CHAPTER 44 Foramen Magnum Meningiomas
INTRODUCTION
Among the meningiomas of the posterior fossa, foramen magnum (FM) meningiomas deserve special consideration because of their characteristics in symptomatology, intriguing surgical anatomy, unique operative requirements, and outcome. When all meningiomas are considered, FM meningiomas have the worst outcome in terms surgical results and operative morbidity.1
DEFINITIONS
FM meningiomas arise at the dura of the craniocervical junction. There was much variability in the older literature regarding the definition of FM tumors. Some authors considered any tumor that was inserted at or passed through the FM as FM tumors.2 Others defined them as craniospinal tumors with anterior extension or spinocranial tumors with posterior extensions.3,4 In the older literature this area was extended down to C3 and even to C4.5–8 The anatomic definition of the FM region by George and colleagues9 gained general acceptance in recent publications. The definition of a FM meningioma is that of a meningioma having its attachment in the FM region. This region is clearly defined: the upper boundaries of the FM region are the lower third of the clivus and the occipital bone while the inferior margins are composed of the body, lamina, and the transverse process of the axis.10 The lateral boundaries are composed of jugular tubercles and the upper aspect of C2 laminas.10 Meningiomas that arise elsewhere but that have an extension into the FM are not included among FM meningiomas. Meningiomas that extend into the FM but with their main origins outside the FM area such as those of the hypoglossal canal, jugular foramen, middle and upper clivus, and cerebellopontine angle are considered as separate entities.
On the axial plane, the FM is also divided into ventral, lateral, and posterior compartments. Ventral and posterior compartments are separated by the coronal plane that spans the first dentate ligament and IX–XIIth cranial nerves.1 FM meningiomas located ventral to the neuraxis pose complex surgical problems to the surgeon due to their close proximity to the brain stem, lower cranial nerves, and the vertebral artery (VA), which increases the surgical risks and the narrow surgical corridor. Controversy still exists regarding the optimal surgical approach for ventral FM meningiomas.
SURGICAL ANATOMY
The Vertebral Artery
For an anatomic description, the VA is divided into four segments.11 The first, “pretransverse” (ostial, proximal) segment extends from its origin to the transverse process of the C6 vertebra. The second “transverse” segment runs inside the transverse processes of C6 through the axis.12 Of special interest for surgery around the FM are the third and fourth segments. The V3 segment is also called the “suboccipital segment” and extends from the transverse process of the axis to the dural penetration of the VA. This segment has three portions: vertical, horizontal, and oblique. The vertical portion lies between the transverse process of axis and atlas. The horizontal portion lies in the groove of the posterior arch of the atlas. In this groove, the atlantooccipital membrane covers the VA. The posterior atlantooccipital membrane stretches between the inferior border and the posterior surface of the occipital one and the posterior arch of the atlas. Ossification or calcification of the occipitoatlantal membrane with complete encasement of the VA in the posterior arch of atlas has been reported in 7.8% to 28% of cases.13,14 The oblique portion leaves this groove and climbs up to the dural penetration. The VA penetrates the atlantooccipital dura on its lateral aspect and becomes the V4 segment, which is also called the “intracranial segment.”11,14 The two V4 segments join at the midline to form the basilar artery (BA).
The VA has four vascular loops. At the transverse foramen of the axis, the artery turns posterolaterally, forming the inferior medial loop. Just next to this loop, the artery turns in the anterosuperior direction before entering the transverse foramen of the atlas, forming the inferior lateral loop. The superior lateral loop is formed where the vertical segment of V3 turns into the horizontal segment. Immediately before penetrating the dura, the VA forms the superior medial loop. This last loop turns around the condyle of the atlas and is attached tightly to the atlantooccipital articulation by the retroglenoid ligament. Therefore, the VA is fixed at its two extremes of the V3 segment: at the transverse foramen of atlas and at the dural penetration. At the same time, the V3 segment is the most mobile part of the VA and rotational movements change anatomic relationships.10 In the neutral position, the vertical and horizontal segments are perpendicular. After head rotation (and also after positioning for the anterolateral approach), the transverse processes of the atlas and axis are pushed away from each other and both segments become stretched and run parallel, separated by the posterior arch of the atlas.15
The V3 segment is cushioned by a venous plexus in its entire length. Parkinson16 first drew attention to its similarity to the cavernous sinus and Arnautovich17 nicknamed it the “suboccipital cavernous sinus.” At the transverse process of atlas, the artery, the surrounding venous cushion, and the periarterial autonomic neural plexus are covered by a periosteal ring. At the dural penetration, the periosteal sheath that covers the V3 segment in its horizontal portion invaginates into the dura and creates a second fibrous ring. At this level, the periosteal sheath is in continuum with both the outer layer of the dura and the adventitia of the VA. As discussed later, attempts at resection of meningiomas that invade this dural portion carry the risk of rupturing the VA.
The posterior circulation may be subject to variations and anomalies.11,18 In 40% of individuals there is an asymmetry of the VA size. In such instances the VA is most commonly larger on the left side.11 In 20% of the cases, the posterior inferior cerebellar artery (PICA) arises extracranially from the V3 or even V2 segments.19 Therefore the PICA must be identified and protected during surgery. Another rare finding is the proatlantal artery, which is a persistent embryonic anastomosis between the VA and the internal carotid artery (ICA). A persistent proatlantal artery is commonly associated with an atretic VA and an extracranial origin of the PICA.
