Chapter 5 Food allergy/intolerance
AETIOLOGY
Food reactions can be divided into toxic and non-toxic reactions.1,2 Toxic food reactions are rare and usually involve food toxins that may be naturally present in food or a consequence of food processing, contaminants or additives, such as aflatoxin found in contaminated grains and peanuts. In the modern world reactions are rare due to diet variety and food-processing standards. They are dose-dependent reactions and have the same effect on everybody. Non-toxic food reactions can be further divided into immune- and non-immune-mediated reactions. Reactions that involve immune activation are mediated by immunoglobulins, especially immunoglobulin E (IgE) but also possibly IgG, IgA and IgM.2 Non-immune-mediated food hypersensitivity reactions are often termed ‘food intolerances’ and include fructose and lactose intolerance. There is also another general category called ‘undefined’. As the name suggests there is little information about this category, which includes idiosyncratic and psychosomatic food reactions.
Food allergy
Most severe food allergies are IgE-mediated.3 IgE is associated with receptors on mast cells, fixed cells in the mucosa and skin, and basophils in the blood,2 and conjugation with an allergen leads to cell granulation and the subsequent release of inflammatory mediators. Vasodilation, exudation, smooth muscle contraction and mucus secretion (largely due to histamine) are common consequences. The greatest concern about an IgE-mediated food allergy is the chance that it will culminate in anaphylaxis. Common food culprits include fish, eggs, cow’s milk and peanuts.4 It is usually a reaction to the protein component of the food such as casein and gluten; if the patient is reacting to another food component, such as lactose, it is more likely to be food intolerance. Other less well-defined food allergies may also occur; these may be IgG-, IgM- or IgA-mediated.
Food intolerance
Food intolerances are much more insidious and often cause delayed symptoms. Food intolerances can be enzymatic, pharmacological or idiosyncratic in nature and are generally thought to be non-immune activated.2,4,5 Below is an outline of the two most common food intolerances: lactose and fructose.
Lactose intolerance
Lactose intolerance is due to the inability of the body to produce enough lactase to break down the lactose in milk. If lactose is undigested it will pass through to the large colon where it is acted on by colonic flora, causing pain, bloating and osmotic diarrhoea. Lactose is a disaccharide and is metabolised by β-galactosidase (lactase is a subclass of this) to glucose and galactose. Lactose maldigestion effects up to 20% of Caucasians,6,7 but incidence in some ethnic groups (African, Asian and those from the Baltic states and the Mediterranean) is up to 60%.6 A recent American study reported the prevalence of lactose intolerance in irritable bowel syndrome to be between 17 and 24%.8 Lactase deficiency can also be transitory (gastroenteritis) or as a result of mucosal damage (coeliac disease or gastroenteritis).
Fructose intolerance
Fructose is a six-carbon monosaccharide. It is ingested as a monosaccharide, as the disaccharide sucrose (glucose + fructose) or in polymerised forms such as oligosaccharides and polysaccharides.9 If the degree of polymerisation (DP) is < 10, they are usually referred to as fructo-oligosaccharides and if the DP is ≥ 10 they are usually called inulins.9 Another form of dietary fructose is the galacto-oligosaccharides (fructose + glucose + galactose), usually present as raffinose. Additional substances also poorly absorbed and readily fermented are:
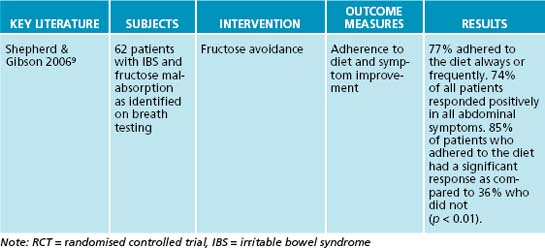
