BTCG also evaluated misonidazole in their study 77-02. In this particular clinical trial, 11 institutions randomized more than 600 patients with supratentorial malignant glioma to one of four treatment groups after surgery: conventional RT to 60 Gy with BCNU chemotherapy, RT plus streptozotocin, hyperfractionated RT (66 Gy in 60 fractions given twice daily) with BCNU, and conventional RT with misonidazole followed by BCNU. Median survival was 10 months, with no statistically significant difference in survival between the four groups. In fact, among patients without glioblastoma, the misonidazole group appeared to have poorer survival.106 Similar lack of efficacy was identified in randomized trials by the MRC116 and the Vienna Study Group despite initial promising results.117 Because of the lack of activity demonstrated by misonidazole, a newer imidazole compound, etanidazole, was tested in a phase I study in which it was administered concurrently with external beam RT to patients with malignant glioma.118 Unfortunately, etanidazole has not found a successful role in the management of these tumors either.
As described previously, efaproxiral (RSR13) is a synthetic small molecule that alters the oxygen-hemoglobin dissociation curve and thereby increases capillary PO2. A phase II study of 50 patients to evaluate its utility for GBM showed a median survival of 12.3 months and a 2-year survival rate of 24%.119 Less than a quarter of the patients experienced grade 2 toxicities, which were almost entirely mild and self-limited. Although median survival surpassed 1 year, efaproxiral has not been evaluated in a randomized setting because of the success in improving survival with concurrent TMZ, as well as the lack of distinct overall survival benefit seen in the efaproxiral phase III study for brain metastases.
The RTOG has extensively evaluated halogenated pyrimidines as potential radiosensitizers in patients with malignant glioma. Halogenated pyrimidines are structurally similar to thymidine, but with a halogen substituted for the methyl group, and are incorporated into the DNA of replicating cells. After incorporation, the cells become more susceptible to single-strand breaks from radiation-induced free radicals and have impaired ability to repair DNA.120 Malignant gliomas seemed to be an ideal target for such agents because the mitotic activity of neoplastic cells is much higher than the nearly quiescent activity of the background glial and vascular cells. Prados and associates analyzed the effect of treatment with a different halogenated pyrimidine, BUdR, during RT. Patient data from several RTOG clinical trials, as well as a single Northern California Oncology Group (NCOG) trial, were analyzed retrospectively. In the entire cohort of 2077 patients, 1743 were treated without BUdR and 334 were treated with it. After adjusting for eligibility, a total of 1774 patients were eligible for evaluation of survival. For patients with glioblastoma, median survival was 9.8 months in the RTOG studies and 18 months in the NCOG trial. For patients with anaplastic astrocytoma, median survival was 35.1 months in the RTOG studies and 42.8 months in the NCOG study. Univariate analysis demonstrated a survival advantage in favor of BUdR. In a proportional hazards regression model, BUdR was found to influence outcome in patients with glioblastoma. The authors concluded that because of patient heterogeneity and treatment variation, absolute conclusions could not be derived, but there appeared to be a favorable treatment effect from BUdR in patients with glioblastoma.121 A single-institution phase II study found no survival benefit with BUdR and RT when compared with historical data.122 Greater than expected myelosuppression and dermatotoxicity were encountered, although PCV was also administered. A follow-up, randomized phase III trial for anaplastic astrocytoma unfortunately found no difference between those who received BUdR, RT, and PCV chemotherapy and those who received RT and PCV chemotherapy alone (4-year survival rate of 51% in both arms).123,124 Although the NCOG data point toward a potential benefit of BUdR in patients with glioblastoma, the failings of any retrospective comparison remain and a phase III trial would ultimately be required to provide convincing evidence of BUdR efficacy for GBM.
MGd, a redox modulator, has demonstrated uptake in vitro in a majority of glioblastoma nuclei125 and has been tolerated well in a phase I trial. MGd will be evaluated in a phase II trial in concert with TMZ and RT.126
Particle Beam Radiation Therapy
Because of the grim prognosis and relative lack of success to date with dose escalation and radiosensitizers, particle beam RT has been explored in patients with malignant glioma. Castro and colleagues reported on 39 patients with primary recurrent glioma of the brain treated with a heavily charged particle beam at the University of California–Lawrence Berkley Laboratory in a phase I/II clinical trial of the NCOG. The data did not show results superior to those obtained with standard therapies, and because of the limited availability of heavily charged particles, substantial investigation in this field is lacking.127 A phase II study of 23 patients with a combination of protons and photons to 90 CGE (cobalt gray equivalent) found a median survival of 20 months, with the majority of patients experiencing recurrence within the 60- to 70-CGE region and only 1 within the high-dose (90 CGE) isodose volume.128 Although a median survival benefit of 5 to 11 months was projected based on median survival from historical data, patient selection excluded individuals with involvement of the thalamus, corpus callosum, or subependymal region or with a postoperative tumor volume exceeding 60 cc.
More attention has been focused on neutron beam RT. Most commonly, a regimen of mixed photons and neutrons has been used because neutrons alone have proved to be too toxic in terms of late radiation injury. In the mid-1980s, the RTOG conducted and completed a phase I trial in patients with malignant glioma to evaluate concomitant neutron boost. A total of 190 patients were randomized to six different neutron dose levels, but no significant difference in overall survival among the six dose levels was detected. For anaplastic astrocytoma, there was a suggestion that patients with higher dose levels had poorer overall survival than did patients with lower dose levels, thus suggesting a possible detrimental effect from neutrons.129 All tumor levels were associated with persistent viable tumor and damage to normal brain parenchyma. An additional four randomized trials found no difference in median survival between the standard photon arm and the experimental arm with neutrons, a neutron boost, or pions.130–133
A second method of using neutron therapy for the management of patients with malignant brain tumors is a technique known as boron neutron capture therapy (BNCT). This method involves incorporating boron 10 into the tumor with an appropriate boronated pharmacologic agent followed by irradiation with thermal or epithermal neutrons. A review of the literature indicates that to date more than 120 patients have been treated in this manner, principally by Japanese investigators. The Japanese reports have for the most part been relatively favorable, and an independent analysis of the Japanese data was conducted by Laramore and Spence. In this particular analysis, the cohort of patients from the United States who received BNCT in Japan was compared with a matched cohort receiving conventional therapy in various RTOG studies. A total of 14 patients receiving BNCT were identified, 12 of whom were evaluable. When compared with a matched set of patients, median survival was 10.5 months in both groups, and no meaningful improvement in survival attributable to this form of therapy could be identified.134 In conclusion, the data to date do not support the routine use of particle beam RT for the management of patients with malignant glioma.