Arterial Branches of the Vertebral Artery
There are several small branches of the V3 segment. The vertical portion has two constant branches.17 The first of these is the muscular branch which originates ventral to the anterior ramus of C2 nerve. It communicates with branches of the ascending pharyngeal artery. The second and also the largest branch of the V3 segment is the radiculomuscular branch originates below the transverse foramen of atlas and divides into radiculomedullar and muscular branches. Both of these branches are coagulated and divided during VA transposition. The horizontal portion also has 2 constant branches.17 The small muscular branch originates just superior to the transverse process of atlas and corresponds to the radicular artery of C2. It supplies surrounding muscles. This small muscular branch usually communicates with the occipital artery. The posterior meningeal artery originates from the posterosuperior surface of the superior medial loop and supplies the posterior fossa dura, posterior tentorium, and the squamous portion of the temporal bone. Anastomoses are reported with (1) branches from occipital artery, (2) the V2 portion of the VA through the anterior meningeal artery, (3) the ascending pharyngeal artery, (4) and the dorsal meningeal artery which is a branch of the meningohypophyseal trunk that arises form the ICA within the cavernous sinus. The posterior spinal artery may also arise from the horizontal portion, and in these cases, it enters the dura where the C1 root exits the spinal canal. The posterior spinal artery most commonly originates intradurally from the V4 segment or the PICA and is located medial to the most rostral attachment of the dentate ligament. Another branch that enters the posterior condylar canal is also reported.17Arterial branches of the V4 are PICA, the anterior spinal artery, and the anterior and posterior meningeal arteries. The major branch of the V4 segment is the PICA, which may also originate extradurally.19
HISTORY
The first case description of an ventral FM meningioma was provided in 1872 by Halopeau at autopsy.20 This was followed in 1922 by Frazier and Spiller’s21 first report of surgical intervention. Several other attempts were all complicated by intraoperative respiratory depression.22,23 Elsberg and Strauss4 performed the first successful surgical removal in 1925 with complete resolution of the patient’s symptoms. Surgical results on small cohorts were reported in the following decades, but meningiomas of the region were either clustered together with other tumors of the craniovertebral junction or with other meningiomas such as posterior fossa or clivus. In his review of the existing literature from 1924 to 1976, Yasargil documented 114 reported cases of FM meningiomas.24 The unique characteristics and challenges of FM meningiomas that separated them from other posterior fossa meningiomas were discovered early. The largest series and the most comprehensive analysis of FM meningiomas was provided by George and colleagues,25 and his work established FM meningiomas as a separate disease entity. In his analysis of the former literature, George identified three periods in the neurosurgical literature, mainly of the first attempts at surgery (1925–1950), then a period concerned with attempts to improve diagnosis (1950–1980) and the last one of technical improvements (1980–present).9 Microsurgery was a major step in decreasing mortality and morbidity but with their popularization and maturation in the last two decades, skull-base approaches and their results have dominated the literature.26 Skull-base techniques have significantly expanded the neurosurgical armamentarium by increasing our surgical capabilities and decreasing morbidity.27–31
INCIDENCE
FM meningiomas are rare and make up 0.3% to 3.2% of all meningiomas, 4% to 6.5% of posterior fossa meningiomas, and 8.6% of all spinal meningiomas.9,32,33 FM is the second most common site for posterior fossa meningiomas.24
Among lesions located at the FM, benign extramedullary tumors comprise one third.9,33–36 Among these lesions meningiomas are the most common tumor type and constitute about 70% of all benign tumors arising in this region.33,37 This is almost three times more frequent than the next most common tumor, neurinomas.1 Most meningiomas are intradural. However, there may be extradural extensions.9,33–36 Strictly extradural tumors are exceedingly rare. FM meningiomas are most commonly found in the fourth to sixth decades of life. FM meningiomas can also be observed in young children. Pediatric FM meningiomas are usually associated with neurofibromatosis.32,38,39 FM meningiomas in children are reported to have a poorer prognosis compared with those in adults, as the tumors tend to grow more rapidly and to a larger size, undergo malignant changes, and have a greater rate of recurrence.40 The female predominance observed in meningiomas in general also holds true for FM meningiomas. The female-to-male ratio is 2–4:1.9 FM meningiomas may present in patients with multiple meningiomas, and this may be a component of the neurofibromatosis type 2 (NF2) complex.
CLINICAL MANIFESTATIONS AND NATURAL COURSE OF THE DISEASE
The clinical manifestations of FM meningiomas are atypical and unpredictable. There are no symptoms or signs that are exclusively diagnostic of FM meningiomas. Before the common use of magnetic resonance imaging (MRI), these lesions were commonly discovered very late in their course, due to their slow growth inside a wide subarachnoid space. A significant delay is reported between the onset of the initial symptom and neurologic findings.35,41,42 In the older literature this period ranged from 20 days to 6.5 years.35,41,42 Newer studies report an average of 30 months between the onset of symptoms and the diagnosis.9 A relapsing and remitting clinical picture has been reported in a significant proportion of the patients and it is common for them to come to clinical attention after minor head–neck trauma.43 Before the frequent use of MRI, the most common first diagnoses in patients with FM tumors were cervical spondylotic myelopathy (12.5%–21%), multiple sclerosis (17.5%–18.8%), syringomyelia (15.8%), intramedullary tumor (14%), cervical disc herniation (12.5%), Arnold Chiari malformation (5.3%), carpal tunnel syndrome (5.3%), subacute combined degeneration (3.5%), amyotrophic lateral sclerosis (3.5%), normal pressure hydrocephalus (1.8%), and brachial plexus injury (1.8%).33,39 The increased availability of advanced neuroimaging has decreased this delay.
The most common symptom is occipital or posterior neck pain, and this presents as the initial symptom in 65.7% to 80% of patients.1,9,35,41,42,44 The pain usually presents in the morning and becomes worse with neck movement (especially extension), maneuvers that increase intracranial pressure or coughing.1,9 Hyperestesia in the C2 dermatome may also be seen with posterior cervical pain. Paresthesia in distal extremities is a late symptom. In advanced cases, spastic weakness in the extremities may be seen. This usually becomes manifest as loss of fine motor skills in the fingers. The presentation of a “rotating palsy,” that of a paresis that starts in one upper extremity and then progress to the ipsilateral lower extremity, then to contralateral lower extremity and finally to the contralateral upper extremity, strongly suggests a FM lesion.
Neurologic examination is commonly positive for ataxia, motor and sensory deficits, cerebellar signs, and lower cranial nerve palsies: Motor weakness is detected as a spastic paresis in the extremities with wasting of intrinsic hand muscles. A spastic gate may be found in more than half of the patients as well as hyperreflexia and a positive Babinski’s sign. Dissociated sensory loss and a Brown Sequard syndrome may be encountered. Lower cranial nerve deficits are uncommon in patients with FM meningiomas except for CN XI palsies, which are reported in up to 44% of patients.42 Findings include dysarthria, dysphagia, atrophy of the sternocleidomastoid or trapezius muscles, hoarseness, and Horner’s syndrome. Respiratory difficulties are a late finding.
PATHOLOGY
Most common histologic types are meningothelial, followed by psammomatous and fibrous meningiomas.24,45–47 The incidence of the meningotheliomatous histology ranges from 22% to 72%, while transitional type is seen in 17% to 28% and the psammomatous type in 11% to 22% of cases.1,33,42,44,48 No association has been reported between the histologic subtype and extent of resection for FM meningiomas.