Fructose intolerance may be a primary or secondary condition. Hereditary fructose intolerance is a rare autosomal recessive disorder that is due to a deficiency of the liver enzyme fructose-1,6-biphosphate aldolase.10,11 It is particularly dangerous and can result in vomiting, failure to thrive, hypoglycaemia and liver failure with jaundice and bleeding in children.10 Secondary fructose intolerance is quite different and much more common. It is usually due to abnormalities in the expression of GLUT5.12 Recent research suggests that it affects 30% of the population.13 If fructose is not absorbed in the small intestine it reaches the distal end of the small intestine and the colon where it is fermented by colonic flora to produce hydrogen and carbon dioxide.9 Fructose is fermented especially quickly, so there is not enough time for gas to be further metabolised or absorbed, increasing intralumen pressure and producing an osmotic effect. Abdominal distension, pain, flatulence and diarrhoea may result. Fructose malabsorption is associated with gastro-oesophageal reflux, small intestinal bacterial overgrowth and depression.12,14
RISK FACTORS
Genetics
It appears that genetic predisposition is a strong determining factor in allergic disease. There is an 11–13% risk of developing allergies if there is no parental history, a 20–30% risk if the patient has one allergic parent and 40–60% risk if both parents are allergic.5 Twin studies show that environmental factors are important in the development of atopic disease.15–18
Gastrointestinal mucosal hyperpermeability
The mucosal barrier in the small intestine is comprised of epithelial cells held together by tight junctions. Various cellular and chemical factors, including extremes of pH, mucus, bile salts, brush border enzymes, together with innate and adaptive immune responses also help to ensnare pathogens or render them harmless.3 Many factors, including gastrointestinal viral infections and stress, appear to increase intestinal permeability.17 If this intestinal barrier is compromised proteins, pathogens and antigens may pass through the intestinal wall. A recent study conducted in 20 patients with food allergies and 21 patients with food sensitivities found that they all had increased intestinal permeability as diagnosed by a lactulose/mannitol test.19 It was also found that the more significant the permeability, the more severe the allergies/intolerances were.
Maternal consumption and early consumption of allergenic foods
Consumption of allergenic foods by the mother during gestation and/or lactation may predispose the child to food allergy.5 This includes foods the mother is sensitive to or common allergenic foods if the mother has any allergic conditions herself. Similarly, consumption of common allergic foods by the child at an early age may also increase risk. A recent meta-analysis found that a hypoallergenic diet during gestation was useful for the prevention of allergic disease in high risk infants.20 The authors also found that the best dietary prevention was breastfeeding for at least 4 to 6 months, together with solid food and cow’s milk avoidance for 4 months.
Lifestyle factors
Factors such as eating habits, meal frequency, lack of exercise, poor sleep and use of analgesic medication are often thought to increase the likelihood of food sensitivities, or increase the intensity of symptoms in some individuals. A recent Norwegian study, however, found that there was no difference between these factors in a group of adults with abdominal discomfort, self-attributed to food intolerance, and a placebo group.21 Stress may be an added risk factor for the development of food sensitivities. Stress (physical, biochemical, psychological) induces the central production of corticotrophin-releasing hormone, which in turn suppresses vagal activity.22
CONVENTIONAL TREATMENT
There is no particular conventional medical treatment available for IgG-mediated food allergies or food intolerances. Patients with IgE-mediated food allergies are often prescribed epinephrine and antihistamines, and cromoglycate may also be given.5,23 Strict dietary avoidance is usually recommended for all known food intolerances or allergies and hyposensitisation may be offered.
KEY TREATMENT PROTOCOLS
Case-taking assessment
The first protocol is to assess the particulars of the suspected digestive intolerance/allergy.
A range of questions should first be asked of the patient, such as:
A food/symptom diary is often useful in identifying potential allergens or intolerances.