Prognostic Factor Analysis
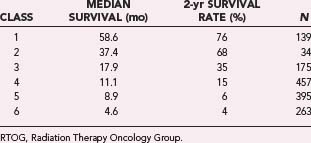
The RTOG used RPA to analyze survival in 1578 patients entered in three RTOG malignant glioma trials from 1974 to 1989.135 In this approach, a regression tree was created from prognostic variables, and patients were classified into homogeneous subsets by survival. Twenty-six pretreatment characteristics and six treatment-related variables were analyzed. This approach permitted the creation of six prognostic classes that primarily used the variables of age, histology, mental status, performance status, symptom duration, and degree of resection. These classes are defined in Table 250-7. Table 250-8 shows survival stratified by prognostic class. This approach allows stratification into appropriate subgroups, which can then be used for further prognostic analysis. It is quite obvious that patients in classes 5 and 6 have the worst outcome, with 2-year survival rates of less than 6%, whereas patients in classes 1 and 2 have 2-year survival rates approaching and exceeding 70%. The 2-year survival rates of patients in classes 3 and 4 are 35% and 15%, respectively. The RTOG RPA maintained prognostic significance between groups with or without the addition of TMZ.136 Methylguanine-deoxyribonucleic acid methyltransferase (MGMT) methylation has also proved to be a powerful predictor of survival in patients receiving RT and TMZ137 and has been incorporated into nomograms that predict survival.138
TABLE 250-7 Definition of Prognostic Classes from RTOG Recursive Partitioning Analysis
| PROGNOSTIC CLASS | DEFINITION |
|---|---|
| 1 | AA, age <50 yr, normal mental status |
| 2 | AA, age ≥50 yr, KPS score ≥70, duration of symptoms >3 mo |
| 3 | AA, age <50 yr, abnormal mental status or GBM, age <50 yr, KPS score ≥90 |
| 4 | AA, age ≥50 yr, KPS ≥70, duration of symptoms ≤3 months; GBM, age <50 yr, KPS score <90; or GBM, age ≥50 yr, KPS score ≥70, partial or total resection, NFS = working |
| 5 | GBM, age ≥50 yr, KPS score ≥70, partial or total resection, NFS < working; GBM, age ≥50 yr, KPS score ≥70, biopsy only; or GBM or AA, age ≥50 yr, KPS score <70, normal mental status |
| 6 | GBM or AA, age ≥50 yr, KPS score <70, abnormal mental status |
AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; KPS, Karnofsky Performance Status; NFS, neurological functional status; RTOG, Radiation Therapy Oncology Group.
 Anaplastic Astrocytoma
Anaplastic Astrocytoma
It appears that patients with anaplastic astrocytoma experience relatively worse outcomes with more aggressive regimens. This phenomenon was initially identified by Laramore in an analysis of 163 patients with anaplastic astrocytoma treated in several RTOG and ECOG trials. Three distinct patient groups were identified: those treated with standard photon RT in the 60- to 70-Gy range, those treated with RT and chemotherapy (BCNU, MeCCNU, or dacarbazine), and those treated with photon radiation plus a neutron boost. The median survival of patients receiving RT alone was 3 years as opposed to 2.3 years for those receiving chemoradiotherapy and 1.7 years for those receiving photon plus neutron therapy. For each subgroup of patients, further prognostic variable analysis using age and KPS scores was carried out. In each category, median survival decreased with more aggressive therapy.139
A similar analysis was conducted in 149 patients with anaplastic astrocytoma treated with RT alone, RT and chemotherapy, or RT, chemotherapy, and misonidazole. The median survival of patients treated with RT alone was 3 years. The median survival of patients treated with chemoradiotherapy was 2.3 years, and for patients treated with chemotherapy and misonidazole, it was 1.2 years. Both these reports suggest that the more aggressive modalities, such as misonidazole sensitization and neutron boost, produce a decrease in survival in patients with anaplastic astrocytoma.140 The use of halogenated pyrimidines, tested in a prospective randomized trial, does not result in a survival advantage.123
Anaplastic Oligodendroglioma
Of the malignant gliomas, anaplastic oligodendroglioma is one of the least common subtypes. This neoplasm is characterized by remarkable sensitivity to both RT and multiagent chemotherapy.141 In addition, anaplastic oligodendrogliomas are also distinguished by a unique constellation of molecular genetic alterations, including loss of 1p and 19q in about 25% to 50% of these tumors. These tumors are also typically characterized by relatively high survival rates. For example, in an analysis of outcome of oligodendroglioma patients based on grade and the concomitant presence of astrocytic features, a group of Japanese investigators found the median survival time for patients with anaplastic oligodendroglioma to be as long as 12.7 years, but the presence of astrocytic features in this tumor (anaplastic oligoastrocytoma) decreased the median survival time to 4.8 years.142
Because these tumors respond significantly to both RT and chemotherapy, a combination approach might logically produce a prolonged disease-free interval for patients with this disease. In two phase III trials, one in North America and the other in Europe, RT alone was compared with RT plus PCV. In the North American trial, powered primarily for survival analysis, patients received PCV for four cycles before RT.143 Survival in the two groups was the same. Notably, patients with 1p and 19q deletions had significantly better outcomes, regardless of treatment. An analysis of progression-free survival (PFS) demonstrated that the benefit from PCV was most notable in patients with 1p and 19q deletions. In the European trial, patients received PCV after RT.144 The results were nearly identical to the North American results. Taken together, these studies demonstrate that chemotherapy potentially improves PFS, but the effect on survival is not statistically obvious, possibly because salvage treatment at recurrence results in equivalent survival. Importantly, both trials confirm the prognostic value of loss of 1p and 19q.
Primary Central Nervous System Lymphoma
A variety of different distinct pathologic and clinical entities are included within the broad designation PCL. In general, this term is meant to reflect lymphoma confined to the CNS, which occurs in less than 2% of all CNS tumors. The overall incidence of this disease increased a minimum of threefold in the 1970s and 1980s,145 at least partly attributable to an increase in the number of immunocompromised patients secondary to acquired immunodeficiency syndrome (AIDS), organ transplantation, and other such conditions.146 However, the incidence appears to have decreased after 1995 in men younger than 60 years, probably as a result of improved treatment of human immunodeficiency virus (HIV) infection with the advent and implementation of highly active antiretroviral therapy (HAART).147 The incidence in patients older than 60 has remained unchanged over that same period. In general, for clinical trial purposes, patients are subdivided into AIDS-associated and non–AIDS-associated PCL.