DIAGNOSTIC STUDIES
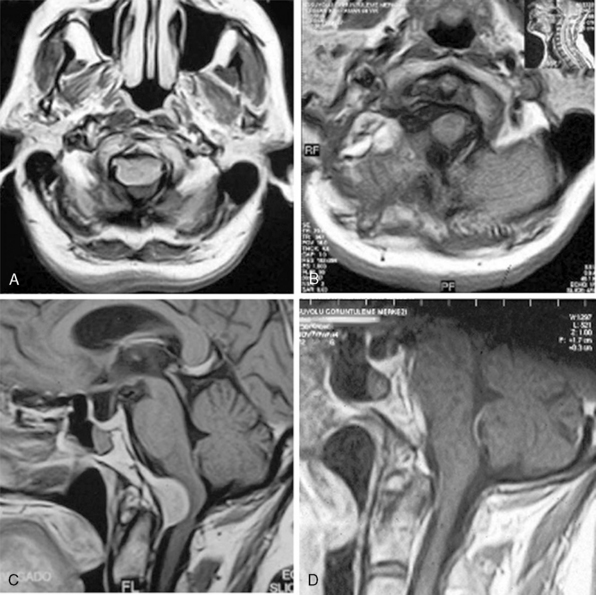
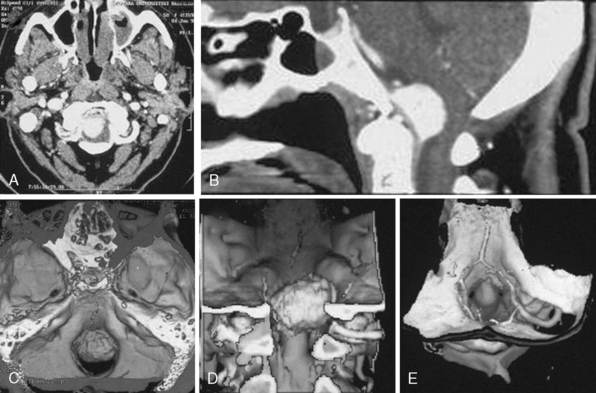
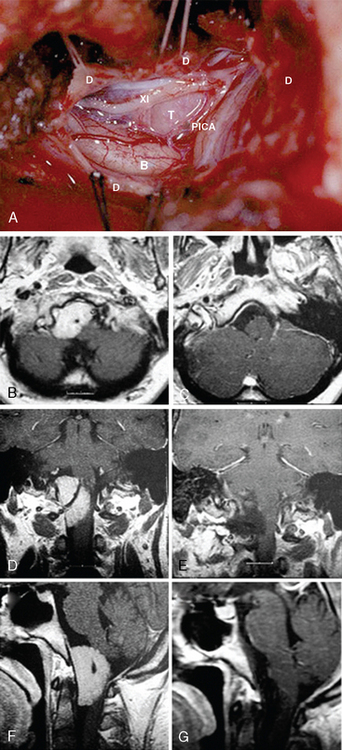
MRI and computed tomography (CT) constitute the basic workup in patients with FM meningiomas. Angiography is seldom required. The information provided by the MRI and CT is useful for the differential diagnosis (from other lesions in the FM) as well as surgical planning (such as the localization of the tumor both on axial and sagittal planes, extent of neuraxis compression and the nature of its displacement, presence of extradural extension, possible encasement of the VA, and presence of other coexistent pathologies such as hydrocephalus) (Figs. 44-1 and 44-2). Among benign extramedullary tumors, meningiomas are the most common pathology. Only a small proportion of meningiomas have an extradural extension. In cases that have an extradural extension, other common pathologies such as metastases and chordomas must be excluded.38,49 The differential diagnosis of intradural mass lesions at the FM includes intra-axial tumors (astrocytomas, ependymomas, subependymomas), extra-axial tumors (meningiomas, schwannomas, glomus jugulare tumors, teratomas, lipomas, hemangioblastomas), non-neoplastic cysts (epidermoid inclusion cysts, neurenteric cysts, arachnoid cysts, dermoid tumors), vascular lesions (cavernomas, giant thrombosed VA aneurysms), tonsillar herniation, and syringomyelia. The differential diagnosis of extradural mass lesions at the FM includes metastases, chordomas, osseous–cartilaginous tumors, sarcomas (including chondrosarcomas), congenital malformations of the craniovertebral (CV) junction, infections, proliferative arthropathies with associated degenerative disease (e.g., Rheumatoid pannus), and synovial cysts. Like meningiomas in other localizations, FM meningiomas appear iso- to slightly hypointense relative to brain parenchyma on T1-weighted images and enhance intensely after intravenous contrast injection. On T2-weighted images meningiomas appear as iso- to hyperintense and the high intensity is correlated with soft consistency of the tumor. Softer tumors are “suckable” and easier to remove surgically. Magnetic resonance angiography (MRA) and magnetic resonance venography (MRV) provide information on the status of arterial and venous structures. CT provides extra information on the presence of hyperostosis or calcifications. The tumor appears iso- to hyperintense and enhances intensely after contrast injection. Bone involvement is also clearly delineated in tumors that have an extradural component. Calcifications, which are seen in 10% of meningiomas, are also easily demonstrated on CT. Anatomic information on the condyles, jugular tubercle and the jugular bulb are also very valuable for preoperative planning, and three-dimensional reconstructions are very helpful in this regard. Plain radiographic studies are usually nondiagnostic in FM meningiomas; however, they may demonstrate bone destruction or hyperostosis in extradural tumors. Angiography is not routinely included in the preoperative workup of FM meningiomas. Vascular supply of FM meningiomas comes from both the vertebral and external carotid (and occasionally internal carotid) arteries, and a complete six- vessel angiogram is required for a complete study. Angiography is superior to MRI in evaluating vascular encasement.38
SURGICAL TREATMENT
Surgical Classification Schemes
Several authors devised classification schemes for FM meningiomas to aid in defining the surgical approach. Cushing and Eisenhard3 classified meningiomas at the craniovertebral junction into “craniospinal meningiomas” that originated from the basal groove at the lower third of the clivus, anterior to the medulla and projected caudally and “spinocranial meningiomas” that originated in the upper cervical area, posterior to the spinal cord and projected cranially into the cerebellomedullary cistern. This classification system is still used by some authors.1
Others described classification schemes according to the zone of dural attachment of the tumor on the axial plane. The boundaries of anterior, lateral, and posterior compartments on the axial plane were previously described. The meningioma may be located ventral (dural attachment on both sides of the anterior midline), ventrolateral (ventral to the dentate ligament), posterolateral (dorsal to the dentate ligament), or posterior (on both sides of the posterior midline). However, the surgical approaches of the posterior and posterolateral cases are the same, and most authors group posterolateral and posterior cases into one category. In their latest classification George and Lot9 differentiated among ventral, lateral, and posterior FM meningiomas.
A ventral FM meningioma is one of the most difficult types of meningiomas to treat. According to different studies, ventral and ventrolateral FM meningiomas comprise 68% to 98% of all cases.42 Most studies have grouped ventral and lateral meningiomas together. In different studies that have differentially analyzed ventral and lateral meningiomas have indicated that 4.7-85.7% of these are in fact anterior FM meningiomas.30,32,35,39,41,42,44,50,51 This wide variability may be due to a selection bias, and it is thought that the incidence of strictly anterior meningiomas is toward the lower end of this range. Ventral FM meningiomas are hidden deep in the craniocervical junction, surrounded by several important neurovascular structures. In strict ventral cases, the lower cranial nerves and the brain stem are usually displaced posteriorly and these vital structures cover the surgical path in a posterior approach. Posterolateral approaches were described, and preoperative classification aids in choosing the optimal approach.
The relation of the tumor to the VA is another factor that influences surgery. In tumors that are located below the VA, the lower cranial nerves are pushed posteriorly and superiorly, out of the surgical path, and no special effort is required for their protection. However, in meningiomas that grow above the VA, the displacement of the lower cranial nerves cannot be anticipated, and all of them need to be identified and protected during surgery. In rare cases, FM meningiomas can also infiltrate the dura at the level of dural penetration of the VA. As indicated earlier, the adventitia of the VA may be in continuum with the dura and resection of the tumor may result in vertebral artery tear. Bruneau and George10 have included all three of these parameters, namely the zone of dural insertion, compartment of development, and the relation of the tumor to the VA into their latest classification.
