32 Follow-up Monitoring of Cardiac Implantable Electronic Devices
Cardiovascular implantable electronic devices (CIEDs) include cardiac pacemakers, implantable cardioverter-defibrillators (ICDs), and leadless devices such as implantable loop recorders and cardiovascular monitors. Implantation is indicated for treatment, diagnosis, and monitoring of bradycardia, tachycardia, and most recently, heart failure.1 Since their inception in 1958, the function of CIEDs and monitoring of patients have become more complex. Functional roles may overlap, and capabilities for ventricular tachyarrhythmia therapy, biventricular stimulation, and collection of heart failure monitoring data may be contained in a single device. Positive results from recent trials for prophylactic ICDs and cardiac resynchronization therapy (CRT) devices have driven an expansion of indications and spurred an exponential increase in CIED use. An estimated 1 million devices were implanted in 2007, and this number will increase as their range and versatility increase.2
 Purpose of CIED Monitoring
Purpose of CIED Monitoring
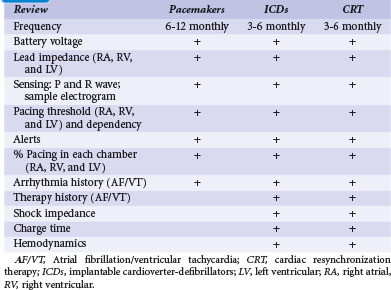
The aims of monitoring are to (1) establish and maintain appropriate CIED function, (2) optimize its programming, (3) identify risks in implanted device (e.g., impending lead failure) or from changes in patient condition (e.g., advent of AF), (4) monitor response to therapy, and (5) provide a communication channel for systematic data review. Current practice generally follows an in-clinic follow-up protocol by physicians and specially trained staff with retrieval of stored diagnostic data. For ICDs and CRT devices, this is performed at short intervals because of safety concerns (e.g., 3 monthly), although optimal frequency has not been tested.2
Significant challenges are increasing volume of patients, complexity of both CIED and patient, maintaining continuous surveillance, capability of prompt problem detection, and management of retrieved data.3,4 These place increasing demands on the health care system.
 In-Person Monitoring
In-Person Monitoring
In-person monitoring with a physician or allied professional in a clinic or medical institution has been the conventional standard for device follow-up. Face-to-face evaluation permits history taking, physical examination, electrocardiography, and radiography as indicated. Interrogation of the device is performed using a programmer for bidirectional communication with the CIED. This has been traditionally wand-based but more recently, with wireless telemetry using a programmer located within 10 feet (3 m). Device function, including capture and sensing thresholds, may be reviewed (Table 32-1). Response to medical therapy may be disclosed, such as for atrial fibrillation (AF). Programmed settings and functions may be reprogrammed if necessary to optimize device operation and individualize parameters according to patient need (“actionable” encounters). The frequency of this may depend on the indication for the encounter; a routine scheduled check differs from unscheduled encounters to follow a particular condition or specific symptoms (Box 32-1).
In-person evaluation is required after implantation and before hospital discharge, for wound checks within 2 weeks, and then again at 6 to 12 weeks to set chronic parameters for pacemakers, ICDs and CRT devices. This period is significant because system-related problems (e.g., threshold increase, perforation, need for revision) tend to cluster in the early postoperative period.5,6
Subsequent follow-up schedules vary according to facility, physician preference, and available resources. Therefore, guidelines based on consensus opinion were issued by the Heart Rhythm Society (HRS) and European Heart Rhythm Association (EHRA). This consensus advocated a minimal recommended schedule of device follow-up of 3 to 6 monthly clinic checks for ICDs and 6 to 12 monthly checks for pacemakers, but with increased frequency (e.g., monthly) in response to product advisories and recalls.2 Such scheduled checks represent an estimated 4 million encounters in the United States alone. Problem discovery rates with this protocol have been unknown until recently.7 The incidence of unscheduled checks occurring with this schedule—an added burden to already-saturated device clinics—is also undetermined. The occurrence of significant cardiac or arrhythmic symptoms (e.g., shock therapy, palpitations) or detection of a CIED alert (e.g., audible alert) traditionally has required emergent clinic attendance and CIED interrogation. In the mid-2000s, a succession of advisories demanding higher frequency of follow-up overwhelmed clinic follow-up capacity, motivating the development and application of remote monitoring technologies.3
 Remote Monitoring
Remote Monitoring
Technologic Advances
Transtelephonic Monitoring Without Interrogation
Historically, remote monitoring of pacemakers has occurred for decades using transtelephonic monitors with modem technology. This system converts electrocardiographic information into sound to communicate over telephone lines to a decoding machine, which changes the sound back into the “rhythm strip.” This was introduced in the early 1970s to monitor the longevity of pacemakers8 and implemented for remote surveillance of sensing and capture as well as lead and device malfunction. It permitted frequent monitoring of pacing rate, determination of the underlying rhythm, and timely detection of battery depletion. Although extensively used, transtelephonic monitoring (TTM) was restricted to pacemakers, depended on active patient participation (and thus vulnerable to adherence issues), and delivered only a “snapshot” of the cardiac rhythm that was likely to miss intermittent problems. Indeed, in a recent trial, only 3 of 190 events were found on TTM, with discovery of the remainder requiring (delayed) inpatient assessment.9