PCL is characterized by dissemination through the craniospinal axis as its natural history advances. It is a radiosensitive tumor with clinical response rates upward of 80%. In a report in the early 1970s, 83 patients with PCL were treated by observation, surgical resection, or RT. In patients who were observed, median survival was 3.3 months; in those treated by surgical excision, median survival was 4.6 months; and in patients receiving external beam RT, median survival was 15.2 months.148 In other studies, median survival times between 14 and 40 months have been documented. In addition to prolonging survival, RT produces rapid responses and therefore quick and sustained clinical improvement.149
Unfortunately, cranial irradiation alone does not result in significant long-term survival despite the high response rates. In a literature review, Leibel and Sheline noted that 1-, 2-, and 5-year survival rates for this disease after RT were 66%, 43%, and 7%, respectively.150
Significant controversy remains regarding the volume of radiation and the appropriate dose. In an attempt to define an appropriate dose, Murray and colleagues evaluated outcomes in 198 patients treated with a dose ranging from less than 40 to greater than 50 Gy.151 The median survival of 54 patients receiving greater than 50 Gy was 17 months versus 15 months for the 144 patients who received less than 50 Gy. More importantly, the actuarial 5-year survival rate for patients receiving greater than 50 Gy was 42% as opposed to 13% for those receiving less than 50 Gy. The latter survival value reached statistical significance, and their data would suggest that doses greater than 50 Gy should be used.
Another contentious issue regarding RT for PCL is the volume to be treated. It has been estimated that spinal relapse, as the first site of progression, occurs in up to half of patients with PCL. It is possible that these numbers may be exaggerated because of the inclusion of patients from older studies in which MRI of the spine before study entry was not conducted. In the series by Murray and associates, 7% of patients relapsed with positive cerebrospinal fluid cytology or overt spinal disease. In addition, in their literature review of 308 patients, they identified 124 who received radiation to the whole brain, 16 who received radiation to the tumor bed alone, 16 who received treatment of the entire neuraxis, and 152 in whom treatment volume could not be assessed. They did not find a significant association between the volume treated and survival outcome. In contrast, the review by Leibel and Sheline suggested that patients receiving RT delivered to the primary tumor alone had a median survival of 39.4 months versus 25.3 months for patients receiving WBRT.150,151
The current trend of using RT for this disease has recognized the role of chemotherapy. The use of intensive pre-RT chemotherapy produces significant responses, and a phase II RTOG trial suggested a significant survival benefit.152 In the RTOG 93-10 study, 102 patients were treated with five cycles of high-dose methotrexate, vincristine, and procarbazine over a 10-week period, followed by 45-Gy WBRT and high-dose cytarabine after RT. Median PFS was 24 months and overall survival was 36.9 months. This trial confirmed that providing radiation to the entire craniospinal axis is unnecessary. A stark age impact was found on survival, with a median survival of 50.4 months in patients younger than 60 and only 21.8 months in those 60 or older. In addition, a 15% rate of severe, delayed neurological toxicity occurred, with 8 of 12 such patients dying of toxicity. This high rate of neurotoxicity, particularly in patients older than 60 years, has brought into question the utility and appropriateness of WBRT for the treatment of PCL.
Multiple chemotherapy regimens have been investigated for the treatment of PCL and are discussed elsewhere. The use of RT, as well as the total dose and fractionation schedule, remains a matter of debate that is unlikely to be resolved outside a randomized, multi-institutional trial. Because PCL does respond avidly to RT, there have been recent attempts to maintain radiation in the treatment regimen, albeit at a lower dose to decrease the risk for neurotoxicity. Shah and coauthors published a regimen with reduced-dose WBRT (23.4 Gy) prescribed to patients with a complete response after an induction regimen consisting of rituximab, methotrexate, procarbazine, and vincristine. At a median follow-up of 37 months, 2-year overall and PFS rates were 67% and 57%, and no late neurotoxicity had occurred.153
Malignant Meningeal Tumors
In the World Health Organization classification, four histopathologic variables are assessed for meningiomas: grade, histologic subtype, proliferation index, and brain invasion. Histologic subtype is intimately associated with the grade, which ranges from I to III.154 In practice, these grades are frequently represented by the descriptors typical meningioma, atypical meningioma, and malignant (anaplastic) meningioma, respectively. Malignant meningioma frequently has histologic evidence of brain parenchyma invasion and, rarely, evidence of distant metastases. Histopathologically, these neoplasms have significant vascular proliferation, increased cellularity, high rates of mitosis, and frequent occurrence of necrosis. Under light microscopy, meningiosarcomas can be categorized as either fibrosarcomas, spindle cell sarcomas, or mixed sarcomas. All these aggressive meningeal neoplasms have poor 5-year survival rates of less than 20%, and the majority of patients are treated with postoperative RT. Because of the rarity of these tumors, identification of the best therapeutic approaches has been difficult. One study of 48 malignant meningeal neoplasms consisting of an eclectic mix of 32 anaplastic meningiomas, 11 hemangiopericytomas, 2 meningiosarcomas, and 3 papillary meningiomas showed an improvement in PFS at 5 years with adjuvant RT after the initial resection.155 The extent of resection strongly influenced PFS, with a 5-year PFS rate of 39% after total resection versus 0% after subtotal resection (P = .001). Although the dose prescribed adjuvantly can vary,156 a dose in the 60-Gy range in 30 to 33 fractions is recommended based on retrospective data.157 GTV is typically expanded by 1.5 to 2 cm, especially in patients with pathologic brain invasion. Close attention should also be paid during RT planning to the dural tails, particularly if a higher Simpson grade resection was performed.