Choosing the Optimal Management Strategy
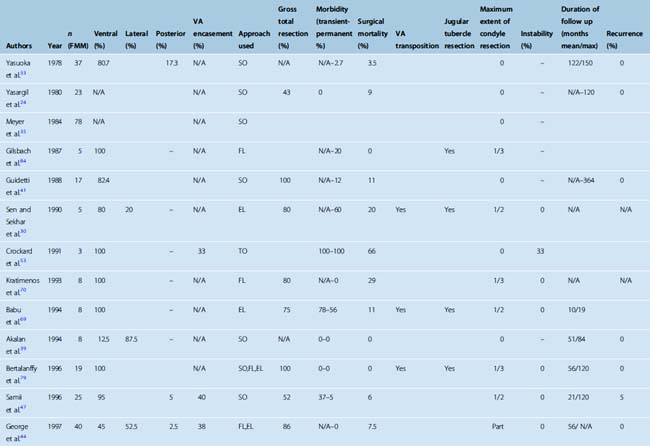
Surgery is the mainstay of treatment in FM meningiomas. Treatment of posteriorly located FM meningiomas is straightforward. These lesions are resected with a classical midline suboccipital approach with excellent results.33,44,45,47,48,50–52 The treatment of ventral FM meningiomas is more challenging. In different series, the incidence of ventral FM meningiomas varies between 12.5% and 100%. It should be kept in mind that most series group lateral and ventral cases together and that strictly ventral meningiomas are less commonly observed (Table 44-1). Controversy still exists about the optimal mode of treatment and the optimal surgical approach for FM meningiomas that are located ventral or ventrolateral to the neuraxis. The two most commonly used approaches for ventral and ventrolateral FM meningiomas are the far-lateral approach and the extreme lateral transcondylar approaches. Posterior midline approaches are also advocated by some surgeons; however, the use of anterior transoral approaches has been abandoned due to their limitations and high complication rate.53
Total resection was shown to have a statistically significant impact on favorable clinical outcome.1 Therefore the ultimate goal of surgery is to increase the extent of resection while minimizing morbidity. Since the era of microneurosurgery, there has been a major improvement in the understanding and surgical management of FM meningiomas. As in many other examples in neurosurgery, skull base surgery for FM meningiomas has evolved through three phases: an initial enthusiasm, a second phase of dissolution, and a third phase of maturation. Initial reports on the use of skull-base techniques in the treatment of FM meningiomas were very encouraging. These techniques were welcomed with much enthusiasm in the neurosurgical community, decreased the mortality and morbidity, and improved surgical outcome. In the following decade techniques were further developed, refined, and entered the routine practice. In the third phase, the advantages and limitations were more realistically appreciated. Initial studies have reported use of the suboccipital midline posterior approach with C1 laminectomy. Such a posterior approach is well suited for most ventral FM meningiomas, especially laterally placed lesions. However, small and strictly ventral lesions or others that have a significant extradural component, or others that grow above the VA, have proven much more difficult to resect with low morbidity. George and colleagues54 introduced the mobilization of the VA and advocated that the majority of FM meningiomas could be resected with a lateral suboccipital approach. Subsequent popularization of skull-base approaches (most importantly, the use of the far-lateral approach) has significantly increased the success rate in the surgical removal of FM meningiomas. Some authors adopted even more aggressive surgical strategies and have reported having achieved Simpson grade 1 resections, that is, excision of the dural attachment and removal of invaded bone with the extreme lateral approach.30 The authors rationalized their decision based on the published significant difference in recurrence rates of Simpson grade 1 and grade 2 resections. In contrast, there have been several reports indicating that the far-lateral transcondylar approach is not necessary and that all FM meningiomas could be resected with a midline posterior approach.55 Currently there is no scientific evidence on the gains, complication rates, and long-term follow-up of either approach and therefore a clear-cut comparison is not possible. We believe that the real situation lies somewhere between these two extremes. Accumulating evidence has shown that most FM meningiomas can in fact be resected with a far-lateral or posterior suboccipital approach.10,42,44,46,49,52–57 However, there is a minority of patients in whom the tumor is hidden from the surgeons view by the neuraxis. A partial resection of the occipital condyle in the far-lateral approach or the use of extreme lateral transcondylar approach provides the additional lateral view to approach the tumor more safely and effectively in this small subset of patients. Mobilization of the VA also requires a circumferential durotomy around the VA penetration and therefore at least a partial resection of the condyle and the superior articulating facet of the atlas.56 Many problems, however, still remain: How aggressive should the treatment be? Is there a role for adjuvant therapies? Therefore, the management strategies for FM meningiomas are still in evolution.
Treatment planning differs substantially between naïve and reoperated FM meningiomas. Results are less satisfactory, as resection rates are far lower and complication rates are higher.1,9,30,44 Meningiomas are surrounded by an arachnoid plane that separates them from surrounding neurovascular structures, and this plane is utilized during surgical resection to protect surrounding tissues from damage.1 The initial surgical attempt violates this arachnoidal plane, and reexplorations carry a much higher risk of damaging surrounding neurovascular structures. In such cases, a subtotal resection was useful for decompressing the tumor if improvement in neurologic function was anticipated after surgery. Alternatively, radiosurgery or radiotherapy may be considered in such cases.57
Surgical Approaches
Anterior approaches have been largely abandoned. Approaches to the FM range from posterior midline to lateral. According to the need for lateral exposure to increase the surgical field, one can use midline suboccipital, lateral suboccipital, retrocondylar, far-lateral, or extreme lateral transcondylar approaches or even combine them with different middle fossa approaches in the case of extradural extensions. Unlike in chordomas, which extensively invade the bony structures, meningiomas grow from an epicenter and mostly stay confined within anatomic boundaries and therefore are resected with more “standard” craniotomies rather than exotic modifications or combinations.58
Midline posterior approach
Posterior FM meningiomas, when they are located medial to the VA, are resected simply with a posterior midline suboccipital craniotomy. Goel and colleagues55 suggested that even ventral FM meningiomas could be resected with the median suboccipital approach. Their approach, however, involves the removal in part of the occipital condyle and therefore basically is a modification of the lateral approach, not a conventional posterior approach.
Anterior transoral approach
Use of the transoral–transpharyngeal approach for ventrally placed FM meningiomas has also been reported by few authors.53,59–61 The transoral–transpharyngeal approach has several drawbacks for management of intradural lesions. First, this is a strictly midline approach and lateral extensions of the tumors are difficult to address without modifications in the technique and total resection rates are low. The risk of cerebrospinal fluid (CSF) fistula and the increased risk of meningitis and pneumonia secondary to the contaminated surgical field remain major concerns among surgeons considering the use of transoral–transpharyngeal approach. Velopalatine insufficiency and craniocervical instability are also significant risks.
The far-lateral approach
As a lateral extension of the suboccipital craniotomy, the dorsolateral approach was first outlined in the book by Koos and colleagues in 1985.31 The “far-lateral” approach was then described in detail by Heros27,28 and the “posterolateral approach to FM” was described by Perneczky.29 With removal of the FM rim toward the condylar fossa, the approach provides an inferolateral view to the ventral surface of the brain stem without the need for parenchymal retraction (Fig. 44-3). Several different modifications have been described since, and in the hands of different surgeons the approach varies in the extent of condyle resection (if any at all) and exposure/mobilization of the VA.
