24 Formation, Modification, and Repair of Neuronal Connections
Both Neurons and Connections Are Produced in Excess during Development
Neurotrophic Factors Ensure That Adequate Numbers of Neurons Survive
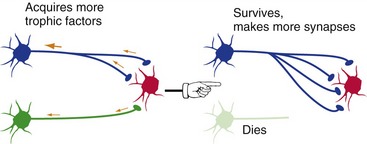
A critical factor that determines whether a given neuron survives or dies during development is its success in accumulating neurotrophic factors of specific kinds, different kinds for different neuronal types (Fig. 24-1). Neurotrophic factors are produced in limited amounts by target tissues (e.g., muscle, glands, other neurons), gobbled up by presynaptic endings, and transported back to the cell body. There they act to prevent apoptosis (programmed cell death) and to promote growth. Going back to the spinal cord example, more dorsal root ganglion cells and motor neurons survive at levels where there’s a lot of target tissue in the periphery (e.g., lower cervical) than at levels where there’s less (e.g., midthoracic). But this isn’t restricted to the spinal cord—throughout the nervous system, something like half of all the neurons produced during development die before birth.
Axonal Branches Are Pruned to Match Functional Requirements
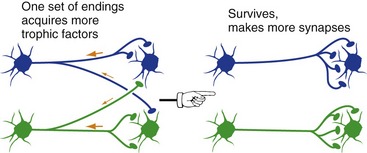
Long after neurons finish competing with each other for survival, they continue to compete for neurotrophic factors in an effort to preserve their connections (Fig. 24-2).
Synaptic Connections Are Adjusted throughout Life
There Are Short-Term and Long-Term Adjustments of Synaptic Strength
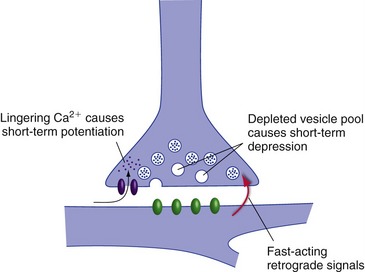
Some of the short-term changes in synaptic strength follow naturally from normal synaptic function (Fig. 24-3). A little extra Ca2+ hanging around in a presynaptic terminal after transmitter release, for example, can result in potentiation of transmitter release in response to the next action potential. High-frequency stimulation of a presynaptic ending can cause depletion of synaptic vesicles, resulting in depression of subsequent release for a little while. Fast-acting retrograde messengers, such as nitric oxide, can also cause short-term changes.
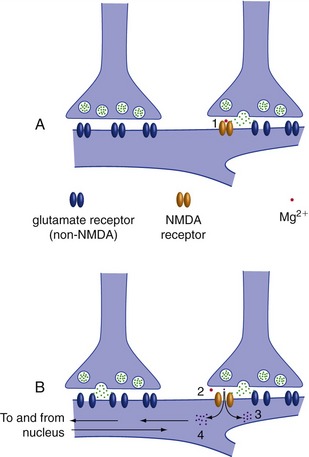
Longer-term changes can involve almost any conceivable part of presynaptic or postsynaptic elements (THB6 Figure 24-10, p. 616). One prominent example is the insertion or removal of postsynaptic transmitter receptors, resulting in long-term potentiation (LTP) or long-term depression (LTD). This can be triggered by postsynaptic Ca2+ entry through NMDA receptors (Fig. 24-4). NMDA receptors (named for N-methyl-d-aspartate, which binds to them) are glutamate receptors with some special properties. First, they only open when they bind glutamate and the membrane is already depolarized, making them great detectors of simultaneous activity at multiple synapses—something that could be a building block for memory formation. Second, they are less selective than other ion channels and let Ca2+ through (in addition to Na+ and K+). Small amounts of Ca2+ entry cause LTD, and larger amounts cause LTP. Subsequent Ca2+-initiated communication with the nucleus can make these changes very long lasting.