Hemangiopericytoma is a rare neoplasm that arises from perivascular pericytes. Accounting for less than 1% of all brain tumors, these neoplasms are characterized by a high local response rate and metastatic potential. They tend to grow most frequently in the fifth decade of life. Nearly 300 cases of hemangiopericytoma have been described in the literature, and they have arisen from virtually every anatomic site. Overall, fewer than 100 cases of meningeal hemangiopericytoma have been reported. Surgical resection has been the historic and prevalent mode of therapy for these tumors. In 1992, a comprehensive review defined the role of RT in the management of this disease. A total of 80 patients with meningeal hemangiopericytoma were identified, and analysis revealed a 90% 9-year actuarial risk for local recurrence after surgical resection only. Interestingly, less than 33% of these recurrences were noted in the first 5 years, which may account for the false assumption that these tumors are highly curable by surgical resection alone. In final analysis, RT appeared to reduce this local recurrence rate and prolong disease-free and overall survival. Radiation responses were noted to be dose dependent, with greater than 50 Gy providing superior long-term disease-free survival. Meningeal hemangiopericytomas are characterized by a slow but progressive radiographic response to ionizing radiation, not unlike other highly vascular brain lesions such as arteriovenous malformations. These tumors also have significant metastatic potential, with the liver, lung, bone, and soft tissue being preferred sites for metastatic dissemination. RT in doses higher than 50 Gy should be incorporated as primary or adjuvant therapy for patients who are being treated with curative intent.158
 Other Malignant Neoplasms
Other Malignant Neoplasms
Primitive Neuroectodermal Tumors
These tumors are exceptionally rare in the adult context and are more frequently seen in the pediatric age groups. PNETs are characterized by sheets of small round blue cells with scant cytoplasm and broadly include supratentorial PNET, pineoblastoma, medulloblastoma, and ependymoblastoma, with the pathologic descriptor frequently based on anatomic location. Given the relative infrequency of the majority of these tumors, the greater part of clinical data on the treatment of PNETs has been accumulated from medulloblastoma studies and trials. RT provides the backbone of therapy in the management of these tumors. For medulloblastomas, a distinction into two prognostic categories is routinely made for patients in the pediatric age group. They are classified as “standard risk” or “high risk” based on four factors: age, Chang M stage, location, and extent of resection. Standard-risk patients have Chang M0 stage disease (no evidence of microscopic or macroscopic dissemination along the craniospinal axis), posterior fossa origin, age older than 3 years, and less than 1.5-cm2 tumor residual after surgery. In the adult situation, prognostic factors appear to be similar to those in children, and therapy is frequently guided by the same risk stratification.159 Although substantial emphasis in the treatment of childhood medulloblastoma has been placed on dropping the dose to the craniospinal axis in an attempt to decrease long-term toxicity related to growth disturbance, this reduction in dose is unnecessary in skeletally mature individuals and has been shown to decrease the control rate in certain settings.160,161 Craniospinal RT at recommended doses of 36 Gy to the craniospinal axis and a posterior fossa boost to 54 Gy remains the standard of care for the majority of these patients. One retrospective study did show equivalent disease-free survival in standard-risk adult patients if chemotherapy was administered concurrently with doses of less than 34 Gy, similar to the results from more recent pediatric cooperative group studies.159 Combination chemotherapy given with craniospinal RT continues to be pursued, particularly within the high-risk group because of relatively poor outcomes with current regimens.162
Germ Cell Neoplasms
Historically, CNS germinomas have been managed with craniospinal RT. The majority of these tumors occur in the pediatric age group, and because of growth impediment from RT, strategies to eliminate the spinal portion of the RT or diminish the craniospinal dose have been aggressively pursued, similar to PNETs. Although single-institution studies suggest that spinal RT may be deleted in some patients, this practice is not universally accepted. In a multicenter prospective trial, all 60 patients with intracranial germinomas treated by craniospinal RT, without any chemotherapy, achieved complete remission. The 5-year relapse-free survival rate was 91%; of the 5 relapsing patients, 4 failed outside the CNS.163 Because growth considerations are not of significant concern in the adult population, the inclusion of chemotherapy in adult patients has taken on less significance. Outcomes with craniospinal RT in patients with germ cell neoplasms in the adult context is excellent, with cure rates upward of 90%. For nongerminomatous germ cell tumors of the CNS, survival is significantly poorer, and both surgical resection and chemotherapy are the primary modalities of treatment. For residual disease, RT may help in prolonging disease-free survival.
Complications
In general, radiation reactions can be broadly classified into three temporal categories: acute, subacute, and delayed.164 Acute reactions occur during and immediately after completion of a course of external beam RT. With current technologies, these reactions are usually rare and clinically readily manageable. Such complications include acute skin reactions, typically dry desquamation and some degree of erythema, which are managed with local ointments. Temporary alopecia within the radiation field is a common sequela. Fatigue may be observed with RT, but it is often a function of several other variables such as age, performance status, underlying medical status, extent of brain being irradiated, and other factors. Acute visual complications during RT are very rare, but occasionally patients report seeing flashes of light while undergoing RT. This is believed to result from retinal stimulation secondary to a phenomenon described as Cerenkov radiation. If both ear canals are included in the radiation field, serous otitis media may develop toward the end of RT, sometimes producing muffled hearing. Nausea is uncommon unless the area postrema is treated with high doses or large fractions. Increased intracranial pressure during RT is uncommon. It may be masked, however, because in the vast majority of patients, steroids have been initiated before RT and are frequently maintained during RT.
Subacute reactions may occur several weeks or months after the completion of RT. When large volumes of the brain are treated, especially in younger patients, lethargy and somnolence may be observed in approximately 3 months. In children, this is specifically described as an acute somnolence syndrome. Rarely, localized demyelination resulting in nausea, vomiting, ataxia, dysphasia, and cerebellar ataxia may develop in some patients. It is usually self-limited. If either lacrimal gland is included in the radiation portal, dry eye may result. Keratoconjunctivitis may develop if the cornea is not protected. Radiation necrosis in the absence of tumor progression is seen clinically during this period, although it has nonlinear dose dependence and is not commonly encountered when total doses are kept lower than 60 Gy.165 However, necrosis in concert with viable tumor is not uncommonly seen, especially in patients with malignant glioma. Radiation necrosis can have a highly variable manifestation, from a purely imaging-based diagnosis in asymptomatic patients to highly symptomatic individuals with mass effect and subsequently increased intracranial pressure, headaches, and symptoms traceable to the anatomic location. Discriminating radiation necrosis from metastases and recurrent glial neoplasms by imaging can be challenging because all can have regions with avid uptake of contrast material on T1-weighted images and can cause mass effect with local edema. Both positron emission tomography166 and magnetic resonance spectroscopy167 have been advocated to differentiate radiation necrosis from recurrent tumor, although volumetric limitations exist and residual neoplasm interspersed with necrosis can confound the diagnosis. Surgical sampling, via either stereotactic biopsy or surgical excision, is frequently required to ultimately differentiate necrosis from recurrence.
Late complications of RT occur several months to several years after completion of therapy. The exact incidence of these complications is unclear because a substantial proportion of patients are either short-term survivors only or have a component of both tumor progression and delayed radiation morbidity. With sequential CT imaging, a mineralizing angiopathy characterized by loss of white matter, enlarged ventricles, and microcalcifications can be identified. It occurs as a consequence of radiation injury to small vessels and may clinically result in impairment of intellectual function, especially memory and mathematical ability. In severe cases it may result in significant dementia, ataxia, and confusion. The clinical sequelae of these changes are frequently manifested as neurocognitive impairment. Historically, patients with the most dramatic radiation-associated cognitive declines have received hypofractionated regimens55 and WBRT for gliomas.168 The exact incidence of such impairment remains inadequately quantified because many patients with malignant neoplasms frequently succumb to the disease process before neurocognitive impairment develops; therefore, the true incidence of this phenomenon is probably greater than reported in most clinical trials. Several factors have now been identified as contributing to overall neurocognitive decline in these patients, including the tumor itself, surgery, RT, and chemotherapy. In addition, host factors such as underlying diseases, especially those characterized by microvascular changes, including diabetes, hypertension, smoking, stroke, cardiovascular insufficiency, and other conditions, also contribute to the greater incidence. Specific RT parameters that influence the risk profile for neurocognitive decline include fraction size, total dose, and volume irradiated. Therefore, current RT paradigms, in general, avoid large-fraction irradiation, with radiosurgery representing an exception to this rule. More importantly, three-dimensional and conformal techniques reduce the volume of normal brain irradiated. These approaches are of significance because no effective therapy exists for patients suffering neurocognitive decline after RT, although symptoms can occasionally be ameliorated with memantine (Namenda) and other Alzheimer-type medications.
Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682-689.
Curran WJJr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704-710.
DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798-805.
Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29-38.
Greco C, Wolden S. Current status of radiotherapy with proton and light ion beams. Cancer. 2007;109:1227-1238.
Gutin PH, Leibel SA, Sheline GE. Radiation Injury to the Nervous System. New York: Raven Press; 1991.
Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1-7.
Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Khuntia D, Brown P, Li J, et al. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295-1304.
Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157-165.
Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470-1476.
Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730-4735.
Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1-9.
Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510-514.
Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-590.
Walker MD, Alexander EJr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-343.
Walker MD, Alexander EJr, Hunt WE, et al. Evaluation of mithramycin in the treatment of anaplastic gliomas. J Neurosurg. 1976;44:655-667.
Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323-1329.
Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
1 Hall EJ, Giaccia AJ. Radiobiology for the Radiologist, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
2 Kelly PJ, Daumas-Duport C, Scheithauer BW, et al. Stereotactic histologic correlations of computed tomography– and magnetic resonance imaging–defined abnormalities in patients with glial neoplasms. Mayo Clin Proc. 1987;62:450-459.
3 Thornton AFJr, Hegarty TJ, Ten Haken RK, et al. Three-dimensional treatment planning of astrocytomas: a dosimetric study of cerebral irradiation. Int J Radiat Oncol Biol Phys. 1991;20:1309-1315.
4 Hermanto U, Frija EK, Lii MJ, et al. Intensity-modulated radiotherapy (IMRT) and conventional three-dimensional conformal radiotherapy for high-grade gliomas: does IMRT increase the integral dose to normal brain? Int J Radiat Oncol Biol Phys. 2007;67:1135-1144.
5 Bova FJ, Buatti JM, Friedman WA, et al. The University of Florida frameless high-precision stereotactic radiotherapy system. Int J Radiat Oncol Biol Phys. 1997;38:875-882.
6 Miranpuri AS, Tome WA, Paliwal BR, et al. Assessment of patient-independent intrinsic error for a noninvasive frame for fractionated stereotactic radiotherapy. Int J Cancer. 2001;96:320-325.
7 Hug EB, Loredo LN, Slater JD, et al. Proton radiation therapy for chordomas and chondrosarcomas of the skull base. J Neurosurg. 1999;91:432-439.
8 Igaki H, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for skull base chordoma. Int J Radiat Oncol Biol Phys. 2004;60:1120-1126.
9 St Clair WH, Adams JA, Bues M, et al. Advantage of protons compared to conventional X-ray or IMRT in the treatment of a pediatric patient with medulloblastoma. Int J Radiat Oncol Biol Phys. 2004;58:727-734.
10 Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1-7.
11 Aoyama H, Westerly DC, Mackie TR, et al. Integral radiation dose to normal structures with conformal external beam radiation. Int J Radiat Oncol Biol Phys. 2006;64:962-967.
12 Greco C, Wolden S. Current status of radiotherapy with proton and light ion beams. Cancer. 2007;109:1227-1238.
13 Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5-14.
14 Larson DA, Rubenstein JL, McDermott MW. Treatment of Metastatic Cancer. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg’s Cancer: Principles & Practice of Oncology. 8th ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008:2461.
15 Hazra T, Mullins GM, Lott S. Management of cerebral metastasis from bronchogenic carcinoma. Johns Hopkins Med J. 1972;130:377-383.
16 Posner JB. Diagnosis and treatment of metastases to the brain. Clin Bull. 1974;4:47-57.
17 Kofman S, Garvin JS, Nagamani D, Taylor SG3rd. Treatment of cerebral metastases from breast carcinoma with prednisolone. JAMA. 1957;163:1473-1476.
18 Chao JH, Phillips R, Nickson JJ. Roentgen-ray therapy of cerebral metastases. Cancer. 1954;7:682-689.
19 Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1980;6:1-9.
20 Borgelt B, Gelber R, Larson M, et al. Ultra-rapid high dose irradiation schedules for the palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1981;7:1633-1638.
21 Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295-1304.
22 Coia LR, Hanks GE, Martz K, et al. Practice patterns of palliative care for the United States 1984-1985. Int J Radiat Oncol Biol Phys. 1988;14:1261-1269.
23 Rades D, Bohlen G, Dunst J, et al. Comparison of short-course versus long-course whole-brain radiotherapy in the treatment of brain metastases. Strahlenther Onkol. 2008;184:30-35.
24 Epstein BE, Scott CB, Sause WT, et al. Improved survival duration in patients with unresected solitary brain metastasis using accelerated hyperfractionated radiation therapy at total doses of 54.4 gray and greater. Results of Radiation Therapy Oncology Group 85-28. Cancer. 1993;71:1362-1367.
25 Murray KJ, Scott C, Greenberg HM, et al. A randomized phase III study of accelerated hyperfractionation versus standard in patients with unresected brain metastases: a report of the Radiation Therapy Oncology Group (RTOG) 9104. Int J Radiat Oncol Biol Phys. 1997;39:571-574.
26 Nieder C, Berberich W, Nestle U, et al. Relation between local result and total dose of radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 1995;33:349-355.
27 Nieder C, Nestle U, Walter K, et al. Dose/effect relationships for brain metastases. J Cancer Res Clin Oncol. 1998;124:346-350.
28 Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795-803.
29 Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583-590.
30 Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994;29:711-717.
31 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494-500.
32 Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470-1476.
33 Diener-West M, Dobbins TW, Phillips TL, et al. Identification of an optimal subgroup for treatment evaluation of patients with brain metastases using RTOG study 7916. Int J Radiat Oncol Biol Phys. 1989;16:669-673.
34 Swift PS, Phillips T, Martz K, et al. CT characteristics of patients with brain metastases treated in RTOG study 79-16. Int J Radiat Oncol Biol Phys. 1993;25:209-214.
35 Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745-751.
36 Nieder C, Andratschke N, Grosu AL, et al. Recursive partitioning analysis (RPA) class does not predict survival in patients with four or more brain metastases. Strahlenther Onkol. 2003;179:16-20.
37 Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485-1489.