The far lateral approach can be performed in the sitting,10,47 lateral, or park bench1,46,48,62 positions. The sitting position carries a high risk of air embolus and requires extra measures to prevent, detect, and treat this and is therefore used by few centers. Extreme care should be given not to flex the head, as compression on the already distorted neuraxis can be aggravated by such a maneuver. Posterior muscles are divided to expose the occipital bone, posterior arch of atlas, and the lamina of axis. An occipital craniotomy (up to the sigmoid sinus) is performed along with resection of the posterior arch of atlas (and axis depending on the extent of caudal extension of the tumor). Most lateral and ventral FMs can be resected with this approach without the need for any occipital condyle resection. However, when treating small and strictly ventral meningiomas the posteriorly pushed neuraxis may stay interposed between the surgeon and the tumor. In other cases, extradural extension of the tumor or VA encasement may necessitate a lateral shift in the angle of approach extension approach. In these instances, a partial resection of the occipital condyle to gain a more lateral view may be required. In our experience, a partial condylectomy, as a part of the far-lateral modification of the paramedian suboccipital approach, significantly increased the surgical corridor.48 Most authors have advocated that drilling less than one third of the condyle is sufficient, and quantitative studies have demonstrated that partial condylar resection provided a wider surgical field and decreased working distance.63,64 A wide condylar resection (more than the posterior two thirds of the condyle) carries the risk of craniocervical instability, but limited drilling of the condyle for meningioma resection is well tolerated.65 No instability or need for instrumentation was reported in large FM meningioma case series that included patients with resection of up to one half of the occipital condyle.1,44,48,50,52,55,62,66 The extent of resection can be judged peroperatively or with radiology (1%–51%: hypoglossal canal not opened, 50%–70%: resection extending to the hypoglossal canal, 70%–90%: resection beyond the hypoglossal canal, 90%–100%: almost complete or complete resection).65 Use of the extreme lateral approach, which requires even more extensive bone resection, does not result in craniospinal instability.65 To gain a more lateral view, the jugular tubercle may also be drilled but this maneuver carries the risk of damaging lower cranial nerves that are in the immediate vicinity.29,56
Encasement of the VA by the meningioma is reported in 33% to 59% of cases.44,47,48,51,53,55 Most FM meningiomas originate close to the area of dural entry of the VA.42 The adventitia of the VA is in direct continuation with the dura, and attempts at radical tumor excision may risk VA tear. Exposure and subsequent mobilization of the vertebral artery for protection and proximal control was pioneered by George and colleagues.54 The authors described medial transposition of the segment from C2 to the dural entry point. VA transposition is seldom indicated in the far lateral approach; however, exposure of the horizontal segment provides necessary proximal control of the VA. We use the transposition only for the extreme lateral approach.67 The technique for exposure of the VA has been described in detail in several publications. We do not use the muscles as a landmark when exposing the V3 segment and prefer use of osseous structures as landmarks for exposing the V3 segment.54,68 In our experience we have not encountered any VA transposition–related complications; however, this problem is cited in the literature. The venous plexus aids in the protection of the artery during surgical exposure. Bleeding from the plexus is easily controlled by packing and gentle compression. It should be kept in mind that coagulating the venous plexus carries the risk of occluding VA branches or even a hypoplastic VA.64
Sectioning of the dentate ligament and if necessary the C1 and C2 rootlets may reduce traction on the spinal cord.47 The arachnoidal plane will facilitate separation of the tumor from the VA. Dural attachment of the tumor must be resected or coagulated. The PICA and the anterior and posterior spinal arteries are frequently found stretched on the capsule. However, PICA may be embedded in the tumor. FM meningiomas frequently grow below lower cranial nerves and displace them superiorly. At the cranial pole of the tumor, they must be dissected away from the tumor.
Extreme lateral approach
The extreme lateral transcondylar approach is used to remove both intradural and extradural tumors located ventral and lateral to the brain stem at the craniovertebral junction. This approach was first described by Sen and Sekhar in 1990;30 however, several variations have been described.62 Although both approaches are aimed at a lateral exposure of the FM, far lateral and the extreme lateral transcondylar approaches are fundamentally different in the trajectory of the approach.64 The extreme lateral approach is an anterolateral exposure of the FM anterior to the sternocleidomastoid muscle and posterior to the jugular vein.64 Resection of the occipital condyle can be done in both approaches but in the extreme lateral approach. VA transposition is required to reach the occipital condyle.1,30,62,67,69,70
The extreme lateral approach involves a vertical retromastoid incision extending down to the neck to expose the VA within the foramen transversarium of axis. Occipital bone, arch and lateral mass of atlas, and the lamina of axis are exposed. A retrosigmoid craniotomy is performed, followed by unroofing of the sigmoid sinus and the jugular bulb and a posterior fossa craniectomy involving the FM. Laminectomy of the atlas and axis is performed depending on the caudal extension of the tumor. The VA is identified within the suboccipital triangle and unroofed at the foramen transversarium of atlas. The exposure and transposition of the V3 segment of the VA is a key step in the extreme lateral approach. This permits safe drilling of the atlanto-occipital joint and the jugular tubercle with less risk of injury to the VA, provides proximal control, and widens the surgical field. The dissection is performed subperiosteally under the operating microscope and requires detailed anatomic knowledge of the V3 segment as well as clinical expertise. For identification of the V3 segment we used bony landmarks.67 Use of muscular landmarks has also been described and used by other authors.64,71 Between atlas and axis the ventral ramus of the C2 nerve root passes lateral to the artery and at this portion (between the atlantooccipital membrane and the posterior fossa dura) the VA courses within a venous plexus. The exposure of the venous plexus greatly facilitates protection of the VA and minor bleeding form the plexus is easily controlled by packing with Surgicel and gentle compression. If present, extradural tumor removal is removed and invaded bone is drilled until healthy tissue is recognized. To mobilize the V3 segment, the posterior wall of the transverse process of atlas is drilled and the condylar veins (which anastomose the suboccipital cavernous sinus with the jugular bulb and vein) are divided. A gentle posteromedial translocation of the artery exposes the lateral mass of C1 and subsequently a durotomy to explore intradural tumor extension may be performed. Calcification of the periosteal sheath around the VA is a common finding.13,14 During transposition care should be taken to manipulate the artery gently and not to harm it by traction or pressure.30,54,68,72 Kinking of the artery may result in brain stem infarction.62,69 Small arterial branches off the vertical and horizontal segments are coagulated and divided. In rare occasions the PICA may arise extradurally, and this may potentially be confused with the muscular branch of the VA above the posterior arch of the atlas.13,14,73 The intradural phase follows in the same as in the far-lateral approach and tumor removal is followed by meticulous hemostasis and watertight dural closure.