Identify and remove offending foods and substances
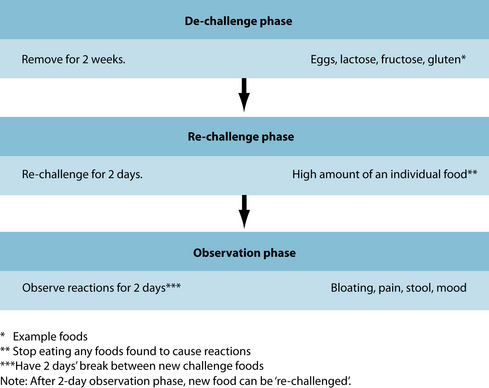
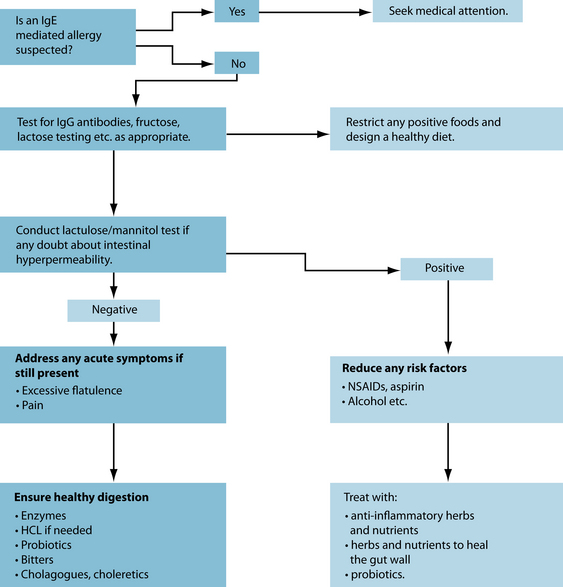
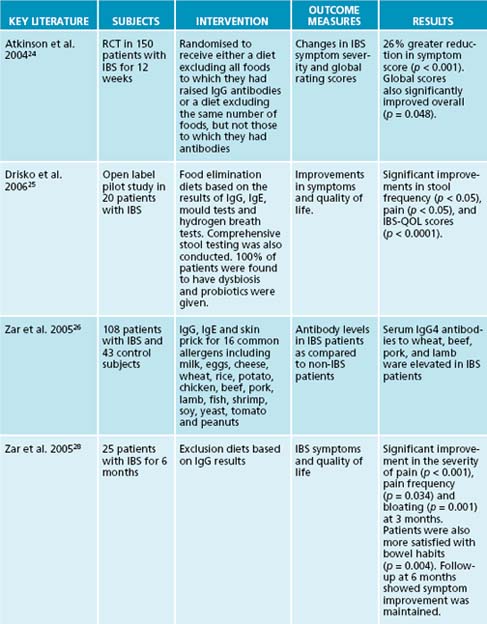
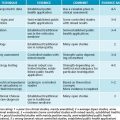
There are various ways to identify problematic foods and substances, depending on what foods are suspected. A de-challenge re-challenge diet is ideal to assess this (see Figure 5.1). Below is a summary of tests that are useful for the identification of food allergies and intolerances:
Once a food has been identified it must be removed from the diet (see Figure 5.1). In the case of IgG-triggered reactions, most of these foods need to be avoided only for a period of time until the reason for their existence is rectified. They can be due to a myriad of causes such as intestinal hyperpermeability, immune dysregulation and poor digestive function. This is also the case for secondary fructose and lactose malabsorption. Foods that provoke an IgE reaction, however, need to be thoroughly avoided.
Design an appropriate healthy diet
Maintenance of a healthy diet is crucial. Many people who have suffered from long-term food allergies or hypersensitivities have self-restricted their diet, in turn leading to various nutrient deficiencies. This is a perilous situation and one that needs addressing immediately if present. Deficiencies of protein, calcium, zinc, iron, vitamin B12 and magnesium are common, and a diet should always be designed with this in mind (see Appendix 4 for nutritional chart). Problematic foods must be avoided and the patient must also be offered as many alternatives as possible as the treatment will often fail if the patient finds compliance difficult.
For patients with lactose intolerance, the severity of symptoms depends on how much lactase is being produced by the small intestine. Many people can tolerate up to 7 g and many up to 12 g.6 This is important when designing a diet for a lactose-intolerant patient as it is not necessary to take patients off all dairy foods. It is also essential to understand hexose transport in the gastrointestinal tract in order to design a diet for fructose intolerant individuals. GLUT5 is a fructose transporter that is responsible for moving fructose across the brush border; it has a low capacity but is present along the whole length of the small intestine.12,29 It is this mechanism that is often deficient in fructose intolerance. Glucose enhances the absorption of fructose by its co-presence in the small intestine. GLUT2 (a low-affinity transporter that will carry glucose, fructose and galactose, found on the basolateral membrane) is shunted into the brush border to facilitate the diffusion of glucose.12,30 This in turn means that higher luminal concentrations of glucose are taken up by the cells via an active process, which in turn activates a system that can more efficiently take up all hexoses including fructose.12 This becomes very important when designing a diet for fructose intolerance as foods with an amount of glucose equal to or higher than the amount of fructose can be included. Hence it is only foods with higher fructose than glucose that are avoided. See Tables 5.1 and 5.2 for a list of foods to avoid if intolerant to lactose or fructose.