38 Regine WF, Rogozinska A, Kryscio RJ, et al. Recursive partitioning analysis classifications I and II: applicability evaluated in a randomized trial for resected single brain metastases. Am J Clin Oncol. 2004;27:505-509.
39 Lutterbach J, Bartelt S, Ostertag C. Long-term survival in patients with brain metastases. J Cancer Res Clin Oncol. 2002;128:417-425.
40 Weltman E, Salvajoli JV, Brandt RA, et al. Radiosurgery for brain metastases: a score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155-1161.
41 Lorenzoni J, Devriendt D, Massager N, et al. Radiosurgery for treatment of brain metastases: estimation of patient eligibility using three stratification systems. Int J Radiat Oncol Biol Phys. 2004;60:218-224.
42 Sperduto PW, Berkey B, Gaspar LE, et al. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008;70:510-514.
43 Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
44 Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483-2491.
45 Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003;2:111-115.
46 Smalley SR, Laws ERJr, O’Fallon JR, et al. Resection for solitary brain metastasis. Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg. 1992;77:531-540.
47 Rades D, Pluemer A, Veninga T, et al. A boost in addition to whole-brain radiotherapy improves patient outcome after resection of 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;110:1551-1559.
48 Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339-348.
49 Knisely JP, Berkey B, Chakravarti A, et al. A phase III study of conventional radiation therapy plus thalidomide versus conventional radiation therapy for multiple brain metastases (RTOG 0118). Int J Radiat Oncol Biol Phys. 2008;71:79-86.
50 Berk L, Seiferheld W, Rich T, et al. RTOG BR-0119: Chronobiological study of the addition of melatonin to radiotherapy for brain metastases. Proc Soc Neuro Oncol. 2004;6:308-387.
51 Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529-2536.
51a Mehta MP, Shapiro WR, Phan SC, et al. Motexafin gadolinium combined with prompt whole brain radiotherapy prolongs time to neurologic progression in non-small-cell lung cancer patients with brain metastases: results of a phase III trial. Int J Radiat Oncol Biol Phys. 2009;73(4):1069-1076.
52 Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106-114.
53 Suh J, Stea B, Tankel K, et al. Results of the phase III ENRICH (RT-016) study of efaproxiral administered concurrent with whole brain radiation therapy (WBRT) in women with brain metastases from breast cancer [abstract 110]. Paper presented at the 2008 Annual Meeting of the American Society for Therapeutic Radiology and Oncology, Boston, Mass, 2008.
54 DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798-805.
55 DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789-796.
56 Meyers CA. Neurocognitive dysfunction in cancer patients. Oncology (Williston Park). 2000;14:75-79.
57 Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22:157-165.
58 Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260-1266.
59 Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388-1395.
60 Grosshans DR, Meyers CA, Allen PK, et al. Neurocognitive function in patients with small cell lung cancer: effect of prophylactic cranial irradiation. Cancer. 2008;112:589-595.
61 Gregor A, Cull A, Stephens RJ, et al. Prophylactic cranial irradiation is indicated following complete response to induction therapy in small cell lung cancer: results of a multicentre randomised trial. United Kingdom Coordinating Committee for Cancer Research (UKCCCR) and the European Organization for Research and Treatment of Cancer (EORTC). Eur J Cancer. 1997;33:1752-1758.
62 Arriagada R, Le Chevalier T, Borie F, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. J Natl Cancer Inst. 1995;87:183-190.
63 Auperin A, Arriagada R, Pignon JP, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med. 1999;341:476-484.
64 Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357:664-672.
65 Abdel-Wahab MM, Wolfson AH, Raub W, et al. The role of hyperfractionated re-irradiation in metastatic brain disease: a single institutional trial. Am J Clin Oncol. 1997;20:158-160.
66 Wong WW, Schild SE, Sawyer TE, et al. Analysis of outcome in patients reirradiated for brain metastases. Int J Radiat Oncol Biol Phys. 1996;34:585-590.
67 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
68 Central Brain Tumor Registry of the United States. 2007-2008 CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004-2006. Hinsdale, Ill: CBTRUS; 2008.
69 Louis DN. Molecular pathology of malignant gliomas. Annu Rev Pathol. 2006;1:97-117.
70 Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190-198.
71 Bouchard J. Radiation therapy of intracranial tumors—long term results. Acta Radiol Ther Phys Biol. 1966;3:11-16.
72 Lindgren M. Roentgen treatment of gliomata. Acta Radiol. 1953;40:325-334.
73 Sheline GE. Radiation therapy of brain tumors. Cancer. 1977;39(suppl 2):873-881.
74 Stage WS, Stein JJ. Treatment of malignant astrocytomas. Am J Roentgenol Radium Ther Nucl Med. 1974;120:7-18.
75 Uihlein A, Colby MYJr, Layton DD, et al. Comparison of surgery and surgery plus irradiation in the treatment of supratentorial gliomas. Acta Radiol Ther Phys Biol. 1966;3:67-78.
76 Walker MD, Alexander EJr, Hunt WE, et al. Evaluation of mithramycin in the treatment of anaplastic gliomas. J Neurosurg. 1976;44:655-667.
77 Walker MD, Alexander EJr, Hunt WE, et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg. 1978;49:333-343.
78 Walker MD, Green SB, Byar DP, et al. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med. 1980;303:1323-1329.
79 Kristiansen K, Hagen S, Kollevold T, et al. Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time: a prospective multicenter trial of the Scandinavian Glioblastoma Study Group. Cancer. 1981;47:649-652.
80 Andersen AP. Postoperative irradiation of glioblastomas. Results in a randomized series. Acta Radiol Oncol Radiat Phys Biol. 1978;17:475-484.
81 Sandberg-Wollheim M, Malmstrom P, Stromblad LG, et al. A randomized study of chemotherapy with procarbazine, vincristine, and lomustine with and without radiation therapy for astrocytoma grades 3 and/or 4. Cancer. 1991;68:22-29.
82 Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527-1535.
83 Combs SE, Wagner J, Bischof M, et al. Postoperative treatment of primary glioblastoma multiforme with radiation and concomitant temozolomide in elderly patients. Int J Radiat Oncol Biol Phys. 2008;70:987-992.
84 Dandy WE. Removal of right cerebral hemisphere for certain tumors with hemiplegia. JAMA. 1928;90:823-835.
85 Concannon JP, Kramer S, Berry R. The extent of intracranial gliomata at autopsy and its relationship to techniques used in radiation therapy of brain tumors. Am J Roentgenol Radium Ther Nucl Med. 1960;84:99-107.
86 Todd DH. Choice of volume in the x-ray treatment of supratentorial gliomas. Br J Radiol. 1963;36:645-649.
87 Marks JE, Baglan RJ, Prassad SC, et al. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7:243-252.
88 Onoyama Y, Abe M, Yabumoto E, et al. Radiation therapy in the treatment of glioblastoma. AJR Am J Roentgenol. 1976;126:481-492.