Surgical Complications
Mortality rates from 0 to 66% are reported in large case series (see Table 44-1). Recent studies report surgical mortality rates of 0 to 3.6%.51 Transient and permanent overall complication rates range from 39.3% to and 7.1% to respectively. The most devastating of those are lower cranial nerve palsies and VA injury. Damage to the medulla is rarely reported. Potential complications of the extreme lateral transcondylar approach are injury to the VA, lower cranial nerves (especially the hypoglossal nerve), jugular bulb, or jugular vein.62,69 The hypoglossal canal is located in the anteromedial one third of the occipital condyle; it contains the jugular nerve, and the jugular bulb and the jugular vein are prone to injury during the removal of the condyle as the last anterior 30% of the condyle can be drilled only beneath or through the hypoglossal nerve.67
RADIOSURGERY
There is only very limited information in the literature regarding the role of radiosurgery in the treatment of FM meningiomas. Muthukumar and colleagues74 reported satisfactory gamma-knife radiosurgery results in five elderly patients: tumor growth control in five (100%) and reduction in tumor volume in one (20%) patients who were poor candidates for surgery. In two (40%) of these cases the gamma-knife was administered as an adjuvant treatment. Nicolato and colleagues75 reported successful use of the gamma-knife in one case of FM meningioma. There is also a reported successful use of the cyber-knife in one FM meningioma.76 With only few published studies and fewer than 10 reported patients, there is currently no clinical evidence to determine the optimal role of radiosurgery in the treatment of FM meningiomas. For meningiomas in other locations in the brain, the gamma-knife has proven effective in providing tumor growth control, and use of radiosurgery has resulted in changes in treatment protocols. One good example is the paradigm shift observed in the treatment of cavernous sinus meningiomas. Early attempts of aggressive surgical resection have resulted in high rates of morbidity and unexpectedly high rates of residual or recurrent tumors. Demonstration of low morbidity and satisfactory tumor growth control rates of gamma-knife radiosurgery in small cavernous sinus meningiomas has resulted in a change of treatment strategy from radical surgery to extracavernous resection followed by gamma-knife radiosurgery with equally good results.77 There is no scientific evidence to support an extrapolation from this experience to FM meningioma treatment. However; despite the lack of definitive evidence, gamma-knife radiosurgery is still advisable in cases in which a radical surgical strategy would significantly increase the risk of surgical morbidity, such as in VA invasion by the FM meningioma. Similarly, results of recurrent surgery are not satisfactory in FM meningiomas, and gamma-knife radiosurgery has become a widely accepted and preferred treatment in residual or recurrent FM meningiomas. Muthukumar and colleagues74 reported their satisfactory results in two cases of recurrent FM meningiomas. Still to be clarified is the role in primary treatment of FM meningiomas. As mentioned earlier, our clinic and others have used gamma-knife radiosurgery as the primary treatment with exclusive tumor growth control in all cases. Immediate vicinity to a compressed medulla is the main factor limiting the dose in FM meningiomas, and long-term results are still needed to ensure efficacy and safety.
RADIOTHERAPY
Radiotherapy is used as a palliative measure in inoperable cases or in the case of recurrence or regrowth. The probability of long-term progression-free survival after radiotherapy exceeded 90% and was comparable to the results of complete resection and radiosurgery.57
OUTCOME
FM meningiomas are rare, and most of the surgical series were blended together with other tumors of the FM or meningiomas of the posterior fossa (see Table 44-1). Therefore clear conclusions on outcome are not easily reached. Yasargil and colleagues,24 in their review of the 114 cases in literature operated between 1924 and 1976, found an operative mortality of approximately 13%, a good outcome in 69%, fair outcome in 8%, and a poor outcome in 10%. A low preoperative Karnofsky Performance Score (KPS), progressive clinical course, and quadriparesis were associated with increased operative risk and poor prognosis.1 Recurrence is a major problem in skull-base meningiomas.78 The work of Mathiessen and colleagues78 supported the view that subtotal resection was not more than a glorified biopsy. The 5-year recurrence rate was 4% for patients undergoing radical surgery (grades 1 and 2) and 25% to 45% for patients undergoing grade 3 or 4 operations. Follow-up periods longer than 5 years revealed that 16% of grade 1 and 20% of grade 2 patients had symptomatic recurrences, whereas a majority of grade 4 and 5 patients showed symptomatic progression.
AUTHORS’ CLINICAL EXPERIENCE
Microsurgical resection is the treatment of choice for FM meningiomas. We have treated 25 patients with microsurgery. Between January 1990 and January 2007, 25 patients were operated at our institution with a diagnosis of FM meningioma. Twenty-two of these cases have been previously reported.48 Nineteen (76%) of the patients were women and 6 were (24%) men. The median age was 47 (ranging from 18 to 74). Presenting signs and symptoms were headache and neck pain in 19 patients (76%), nausea and vomiting in 15 patients (60%), difficulty in swallowing in 8 patients (32%), monoparesis in 6 patients (24%), brachialgia and upper extremity paresthesias in 5 patients (20%), dysarthria in 4 patients (16%), tongue hemiatrophy and fasciculations in 4 patients (16%), quadriparesis in 3 patients (12%), and gait imbalance in 2 patients (8%). Median duration of symptoms was 12 months (ranging from 1 to 76 months). Two patients were asymptomatic at hospitalization but were diagnosed on radiological grounds at follow up for other lesions. Three patients were diagnosed with neurofibromatosis (12%). The tumor was located ventrally or ventrolaterally in 23 (92%) and posteriorly in 2 (8%) patients. Early in this series, a suboccipital, paramedian, retrosigmoid approach was performed in 3 patients (for 2 harboring posterior and 1 ventrolateral located tumors). For all the remaining 22 patients (88%) a far-lateral approach with limited (fewer than one third) resection of the occipital condyle was performed. Partial resection of the lateral mass of C1 was performed in all of the far-lateral approaches. On the contrary, we performed a limited drilling of the occipital condyle (none to fewer than one third) and/or of the lateral mass of the atlas (none to fewer than one half) when required, mostly for small ventral meningiomas. No atlanto-occipital instability was observed in any of our patients.
Surgical gross total removal was achieved in 24 of these 25 FM meningiomas (96%).
CONCLUSIONS
[1] Arnautovic K.I., Al-Mefty O., Husain M. Ventral foramen magnum meningiomas. J Neurosurg. 2000;92:71-80.
[2] Barre Y., Alfandary A., Phillipides O. Etude clinique et operatoire d`une tumeur du trou occipital. Rev Neurol. 1950;82:401-404.
[3] Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History and Surgical End Results. Springfield, IL: Charles C Thomas, 1938.
[4] Elsberg C.A., Strauss I. Tumors of the spinal cord which project into the posterior cranial fossa: Report of a case in which a growth was removed from the ventral and lateral aspects of the medulla oblongata and upper cervical cord. Arch Neurol Psychiatry. 1929;21:261-273.
[5] Heiskanen O. Benign extramedullary tumors in the high cervical region. Ann Chir Gynaecol Fenn. 1968;57:59-62.
[6] Lazorthes G., Espagno J. [Tumors of the occipital foramen]. Neurochirurgie. 1971;17:443-446.
[7] Lereboullet J., Puech P. Tumeurs sous-durales du trou occipital. Paris. 1940;21:607-616.
[8] Martin P., Kleyntjens F. Tumeurs sous durales du trou occipital. Rev Neurol. 1950;82:313-314.
[9] George B., Lot G. Foramen magnum meningiomas: A review from personal experience of 37 cases and from a cooperative study of 106 cases. Neurosurgery Quarterly. 1995;5:149-167.