| Milk—cow’s, sheep’s, goat’s. More than ½ a cup at any serving. |
| Yoghurt—more than 100 g a serving (1/2 an average tub). |
| Ice-cream |
| Cream cheese |
Table 5.2 High fructose foods to avoid (fructose higher than glucose)9,31
| Fruits—apple, coconut (also coconut milk and cream), grape, guava, honeydew melon, mango, nashi fruit, paw paw/papaya, pear, quince, star fruit, tomato and watermelon. Also all fruit juice, fruit juice concentrate, dried fruit and tinned fruit. |
| Vegetables—Lebanese cucumber, sweet potatoes |
| Other—tomato sauce, tomato paste, chutney, relish, plum sauce, sweet and sour sauce, BBQ sauce, high fructose corn syrup, fructose, honey and fortified wines. Inulin and fructo-oligosaccharides should also be avoided. |
Note: Spring onion, garlic and leek contain longer chains of fructans and may therefore be easier to tolerate for some. Rye, barley, banana and lettuce all contain fructans; however, they appear to be tolerated by most patients.9
If the patient is avoiding fructose and lactose, or lactose only, it is probably best to avoid fructans, galactans (galacto-oligosaccharides such as raffinose and stacchyose) and polyols (sorbitol, xylitol, mannitol, maltilol) as well.12 While glucose enhances the absorption of fructose it does not enhance the absorption of fructans, galactans and polyols.
Reduce inflammation
While there is excessive gut inflammation driven by food hypersensitivities, the small intestine does not get a chance to repair.1 Inflammation causes increased local immune activation, which further damages the gut mucosa and potentially leads to more sensitivities. Inflammation also increases gut permeability, therefore increasing the amount of food antigens that can cross the bowel wall and provoke an immune response.19
Glutamine appears to down-regulate inflammatory mediators in the gastrointestinal system by stimulating the protective stress response in gut cells.32 The anti-inflammatory effects of fish oil have been touted for some time now and these also appear to be relevant to the gut. Although no clinical trials have investigated the effects of fish oil in food intolerance/allergy, randomised clinical trials have demonstrated anti-inflammatory effects in other gastrointestinal disorders such as ulcerative colitis.33
Many constituents of C. recutita have been found to be anti-inflammatory, including the flavonoids apigenin, apigetrin, rutin and quercetin. C. recutita is also antispasmodic, choleretic and antioxidant;34 however, clinical trials need to be conducted to confirm C. recutita’s role as an anti-inflammatory in the gut. U. dioica is a traditional treatment for inflammation and allergy. The leaf extract IDS 30 was shown to reduce nuclear factor kappa B (NFkB), leading to reductions in cyclooxygenase (COX) and lipooxygenase (LOX) reactions in vitro.35 The extract may also reduce tumour necrosis factor alpha (TNF-alpha).36 Curcuma longa and in particular its chief constituent, curcumin, inhibit multiple inflammatory mediators such as NFkB, cyclooxygenase-2 (COX-2), LOX and inducible nitric oxide synthase (iNOS).37 Clinical trials of C. longa’s anti-inflammatory effects in allergy are lacking; however, these effects are biologically plausible due to the plant’s demonstrated efficacy in inflammatory bowel disease.38,39
Recent research has shown that inflammation in the small bowel is frequently caused by an immunological cytokine TNF-alpha.40 It is this cytokine that causes an increase in gastrointestinal permeability, leading to leaky gut syndrome. Both fish oil and C. longa have been shown to inhibit TNF-alpha.41–44
Repair mucosal hyperpermeability