Multiple Memory Systems Depend on Adjustments of Synaptic Strength
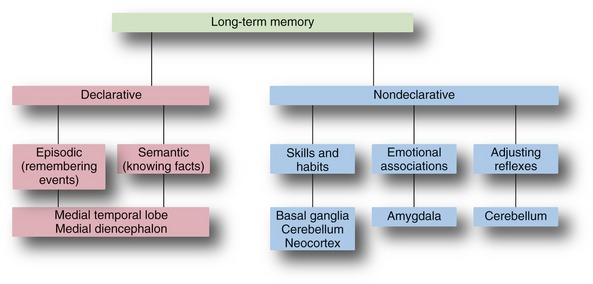
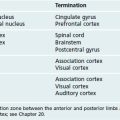
Most of us associate memory with things like learning lists of items before taking a test or writing a book, but there are actually multiple types of memory, each related to particular parts of the CNS (Fig. 24-5). Memories of facts (e.g., whose picture is on a dollar bill) and events (e.g., the particulars of the last handball game) are declarative memories, meaning you can declare them to be true. Memories of facts, also called semantic memories, span a range of categories, including historical facts, mathematical relationships, and the meanings of words; episodic memories include the specifics of events, including their timing. Nondeclarative memories, such as skills, patterns of behavior, and emotional reactions, are learned over time but show up more subconsciously.
The Hippocampus and Nearby Cortical Regions Are Critical for Declarative Memory
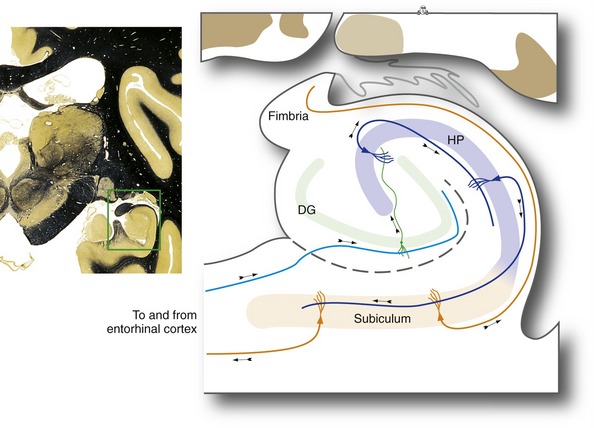
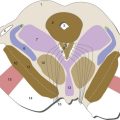
The hippocampus is a distinctive area of cerebral cortex folded into the temporal lobe. It is made up of the dentate gyrus and the hippocampus proper, two interlocking strips of three-layered cortex (unlike the six-layered neocortex described in Chapter 22), together with the subiculum, a transition zone between the hippocampus proper and temporal lobe neocortex (Fig. 24-6). The anterior part of the parahippocampal gyrus (entorhinal cortex) is the major interface between the hippocampus and vast areas of association cortex (see Figs. 24-7 and 24-8), allowing the hippocampus to somehow serve as a key link underlying declarative memory. Bilateral damage to the hippocampus and neighboring areas of cortex, or to the diencephalic areas they are interconnected with, causes anterograde amnesia, in which new memories for facts and events cannot be formed. The retrograde amnesia following such damage is not as severe, indicating that long-term memories mostly live outside the hippocampus.
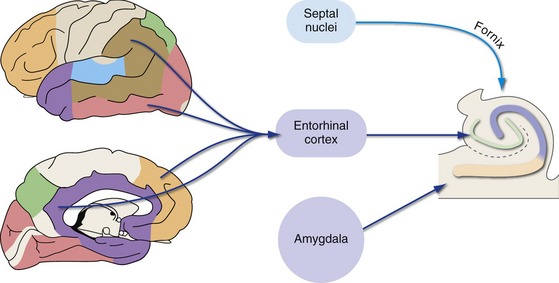
Inputs to entorhinal cortex, and from there to the hippocampus, come from widespread unimodal, multimodal, and limbic areas (Fig. 24-7). In addition, modulatory cholinergic inputs from the septal nuclei reach the hippocampus directly by traveling “backward” through the fornix, which is a major output route from the hippocampus (Fig. 24-8). Finally, there are direct projections from the amygdala to the hippocampus. As discussed later in this chapter, the amygdala is important for marking the emotional significance of situations and events; this connection affects the probability that something will be recorded as a declarative memory, depending on our emotional reaction to it.