[10] Bruneau M., George B. Foramen magnum meningiomas: detailed surgical approaches and technical aspects at Lariboisiere Hospital and review of the literature. Neurosurg Rev. 2008;31:192.
[11] George B., Laurian C. The Vertebral Artery. Pathology and Surgery. Vienna: Springer Verlag, 1987.
[12] Ozgen S., Pait T.G., Caglar Y.S. The V2 segment of the vertebral artery and its branches. J Neurosurg Spine. 2004;1:299-305.
[13] Abd el-Bary T.H., Dujovny M., Ausman J.I. Microsurgical anatomy of the atlantal part of the vertebral artery. Surg Neurol. 1995;44:392-400.
[14] de Oliveira E., Rhoton A.L.Jr, Peace D. Microsurgical anatomy of the region of the foramen magnum. Surg Neurol. 1985:293-352.
[15] Bruneau M., Cornelius J.F., George B. Antero-lateral approach to the V3 segment of the vertebral artery. Neurosurgery. 2006;58:ONS29-35. discussion ONS29–35
[16] Parkinson D. Cavernous sinus or lateral sellar compartment. J Neurosurg. 1996;85:190-191.
[17] Arnautovic K.I., al-Mefty O., Pait T.G., Krisht A.F., Husain M.M. The suboccipital cavernous sinus. J Neurosurg. 1997;86:252-262.
[18] Fine A.D., Cardoso A., Rhoton A.L.Jr. Microsurgical anatomy of the extracranial-extradural origin of the posterior inferior cerebellar artery. J Neurosurg. 1999;91:645-652.
[19] Margolis M.T., Newton R.H. Borderlands of the normal and abnormal PICA. Acta Radiol (Diagn). 1972;13:163-176.
[20] Hallopeau H. Note sur deux faits du tumeur du mésocéphale. Gaz Med Paris. 1874;3:111-112.
[21] Frazier C., Spiller W. An analysis of fourteen consecutive cases of spinal cord tumor. Arch Neurol Psychiatr (Chicago). 1922;8:455-498.
[22] Rhein J.H.V. Tumor in the region of the foramen magnum. Arch Neurol Psychiatr (Chicago). 1924;11:432-435.
[23] Abrahamson I., Grossmann M. Tumor of the upper cervical cord. J Nerv Ment Dis. 1923;57:342-363.
[24] Yasargil M., Mortara R., Curcic M. Meningiomas of basal posterior cranial fossa. In: Krayenbühl H., editor. Advances and Technical Standards in Neurosurgery. Vienna: Springer-Verlag; 1980:1-115.
[25] George B., Lot G., Velut S., Gelbert F., Mourier K.L. [French language Society of Neurosurgery. 44th Annual Congress. Brussels, June 8–12, 1993. Tumors of the foramen magnum]. Neurochirurgie. 1993;39(Suppl. 1):1-89.
[26] Yasargil M. Microneurosurgery. Stuttgart/New York: Thieme Verlag, 1996.
[27] Heros R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
[28] Heros R.C. The far lateral inferior suboccipital approach. In: Wilkins R., Rengachary S., editors. Neurosurgery Update. New York: McGraw-Hill; 1991:106-109.
[29] Perneczky A. The posterolateral approach to the foramen magnum. In: Samii M., editor. Surgery in and around the brain stem and the third ventricle. Stuttgart/New York: Thieme Verlag; 1986:460-466.
[30] Sen C.N., Sekhar L.N. An extreme lateral approach to intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
[31] Koos W.T.H., Spetzler R.F., Pendl G., Perneczky A., Lang J. Color Atlas of Microneurosurgery. Stuttgart: Thieme Verlag, 1985.
[32] Castellano F., Ruggiero G. Meningiomas of the posterior fossa. Acta Radiol. 1953;104:1-177.
[33] Yasuoka S., Okazaki H., Daube J.R., MacCarty C.S. Foramen magnum tumors. Analysis of 57 cases of benign extramedullary tumors. J Neurosurg. 1978;49:828-838.
[34] Guidetti B., Spallone A. Benign extramedullary tumors of the foramen magnum. Surg Neurol. 1980;13:9-17.
[35] Meyer F.B., Ebersold M.J., Reese D.F. Benign tumors of the foramen magnum. J Neurosurg. 1984;61:136-142.
[36] Sarat Chandra P., Jaiswal A.K., Mehta V.S. Foramen magnum tumors: a series of 30 cases. Neurol India. 2003;51:193-196.
[37] Scott E.W., Rhoton A.L.Jr. Foramen magnum meningiomas. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:543-568.
[38] Menezes A.H. Craniovertebral junction neoplasms in the pediatric population. Childs Nerv Syst. 2008;10:1173-1186. [Epub ahead of print].
[39] Akalan N., Seckin H., Kilic C., Ozgen T. Benign extramedullary tumors in the foramen magnum region. Clin Neurol Neurosurg. 1994;96:284-289.
[40] Sheikh B.Y., Siqueira E., Dayel F. Meningioma in children: a report of nine cases and a review of the literature. Surg Neurol. 1996;45:328-335.
[41] Guidetti B., Spallone A. Benign extramedullary tumors of the foramen magnum. Adv Tech Stand Neurosurg. 1988;16:83-120.
[42] Stein B.M., Leeds N.E., Taveras J.M., Pool J.L. Meningiomas of the foramen magnum. J Neurosurg. 1963;20:740-751.
[43] Howe J.R., Taren J.A. Foramen magnum tumors. Pitfalls in diagnosis. JAMA. 1973;225:1061-1066.
[44] George B., Lot G., Boissonnet H. Meningioma of the foramen magnum: a series of 40 cases. Surg Neurol. 1997;47:371-379.
[45] Sharma B.S., Gupta S.K., Khosla V.K., Mathuriya S.N., Khandelwal N., Pathak A., et al. Midline and far lateral approaches to foramen magnum lesions. Neurol India. 1999;47:268-271.
[46] Sekhar L.N., Wright D.C., Richardson R., Monacci W. Petroclival and foramen magnum meningiomas: surgical approaches and pitfalls. J Neurooncol. 1996;29:249-259.
[47] Samii M., Klekamp J., Carvalho G. Surgical results for meningiomas of the craniocervical junction. Neurosurgery. 1996;39:1086-1094. discussion 1094–5
[48] Pamir M.N., Kilic T., Ozduman K., Ture U. Experience of a single institution treating foramen magnum meningiomas. J Clin Neurosci. 2004;11:863-867.
[49] Osborn A.G. Brain tumors and tumorlike masses: Classification and differential diagnosis. In: Osborn A.G., editor. Diagnostic Neuroradiology. St. Louis, MO: CV Mosby; 1994:401-528.
[50] Boulton M.R., Cusimano M.D. Foramen magnum meningiomas: concepts, classifications, and nuances. Neurosurg Focus. 2003;e10:14.