This is an essential part of the process as a damaged gut wall leads to further inflammation. Limit alcohol, antibiotics, aspirin and other non-steroidal anti-inflammatory drugs if possible as they all damage the gut wall.45–47 Additionally, encourage reduced exposure to xenobiotics such as pesticides and insecticides as early in vitro evidence suggests they may increase intestinal permeability.48,49 Support liver function and increase antioxidant status by increased consumption of fruit and vegetables. Encourage the consumption of cruciferous vegetables such as broccoli, cabbage, Brussels sprouts and cauliflower as they all support healthy liver function.50
Glutamine, zinc, slippery elm and probiotics may help to heal a damaged gut wall. Glutamine has also been shown to decrease intestinal permeability in animal models51,52 and two recent randomised clinical trials have demonstrated promising results.53,54 Zinc sulfate (110 mg three times a day, for 8 weeks) decreased intestinal hyperpermeability in Crohn’s disease patients in remission;55 however, zinc supplementation for intestinal permeability in food intolerance/allergy patients has not been studied to date. The dose in this study is extremely high and should only be used for short periods of time. Slippery elm is also a prebiotic, which will encourage the growth of healthy bacteria. Probiotics including Lactobacillus spp. and Bifidobacterium spp. will also help to heal the gut wall and may also help to prevent bacterial translocation. Probiotics have been shown to improve immunity, decrease allergies and food intolerances, normalise bowel function, reduce intestinal inflammation and decrease intestinal permeability.56–58
Reduce flatulence and abdominal spasm
Herbs such as Mentha X piperita, Zingiber officinale, Foeniculum vulgare and Melissa officinalis are useful to decrease abdominal spasm and flatulence.59
MO piperita is used mainly as a whole plant extract, either as a tea, tincture or fluid extract. It is antispasmodic, antiemetic, antimicrobial, cholagogue, carminative and bitter.34 Peppermint oil contains on average between 35 and 55% menthol,34 which is thought to be largely responsible for the plant’s spasmolytic activity.60 M. piperita appears to relax the sphincter of Oddi and inhibits the movement of calcium across the cell membrane.60,61 Nine human studies (n = 269) found that M. piperita (0.1–0.24 mL) significantly reduced spasm of the smooth muscle of the gastrointestinal tract.62 M. piperita has also been shown to reduce histamine release in vitro.63 Z. officinale has a spasmolytic effect on the gastrointestinal tract also via a calcium antagonist mechanism.64 Z. officinale is also antiemetic and anti-inflammatory.59 A combination of Foeniculum vulgare and C. recutita has been shown to significantly reduce infantile colic in 93 breastfed infants in a randomised clinical trial after 1 week.65 Clinical studies are needed to assess the spasmolytic and carminative effects of these herbs in adults.
Ensure healthy gastrointestinal function
Although no research is currently available to support the following protocols, they describe a very traditional way of treating the digestive system. Bitters, cholagogues and choleretics are employed to facilitate and enhance digestion.66 Bitter digestives may be useful to ensure healthy stomach function. These include Gentiana lutea, Taraxacum officinale radix and Hydrastis canadensis.59 Choleretics are substances that improve the production of bile by the liver. These could be useful if bile production is compromised; consider Berberis vulgaris, Taraxacum officinale radix, Cynara scolymus, Silybum marianum, Curcuma longa and Hydrastis canadensis.59,66 A cholagogue may also be helpful. Cholagogues are substances that facilitate the release of bile from the gallbladder. They include Gentiana lutea, Berberis vulgaris, Cynara scolymus, Rumex crispus and Iris versicolor.59,66 See Table 5.3, summarising the actions for relevant herbs.