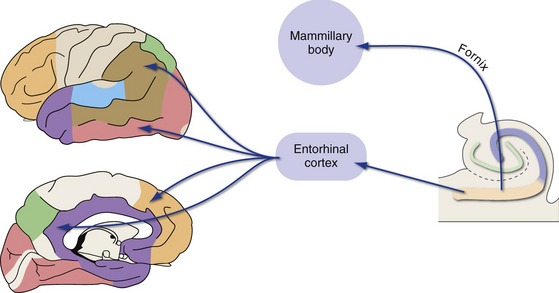
The hippocampus then projects back, by way of entorhinal cortex, to widespread unimodal, multimodal, and limbic areas (Fig. 24-8). Hippocampal outputs also reach limbic cortex indirectly, by way of projections to the mammillary bodies through the fornix. The fornix curves around with the lateral ventricle; it separates from the hippocampus near the splenium of the corpus callosum, travels forward along the inferior edge of the septum pellucidum, turns downward in front of the interventricular foramen, and enters the hypothalamus (THB6 Figure 24-17, p. 623). The mammillary bodies project to the anterior nucleus of the thalamus through the mammillothalamic tract, and this link forms part of a hippocampal loop known as the Papez circuit (hippocampus → mammillary body → anterior nucleus → cingulate and parahippocampal gyri → hippocampus). The relative roles of these direct and indirect outputs to limbic cortex in the formation of declarative memories are still not understood.
The Amygdala Is Centrally Involved in Emotional Memories
One form of learning critical for survival is figuring out which situations are likely to be enjoyable and which are likely to be unpleasant or dangerous. The connections of the amygdala (see Fig. 23-7Fig. 23-8) suit it well for a role in such emotional learning. The amygdala receives inputs about things that are intrinsically pleasant or unpleasant (a full stomach, a painful jab) and is hardwired to have such events cause autonomic reactions (through the hypothalamus) and conscious feelings (through projections to limbic cortex). Information from the thalamus and unimodal sensory areas reaches the same neurons in the amygdala; pairing an otherwise neutral stimulus (e.g., the sight of chocolate or a scorpion) with pleasant or unpleasant outcomes causes activation of NMDA receptors, LTP, and an emotional response to subsequent experience with the previously neutral stimuli.
The Basal Ganglia Are Important for Some Forms of Nondeclarative Memory
Both the basal ganglia and cerebellum collaborate with motor areas of cerebral cortex as we learn to move more rapidly and accurately, as in learning to play a musical instrument; this collaboration is based on the long loops from cortex → basal ganglia or cerebellum → thalamus → cortex (see Fig. 19-2Fig. 20-7). The basal ganglia, however, have a broader role in learning patterns of behavior. We routinely make decisions about how to act based on subconsciously accumulated experiences that allow “educated guesses” about probable outcomes (e.g., deciding whether to bring an umbrella on the basis of what the sky looks like in the morning). Learning to make decisions this way is based largely on interconnections between the caudate nucleus and association cortex (see Fig. 19-4).
The Cerebellum Is Important for Some Forms of Nondeclarative Memory
The cerebellum has a special role in learning how to adjust movements to fit changing circumstances. This ranges from adjusting the gain of reflexes (e.g., the flocculus changes the gain of the vestibulo-ocular reflex to compensate for wearing eyeglasses) to modifying limb movements (THB6 Figure 20-25, p. 518). The widespread interconnections of the lateral cerebellar hemispheres with nonmotor areas of cerebral cortex indicate that they probably have a broader role in learning various mental skills as well (THB6 Figure 24-24, p. 630).
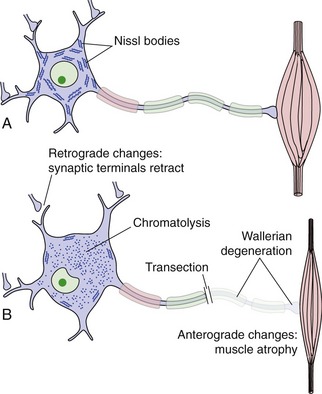
PNS Repair Is More Effective Than CNS Repair
If a neuron in the PNS or CNS is killed by disease or injury, it is generally not replaced (although stem cells, as described a little later, offer some hope for this in the future). If an axon is transected, the part separated from the cell body degenerates (Wallerian degeneration, Fig. 24-9). Trophic interactions of the neuron are disrupted, and there may be anterograde changes in cells it used to synapse on and retrograde changes in neurons that synapse on it. Depending on the neuron’s success in regrowing an axon, all of these changes may be reversed or the neuron (or even its synaptic partners) may die.