[51] Bassiouni H., Ntoukas V., Asgari S., Sandalcioglu E.I., Stolke D., Seifert V. Foramen magnum meningiomas: clinical outcome after microsurgical resection via a posterolateral suboccipital retrocondylar approach. Neurosurgery. 2006;59:1177-1185. discussion 1185–7
[52] Marin Sanabria E.A., Ehara K., Tamaki N. Surgical experience with skull base approaches for foramen magnum meningioma. Neurol Med Chir (Tokyo). 2002;42:472-478. discussion 479–80
[53] Crockard H.A., Sen C.N. The transoral approach for the management of intradural lesions at the craniovertebral junction: review of 7 cases. Neurosurgery. 1991;28:88-97. discussion 97–8
[54] George B., Laurian C. Surgical approach to the whole length of the vertebral artery with special reference to the third portion. Acta Neurochir (Wien). 1980;51:259-272.
[55] Goel A., Desai K., Muzumdar D. Surgery on anterior foramen magnum meningiomas using a conventional posterior suboccipital approach: a report on an experience with 17 cases. Neurosurgery. 2001;49:102-106. discussion 106–7
[56] Matsushima T., Ikezaki K., Nagata S., Inoue K., Natori Y., Fukui M., et al. Microsurgical anatomy for lateral approaches to the foramen magnum: With special reference to the far-lateral approach and the transcondylar approach. In: Nakagawa E., editor. Surgical Anatomy for Microneurosurgery. Tokyo: SciMed Publications; 1995:81-89.
[57] Mendenhall W.M., Morris C.G., Amdur R.J., Foote K.D., Friedman W.A. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer. 2003;98:1473-1482.
[58] Pamir M.N., Ozduman K. Tumor-biology and current treatment of skull-base chordomas. Adv Tech Stand Neurosurg. 2008;33:35-129.
[59] Miller E., Crockard H.A. Transoral transclival removal of anteriorly placed meningiomas at the foramen magnum. Neurosurgery. 1987;20:966-968.
[60] Bonkowski J.A., Gibson R.D., Snape L. Foramen magnum meningioma: transoral resection with a bone baffle to prevent CSF leakage. Case report. J Neurosurg. 1990;72:493-496.
[61] Imamura J., Ikeyama Y., Tsutida E., Moroi J. Transoral transclival approach for intradural lesions using a protective bone baffle to block cerebrospinal fluid pulse energy–two case reports. Neurol Med Chir (Tokyo). 2001;41:222-226.
[62] Salas E., Sekhar L.N., Ziyal I.M., Caputy A.J., Wright D.C. Variations of the extreme-lateral craniocervical approach: anatomical study and clinical analysis of 69 patients. J Neurosurg. 1999;90:206-219.
[63] Spektor S., Anderson G.J., McMenomey S.O., Horgan M.A., Kellogg J.X., Delashaw J.B.Jr. Quantitative description of the far-lateral transcondylar transtubercular approach to the foramen magnum and clivus. J Neurosurg. 2000;92:824-831.
[64] Rhoton A.L.Jr. The far-lateral approach and its transcondylar, supracondylar and paracondylar extensions. Neurosurgery. 2000;47:s195-s209.
[65] Bejjani G.K., Sekhar L.N., Riedel C.J. Occipitocervical fusion following the extreme lateral transcondylar approach. Surg Neurol. 2000;54:109-115. discussion 115–6
[66] Pirotte B., David P., Noterman J., Brotchi J. Lower clivus and foramen magnum anterolateral meningiomas: surgical strategy. Neurol Res. 1998;20:577-584.
[67] Ture U., Pamir M.N. Extreme lateral-transatlas approach for resection of the dens of the axis. J Neurosurg. 2002;96:73-82.
[68] George B., Dematons C., Cophignon J. Lateral approach to the anterior portion of the foramen magnum. Application to surgical removal of 14 benign tumors: technical note. Surg Neurol. 1988;29:484-490.
[69] Babu R.P., Sekhar L.N., Wright D.C. Extreme lateral transcondylar approach: technical improvements and lessons learned. J Neurosurg. 1994;81:49-59.
[70] Kratimenos G.P., Crockard H.A. The far lateral approach for ventrally placed foramen magnum and upper cervical spine tumours. Br J Neurosurg. 1993;7:129-140.
[71] Matsushima T., Natori Y., Katsuta T., Ikezaki K., Fukui M., Rhoton A.L. Microsurgical anatomy for lateral approaches to the foramen magnum with special reference to transcondylar fossa (supracondylar transjugular tubercle) approach. Skull Base Surg. 1998;8:119-125.
[72] Bertalanffy H., Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815-821.
[73] Lang J. Craniocervical region, surgical anatomy. Neuro Orthop. 1987;3:1-26.
[74] Muthukumar N., Kondziolka D., Lunsford L.D., Flickinger J.C. Stereotactic radiosurgery for anterior foramen magnum meningiomas. Surg Neurol. 1999;51:268-273.
[75] Nicolato A., Foroni R., Pellegrino M., Ferraresi P., Alessandrini F., Gerosa M., et al. Gamma knife radiosurgery in meningiomas of the posterior fossa. Experience with 62 treated lesions. Minim Invasive Neurosurg. 2001;44:211-217.
[76] Bhatnagar A.K., Gerszten P.C., Ozhasaglu C., Vogel W.J., Kalnicki S., Welch W.C., et al. CyberKnife frameless radiosurgery for the treatment of extracranial benign tumors. Technol Cancer Res Treat. 2005;4:571-576.
[77] Pamir M.N., Kilic T., Bayrakli F., Peker S. Changing treatment strategy of cavernous sinus meningiomas: experience of a single institution. Surg Neurol. 2005;64((Suppl. 2):S58-S66.
[78] Mathiesen T., Lindquist C., Kihlstrom L., Karlsson B. Recurrence of cranial base meningiomas. Neurosurgery. 1996;39:2-7. discussion 8–9.
[79] Bertalanffy H., Gilsbach J.M., Mayfrank L., Klein H.M., Kawase T., Seeger W. Microsurgical management of ventral and ventrolateral foramen magnum meningiomas. Acta Neurochir Suppl. 1996;65:82-85.
[80] Roberti F., Sekhar L.N., Kalavakonda C., Wright D.C. Posterior fossa meningiomas: surgical experience in 161 cases. Surg Neurol. 2001;56:8-20. discussion 20–1
[81] Nanda A., Vincent D.A., Vannemreddy P.S., Baskaya M.K., Chanda A. Far-lateral approach to intradural lesions of the foramen magnum without resection of the occipital condyle. J Neurosurg. 2002;96:302-309.
[82] Pamir M.N., Kılıç T., Özduman K., Türe U. Experience of a single institution treating foramen magnum meningiomas. J Clin Neurosci. 2004;11:863-867.
[83] Margalit N.S., Lesser J.B., Singer M., Sen C. Lateral approach to anterolateral tumors at the foramen magnum: factors determining surgical procedure. Neurosurgery. 2005;56:324-336. discussion 324–36
[84] Gilsbach J.M., Eggert H.R., Seeger W. The dorsolateral approach in ventrolateral craniospinal lesions. In: Voth D., von Goethe J.W., editors. Diseases in the Craniocervical Junction. Berlin: Walter de Gruyter; 1987:359-364.
[85] Parlato C., Tessitore E., Schonauer C., Moraci A. Management of benign craniovertebral junction tumors. Acta Neurochir. 2003;145:31-36.