Table 5.3 ‘Digestive’ herbal medicines: summary of main actions59
| HERB | ACTIONS |
|---|---|
| Berberis vulgaris | Bitter, choleretic, cholagogue |
| Chamomilla recutita | Anti-inflammatory, bitter, carminative, spasmolytic |
| Curcuma longa | Anti-inflammatory |
| Cynara scolymus | Antiemetic, bitter, choleretic, cholagogue, hepatoprotective, hepatic-trophorestorative |
| Foeniculum vulgare | Appetite stimulating, carminative, digestive, spasmolytic |
| Gentiana lutea | Antiemetic, bitter, cholagogue |
| Hydrastis canadensis | Anti-inflammatory, bitter, choleretic |
| Iris versicolor | Cholagogue |
| Melissa officinalis | Carminative, spasmolytic |
| Mentha piperita | Antiemetic, bitter, carminative, cholagogue, spasmolytic |
| Rumex crispus | Cholagogue, laxative |
| Silybum marianum | Antioxidant, choleretic, hepatoprotective, hepatic-trophorestorative |
| Taraxacum officinale radix | Bitter, choleretic, laxative |
| Urtica dioica | Antiallergic, anti-inflammatory |
| Zingiber officinale | Antiemetic, anti-inflammatory, carminative, digestive stimulant, spasmolytic |
INTEGRATIVE MEDICAL CONSIDERATIONS
There are very few integrative medical therapies that can be considered at this present time. Therapies such as homoeopathy and kinesiology are often utilised by patients with food allergies or intolerances; however, there is no current research to support this use. There is a small amount of research to support the use of traditional Chinese medicine in the treatment of asthma and food allergy and two double-blind clinical trials are currently underway in the United States of America to assess the benefit of two different traditional Chinese medicine herbal products.67
Example treatment
Dietary and lifestyle modification
Dietary modification
The low fructose and lactose diet could be prescribed, advising her to avoid foods that contain fructose in higher or equal amounts to glucose, including apple, coconut (also coconut milk and cream), grape, guava, honeydew melon, mango, nashi fruit, paw paw/papaya, pear, quince, star fruit, tomato, and watermelon. She should also avoid fruit juice, fruit juice concentrate, dried fruit and tinned fruit.9,31 Vegetables such as Lebanese cucumber and sweet potatoes need to be avoided, along with condiments and miscellaneous objects such as tomato sauce, tomato paste, chutney, relish, plum sauce, sweet and sour sauce, BBQ sauce, high fructose corn syrup, fructose, honey and fortified wines. Stone fruits such as peaches, plums and apricots contain sorbitol, which can have similar effects on the gastrointestinal system as free fructose, so effects should be observed.
Avoidance of foods that contain over 7 g of lactose per serve, which include cow’s milk, sheep’s milk and goat’s milk (more than 1/3 of a cup on average), yoghurt (more than 100 g), ice-cream, cream cheese (more than 100 g) and cream cheese-based dips is also recommended.31 Exercise caution with low-fat milk and cheddar cheese as some contain high amounts of lactose.
Expected outcomes and follow-up protocols
It is expected that abdominal symptoms such as distension and flatulence would be alleviated within 2 or 3 weeks of adhering to a fructose- and lactose-free diet. The herbal mix should also help to reduce these symptoms, while the causes are rectified. After 3 or 4 months it is expected that the patient will be able to tolerate small portions of foods that produced a 1+ or 2+ response on the IgG antibody test (eggs and rye in this case). These foods should be re-introduced slowly every 3 to 4 days. If worrying symptoms such as diarrhoea are still present the patient needs to be referred for further testing.
David T.J. Adverse reactions and intolerance to foods. Br Med Bull. 2000;56(1):34-50.
Gibson P.R., et al. Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther. 2007;25(4):349-363.
Ortolani C., Pastorello E.A. Food allergies and food intolerances. Best Pract Res Clin Gastroenterol. 2006;20(3):467-483.
1. Montalto M., et al. Adverse reactions to food: allergies and intolerances. Dig Dis. 2008;26(2):96-103.
2. Ortolani C., Pastorello E.A. Food allergies and food intolerances. Best Pract Res Clin Gastroenterol. 2006;20(3):467-483.
3. Sampson H.A. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805-819. quiz 820
4. David T.J. Adverse reactions and intolerance to foods. Br Med Bull. 2000;56(1):34-50.
5. Samartín S. Food hypersensitivity. Nutrition Research. 2001;21(3):473-497.
6. Savaiano D., et al. Nutrient considerations in lactose intolerance in nutrition in the prevention and treatment of disease. San Diego: Academic Press, 2001. 563–575
7. Suarez F., et al. Lactose intolerance. In: Encyclopedia of food sciences and nutrition. Oxford: Academic Press; 2003:2634-2642.
8. Pimentel M. Breath testing to evaluate lactose intolerance in irritable bowel syndrome correlates with lactulose testing and may not reflect true lactose malabsorption. Am J Gastroenterol. 2003;98(12):2700-2704.
9. Shepherd S.J., Gibson P.R. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106(10):1631-1639.
10. Wong D. Hereditary fructose intolerance. Mol Genet Metab. 2005;85(3):165-167.
11. Yasawy M.I., et al. Adult hereditary fructose intolerance. World J Gastroenterol. 2009;15(19):2412-2413.
12. Gibson P.R., et al. Review article: fructose malabsorption and the bigger picture. Aliment Pharmacol Ther. 2007;25(4):349-363.