89 Ramsey RG, Brand WN. Radiotherapy of glioblastoma multiforme. J Neurosurg. 1973;39:197-202.
90 Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30:907-911.
91 Kelly PJ, Daumas-Duport C, Kispert DB, et al. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987;66:865-874.
92 Shapiro WR, Green SB, Burger PC, et al. Randomized trial of three chemotherapy regimens and two radiotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma. Brain Tumor Cooperative Group Trial 8001. J Neurosurg. 1989;71:1-9.
93 Sharma RR, Singh DP, Pathak A, et al. Local control of high-grade gliomas with limited volume irradiation versus whole brain irradiation. Neurol India. 2003;51:512-517.
94 Chang EL, Akyurek S, Avalos T, et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int J Radiat Oncol Biol Phys. 2007;68:144-150.
95 Pirzkall A, McKnight TR, Graves EE, et al. MR-spectroscopy guided target delineation for high-grade gliomas. Int J Radiat Oncol Biol Phys. 2001;50:915-928.
96 Oppitz U, Maessen D, Zunterer H, et al. 3D-recurrence-patterns of glioblastomas after CT-planned postoperative irradiation. Radiother Oncol. 1999;53:53-57.
97 Lee SW, Fraass BA, Marsh LH, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys. 1999;43:79-88.
98 Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5:1725-1731.
99 Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769-774.
100 Taghian A, Suit H, Pardo F, et al. In vitro intrinsic radiation sensitivity of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1992;23:55-62.
101 Nelson DF, Diener-West M, Horton J, et al. Combined modality approach to treatment of malignant gliomas—re-evaluation of RTOG 7401/ECOG 1374 with long-term follow-up: a joint study of the Radiation Therapy Oncology Group and the Eastern Cooperative Oncology Group. NCI Monogr. 1988;6:279-284.
102 Nelson DF, Curran WJJr, Scott C, et al. Hyperfractionated radiation therapy and bis-chlorethyl nitrosourea in the treatment of malignant glioma—possible advantage observed at 72.0 Gy in 1.2 Gy B.I.D. fractions: report of the Radiation Therapy Oncology Group Protocol 8302. Int J Radiat Oncol Biol Phys. 1993;25:193-207.
103 Fulton DS, Urtasun RC, Scott-Brown I, et al. Increasing radiation dose intensity using hyperfractionation in patients with malignant glioma. Final report of a prospective phase I-II dose response study. J Neurooncol. 1992;14:63-72.
104 Murray KJ, Nelson DF, Scott C, et al. Quality-adjusted survival analysis of malignant glioma. Patients treated with twice-daily radiation (RT) and carmustine: a report of Radiation Therapy Oncology Group (RTOG) 83-02. Int J Radiat Oncol Biol Phys. 1995;31:453-459.
105 Coughlin C, Scott C, Langer C, et al. Phase II, two-arm RTOG trial (94-11) of bischloroethyl-nitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (> 20 or < or = 20 cm(2), respectively) in the treatment of newly-diagnosed radiosurgery-ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351-1358.
106 Deutsch M, Green SB, Strike TA, et al. Results of a randomized trial comparing BCNU plus radiotherapy, streptozotocin plus radiotherapy, BCNU plus hyperfractionated radiotherapy, and BCNU following misonidazole plus radiotherapy in the postoperative treatment of malignant glioma. Int J Radiat Oncol Biol Phys. 1989;16:1389-1396.
107 Chang CH, Horton J, Schoenfeld D, et al. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer. 1983;52:997-1007.
108 Prados MD, Wara WM, Sneed PK, et al. Phase III trial of accelerated hyperfractionation with or without difluromethylornithine (DFMO) versus standard fractionated radiotherapy with or without DFMO for newly diagnosed patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;49:71-77.
109 Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20:1635-1642.
110 Laperriere NJ, Leung PM, McKenzie S, et al. Randomized study of brachytherapy in the initial management of patients with malignant astrocytoma. Int J Radiat Oncol Biol Phys. 1998;41:1005-1011.
111 Selker RG, Shapiro WR, Burger P, et al. The Brain Tumor Cooperative Group NIH Trial 87-01: a randomized comparison of surgery, external radiotherapy, and carmustine versus surgery, interstitial radiotherapy boost, external radiation therapy, and carmustine. Neurosurgery. 2002;51:343-355.
112 Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987-996.
113 Chang CH. Hyperbaric oxygen and radiation therapy in the management of glioblastoma. Natl Cancer Inst Monogr. 1977;46:163-169.
114 Urtasun R, Band P, Chapman JD, et al. Radiation and high-dose metronidazole in supratentorial glioblastomas. N Engl J Med. 1976;294:1364-1367.
115 Nelson DF, Schoenfeld D, Weinstein AS, et al. A randomized comparison of misonidazole sensitized radiotherapy plus BCNU and radiotherapy plus BCNU for treatment of malignant glioma after surgery; preliminary results of an RTOG study. Int J Radiat Oncol Biol Phys. 1983;9:1143-1151.
116 A study of the effect of misonidazole in conjunction with radiotherapy for the treatment of grades 3 and 4 astrocytomas. A report from the MRC Working Party on misonidazole in gliomas. Br J Radiol. 1983;56:673-682.
117 Stadler B, Karcher KH, Kogelnik HD, et al. Misonidazole and irradiation in the treatment of high-grade astrocytomas: further report of the Vienna Study Group. Int J Radiat Oncol Biol Phys. 1984;10:1713-1717.
118 Riese NE, Loeffler JS, Wen P, et al. A phase I study of etanidazole and radiotherapy in malignant glioma. Int J Radiat Oncol Biol Phys. 1994;29:617-620.
119 Kleinberg L, Grossman SA, Carson K, et al. Survival of patients with newly diagnosed glioblastoma multiforme treated with RSR13 and radiotherapy: results of a phase II new approaches to brain tumor therapy CNS consortium safety and efficacy study. J Clin Oncol. 2002;20:3149-3155.
120 McGinn CJ, Shewach DS, Lawrence TS. Radiosensitizing nucleosides. J Natl Cancer Inst. 1996;88:1193-1203.
121 Prados MD, Scott CB, Rotman M, et al. Influence of bromodeoxyuridine radiosensitization on malignant glioma patient survival: a retrospective comparison of survival data from the Northern California Oncology Group (NCOG) and Radiation Therapy Oncology Group trials (RTOG) for glioblastoma multiforme and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 1998;40:653-659.
122 Groves MD, Maor MH, Meyers C, et al. A phase II trial of high-dose bromodeoxyuridine with accelerated fractionation radiotherapy followed by procarbazine, lomustine, and vincristine for glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1999;45:127-135.