13. Gibson P.R., Shepherd S.J. Personal view: food for thought –western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21(12):1399-1409.
14. Varea V., et al. Malabsorption of carbohydrates and depression in children and adolescents. J Pediatr Gastroenterol Nutr. 2005;40(5):561-565.
15. Thomsen S.F., et al. Importance of genetic factors in the etiology of atopic dermatitis: a twin study. Allergy Asthma Proc. 2007;28(5):535-539.
16. Thomsen S.F., et al. Multivariate genetic analysis of atopy phenotypes in a selected sample of twins. Clin Exp Allergy. 2006;36(11):1382-1390.
17. Lichtenstein P., Svartengren M. Genes environments and sex: factors of importance in atopic diseases in 7–9-year-old Swedish twins. Allergy. 1997;52(11):1079-1086.
18. Wuthrich B., et al. Total and specific IgE (RAST) in atopic twins. Clin Allergy. 1981;11(2):147-154.
19. Ventura M.T., et al. Intestinal permeability in patients with adverse reactions to food. Dig Liver Dis. 2006;38(10):732-736.
20. Host A., et al. Dietary prevention of allergic diseases in infants and small children. Pediatr Allergy Immunol. 2008;19(1):1-4.
21. Lind R., et al. Lifestyle of patients with self-reported food hypersensitivity differ little from controls. Gastroenterol Nurs. 2008;31(6):401-410.
22. Berstad A., et al. Food hypersensitivity – immunologic (peripheral) or cognitive (central) sensitisation? Psychoneuroendocrinology. 2005;30(10):983-989.
23. Pastar Z., Lipozencic J. Adverse reactions to food and clinical expressions of food allergy. Skinmed. 2006;5(3):119-125. quiz 126–127
24. Atkinson W., et al. Food elimination based on IgG antibodies in irritable bowel syndrome: a randomised controlled trial. Gut. 2004;53(10):1459-1464.
25. Drisko J., et al. Treating irritable bowel syndrome with a food elimination diet followed by food challenge and probiotics. J Am Coll Nutr. 2006;25(6):514-522.
26. Zar S. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am J Gastroenterol. 2005;100(7):1550-1557.
27. Zar S. Food hypersensitivity and irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15(4):439-449.
28. Zar S., et al. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand J Gastroenterol. 2005;40(7):800-807.
29. Wright E.M. Intestinal absorption in health and disease–sugars. Best Pract Res Clin Gastroenterol. 2003;17(6):943-956.
30. Sibley E. Carbohydrate intolerance. Curr Opin Gastroenterol. 2004;20(2):162-167.
31. FSANZ. Online. Available: http://www.foodstandards.gov.au/2006 May 2009.
32. Wischmeyer P.E. Glutamine: role in gut protection in critical illness. Curr Opin Clin Nutr Metab Care. 2006;9(5):607-612.
33. De Ley M., et al. Fish oil for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. (4):2007;. CD005986
34. Braun L., Cohen M. Herbs and natural supplements: an evidence-based guide, 2nd edition. Sydney: Elsevier; 2007.
35. Broer J., Behnke B. Immunosuppressant effect of IDS 30, a stinging nettle leaf extract on myeloid dendritic cells in vitro. J Rheumatol. 2002;29(4):659-666.
36. Konrad A., et al. Ameliorative effect of IDS 30, a stinging nettle leaf extract, on chronic colitis. Int J Colorectal Dis. 2005;20(1):9-17.
37. Bengmark S. Curcumin, an atoxic antioxidant and natural NFkappaB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases. J Parenter Enteral Nutr. 2006;30(1):45-51.
38. Hanai H., et al. Curcumin maintenance therapy for ulcerative colitis: randomized, multicenter, double-blind, placebo-controlled trial. Clinical Gastroenterology and Hepatology. 2006;4(12):1502-1506.