123 Prados MD, Seiferheld W, Sandler HM, et al. Phase III randomized study of radiotherapy plus procarbazine, lomustine, and vincristine with or without BUdR for treatment of anaplastic astrocytoma: final report of RTOG 9404. Int J Radiat Oncol Biol Phys. 2004;58:1147-1152.
124 Prados MD, Scott C, Sandler H, et al. A phase 3 randomized study of radiotherapy plus procarbazine, CCNU, and vincristine (PCV) with or without BUdR for the treatment of anaplastic astrocytoma: a preliminary report of RTOG 9404. Int J Radiat Oncol Biol Phys. 1999;45:1109-1115.
125 De Stasio G, Rajesh D, Ford JM, et al. Motexafin-gadolinium taken up in vitro by at least 90% of glioblastoma cell nuclei. Clin Cancer Res. 2006;12:206-213.
126 Ford JM, Seiferheld W, Alger JR, et al. Results of the phase I dose-escalating study of motexafin gadolinium with standard radiotherapy in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69:831-838.
127 Castro JR, Saunders WM, Austin-Seymour MM, et al. A phase I-II trial of heavy charged particle irradiation of malignant glioma of the brain: a Northern California Oncology Group Study. Int J Radiat Oncol Biol Phys. 1985;11:1795-1800.
128 Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251-260.
129 Laramore GE, Diener-West M, Griffin TW, et al. Randomized neutron dose searching study for malignant gliomas of the brain: results of an RTOG study. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 1988;14:1093-1102.
130 Griffin TW, Davis R, Laramore G, et al. Fast neutron radiation therapy for glioblastoma multiforme. Results of an RTOG study. Am J Clin Oncol. 1983;6:661-667.
131 Duncan W, McLelland J, Jack WJ, et al. Report of a randomised pilot study of the treatment of patients with supratentorial gliomas using neutron irradiation. Br J Radiol. 1986;59:373-377.
132 Duncan W, McLelland J, Jack WJ, et al. The results of a randomised trial of mixed-schedule (neutron/photon) irradiation in the treatment of supratentorial grade III and grade IV astrocytoma. Br J Radiol. 1986;59:379-383.
133 Pickles T, Goodman GB, Rheaume DE, et al. Pion radiation for high grade astrocytoma: results of a randomized study. Int J Radiat Oncol Biol Phys. 1997;37:491-497.
134 Laramore GE, Spence AM. Boron neutron capture therapy (BNCT) for high-grade gliomas of the brain: a cautionary note. Int J Radiat Oncol Biol Phys. 1996;36:241-246.
135 Curran WJJr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704-710.
136 Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563-2569.
137 Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997-1003.
138 Gorlia T, van den Bent MJ, Hegi ME, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981-22981/CE.3. Lancet Oncol. 2008;9:29-38.
139 Laramore GE, Martz KL, Nelson JS, et al. Radiation Therapy Oncology Group (RTOG) survival data on anaplastic astrocytomas of the brain: does a more aggressive form of treatment adversely impact survival? Int J Radiat Oncol Biol Phys. 1989;17:1351-1356.
140 Fischbach AJ, Martz KL, Nelson JS, et al. Long-term survival in treated anaplastic astrocytomas. A report of combined RTOG/ECOG studies. Am J Clin Oncol. 1991;14:365-370.
141 Cairncross JG, Ueki K, Zlatescu MC, et al. Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst. 1998;90:1473-1479.
142 Tamura M, Zama A, Kurihara H, et al. Clinicohistological study of oligodendroglioma and oligoastrocytoma. Brain Tumor Pathol. 1997;14:35-39.
143 Cairncross G, Berkey B, Shaw E, et al. Phase III trial of chemotherapy plus radiotherapy compared with radiotherapy alone for pure and mixed anaplastic oligodendroglioma: Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006;24:2707-2714.
144 van den Bent MJ, Carpentier AF, Brandes AA, et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: a randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006;24:2715-2722.
145 Eby NL, Grufferman S, Flannelly CM, et al. Increasing incidence of primary brain lymphoma in the US. Cancer. 1988;62:2461-2465.
146 Corn BW, Marcus SM, Topham A, et al. Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer. 1997;79:2409-2413.
147 Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing incidence rates of primary central nervous system lymphoma. Cancer. 2002;95:193-202.
148 Henry JM, Heffner RRJr, Dillard SH, et al. Primary malignant lymphomas of the central nervous system. Cancer. 1974;34:1293-1302.
149 Berry MP, Simpson WJ. Radiation therapy in the management of primary malignant lymphoma of the brain. Int J Radiat Oncol Biol Phys. 1981;7:55-59.
150 Leibel SA, Sheline GE. Radiation therapy for neoplasms of the brain. J Neurosurg. 1987;66:1-22.
151 Murray K, Kun L, Cox J. Primary malignant lymphoma of the central nervous system. Results of treatment of 11 cases and review of the literature. J Neurosurg. 1986;65:600-607.
152 DeAngelis LM, Seiferheld W, Schold SC, et al. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10. J Clin Oncol. 2002;20:4643-4648.
153 Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25:4730-4735.
154 Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215-225.
155 Dziuk TW, Woo S, Butler EB, et al. Malignant meningioma: an indication for initial aggressive surgery and adjuvant radiotherapy. J Neurooncol. 1998;37:177-188.
156 Pasquier D, Bijmolt S, Veninga T, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388-1393.
157 Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151-160.
158 Bastin KT, Mehta MP. Meningeal hemangiopericytoma: defining the role for radiation therapy. J Neurooncol. 1992;14:277-287.
159 Padovani L, Sunyach MP, Perol D, et al. Common strategy for adult and pediatric medulloblastoma: a multicenter series of 253 adults. Int J Radiat Oncol Biol Phys. 2007;68:433-440.
160 Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000;18:3004-3011.
161 Bailey CC, Gnekow A, Wellek S, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol. 1995;25:166-178.
162 Brandes AA, Ermani M, Amista P, et al. The treatment of adults with medulloblastoma: a prospective study. Int J Radiat Oncol Biol Phys. 2003;57:755-761.
163 Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: results of the German cooperative prospective trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585-2592.
164 Gutin PH, Leibel SA, Sheline GE. Radiation Injury to the Nervous System. New York: Raven Press; 1991.
165 Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093-1112.
166 Hustinx R, Pourdehnad M, Kaschten B, et al. PET imaging for differentiating recurrent brain tumor from radiation necrosis. Radiol Clin North Am. 2005;43:35-47.
167 Rock JP, Hearshen D, Scarpace L, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51:912-919.
168 Scott JN, Rewcastle NB, Brasher PM, et al. Long-term glioblastoma multiforme survivors: a population-based study. Can J Neurol Sci. 1998;25:197-201.