39. Holt P.R. Curcumin therapy in inflammatory bowel disease: a pilot study. Dig Dis Sci. 2005;50(11):2191-2193.
40. Ye D. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol. 2006;290(3):G496-G504.
41. Jagetia G.C., Aggarwal B.B. ‘Spicing up’ of the immune system by curcumin. J Clin Immunol. 2007;27(1):19-35.
42. Kim Y.S., et al. Curcumin attenuates inflammatory responses of TNF-alpha-stimulated human endothelial cells. J Cardiovasc Pharmacol. 2007;50(1):41-49.
43. Mehra M.R., et al. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25(7):834-838.
44. Zhao Y., et al. Eicosapentaenoic acid prevents LPS-induced TNF-alpha expression by preventing NF-kappaB activation. J Am Coll Nutr. 2004;23(1):71-78.
45. DeMeo M.T., et al. Intestinal permeation and gastrointestinal disease. J Clin Gastroenterol. 2002;34(4):385-396.
46. Bode C., Bode J.C. Effect of alcohol consumption on the gut. Best Pract Res Clin Gastroenterol. 2003;17(4):575-592.
47. Viljoen M. Gastro intestinal hyperpermeability: a review. East Afr Med J. 2003;80(6):324-330.
48. Isoda H., et al. Effects of organophosphorous pesticides used in China on various mammalian cells. Environ Sci. 2005;12(1):9-19.
49. Singer M.M., Tjeerdema R.S. Fate and effects of the surfactant sodium dodecyl sulfate. Rev Environ Contam Toxicol. 1993;133:95-149.
50. Tanii H., et al. Effects of cruciferous allyl nitrile on phase 2 antioxidant and detoxification enzymes. Med Sci Monit. 14(10), 2008. BR189–92
51. Basivireddy J. Oral glutamine attenuates indomethacin-induced small intestinal damage. Clin Sci (Lond). 2004;107(3):281-289.
52. Salman B., et al. Effect of timing of glutamine-enriched enteral nutrition on intestinal damage caused by irradiation. Adv Ther. 2007;24(3):648-661.
53. Peng X., et al. Effects of enteral supplementation with glutamine granules on intestinal mucosal barrier function in severe burned patients. Burns. 2004;30(2):135-139.
54. Zhou Y.P., et al. The effect of supplemental enteral glutamine on plasma levels gut function and outcome in severe burns: a randomized, double-blind, controlled clinical trial. JPEN J Parenter Enteral Nutr. 2003;27(4):241-245.
55. Sturniolo G.C., et al. Zinc supplementation tightens ‘leaky gut’ in Crohn’s disease. Inflamm Bowel Dis. 2001;7(2):94-98.
56. Forsyth C.B., et al. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43(2):163-172.
57. White J.S., et al. The probiotic bacterium Lactobacillus plantarum species 299 reduces intestinal permeability in experimental biliary obstruction. Lett Appl Microbiol. 2006;42(1):19-23.
58. Limdi J.K. Do probiotics have a therapeutic role in gastroenterology? World J Gastroenterol. 2006;12(34):5447-5457.
59. Bone K. A clinical guide to blending liquid herbs. St Louis: Elsevier, 2003.
60. Grigoleit H.G., Grigoleit P. Pharmacology and preclinical pharmacokinetics of peppermint oil. Phytomedicine. 2005;12(8):612-616.
61. Giachetti D. Pharmacological activity of Mentha piperita, Salvia officinalis and Rosmarinus officinalis essences on Oddi’s sphincter. Planta Med. 1986;52(6):543-544.
62. Grigoleit H.G., Grigoleit P. Gastrointestinal clinical pharmacology of peppermint oil. Phytomedicine. 2005;12(8):607-611.
63. Inoue T., et al. Antiallergic effect of flavonoid glycosides obtained from Mentha piperita L. Biol Pharm Bull. 2002;25(2):256-259.
64. Ghayur M.N., Gilani A.H. Pharmacological basis for the medicinal use of ginger in gastrointestinal disorders. Dig Dis Sci. 2005;50(10):1889-1897.
65. Savino F., et al. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil) in the treatment of breastfed colicky infants. Phytother Res. 2005;19(4):335-340.
66. Mills S., Bone K. Principles and practice of phytotherapy. St Louis: Churchill Livingstone, 2000.
67. Li X.M. Complementary and alternative medicine in pediatric allergic disorders. Curr Opin Allergy Clin Immunol. 2009;9(2):161-167.