Modern Systems
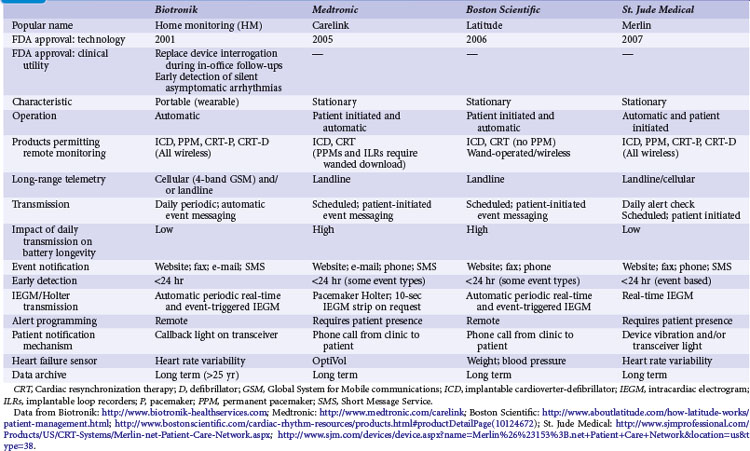
Remote monitoring systems permit access to the extensive data recorded in diagnostic memory of current-generation CIEDs. Each system is proprietary and works only with compatible devices from the same manufacturer: Home Monitoring (HM; Biotronik, Berlin), CareLink Network (Medtronic, Minneapolis), Latitude Patient Management System (Boston Scientific, St. Paul, Minn.), and HouseCall Plus (St. Jude Medical, Sylmar, Calif.). Their characteristics are summarized in Table 32-2. All technologies from a single manufacturer may not be available in all countries. CareLink may employ wand-operated or wireless transmitters. HM has been most extensively studied,5,10,11 in contrast to CareLink, although this is widely used. Latitude and Merlin (St. Jude Medical) have no published data regarding their technical or clinical utility. Mode of operation and level of patient involvement during transmission vary among monitoring systems. Operating differences pertain to the frequency of data transmission and report generation, type of reports generated, sensor technologies, and degree of patient involvement required for successful transmissions. For example, a high level of patient interaction is required by wand-based systems (e.g., CareLink), but virtually none by automatic systems (e.g., HM). Most remote monitoring systems transmit data via standard telephone lines, whereas HM uses a fully mobile, wandless, cellular-network transmitter that can be worn on the belt or carried in a purse to deliver automatic, daily device interrogations and alert reports (Fig. 32-1).
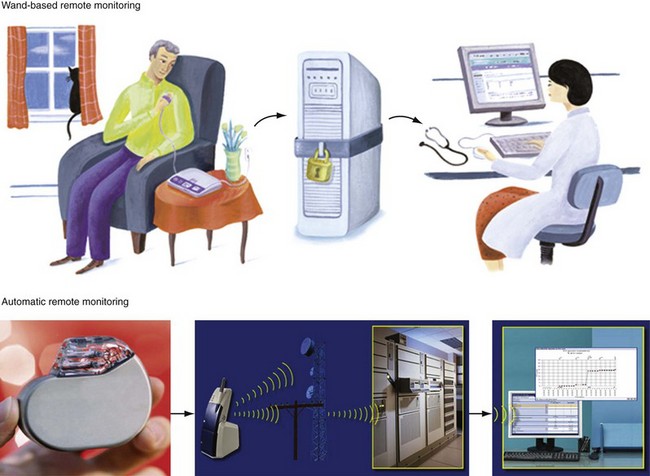
Figure 32-1 Contrasting remote monitoring technologies.
Top, Patient-activated system from home. Bottom, Example of automatic remote home monitoring by Biotronik Home Monitoring (HM). HM transmits data daily at a preset time, and immediately in response to a critical event. Transmission steps are as follows. A very-low-power radiofrequency transmitter circuitry is integrated within the pulse generator. The implanted device wirelessly transmits stored data on a daily basis to a mobile transceiver (typically placed at bedside at night). The transceiver automatically and silently accepts patient data from the implant and transfers this digital information using a cellular short-messaging system. The data are relayed wirelessly or via landline (automatically seeking first path available) to a dedicated service center. The service center generates a customized summary with informative trends, charts, parameters, electrograms, and graphs, available to the physician online via secure Internet access. Thus, daily patient monitoring may occur between scheduled office device interrogations providing trended information (e.g., battery status, lead impedance, sensing function). Service center processing and data upload on the HM webpage is automatic, bypassing potential delays (and errors) associated with manual processing. Data are stored in service center repository and analyzed and disseminated electronically. Critical and clinically relevant status changes data may be transmitted immediately without the need for patient interaction and flagged for attention on the HM webpage (see Fig. 32-2). Automatic alerts occur for silent but potentially dangerous events (e.g., device/lead failure, onset of asymptomatic AF). Event details include transmission of intracardiac electrograms similar to those available during office device interrogations. Physicians may be notified of alert conditions via e-mail or fax, if required. Useful information about the most important device or patient status changes can be delivered to the physician as quickly as a few minutes after the event. HM uses a triple-band and a quad-band exterior wireless transceiver connected to available worldwide cellular telephone networks (>55 countries).
(Modified from Varma N: Rationale and design of a prospective study of the efficacy of a remote monitoring system used in implantable cardioverter defibrillator follow-up: the Lumos-T Reduces Routine Office Device Follow-Up Study (TRUST). Am Heart J 154:1029-1034, 2007.)
An important potential of remote monitoring systems is the ability to perform early detection, especially of asymptomatic events, although systems vary in this ability. Clinically important events may be considerably delayed with systems relying on patient activation but, in contrast, can be notified within minutes by HM.12 However, remote manual testing or reprogramming of devices, although possible from an engineering perspective, is not available because of safety and security concerns. Actionable events, even if straightforward and easily resolved with simple reprogramming, demand in-person assessment.
Patient-Activated Remote Monitoring
These versions require patient-driven communication, whereby wand-derived data are relayed via telephone connections to following facilities (see Fig. 32-1). This requires the patient at home to manipulate a programmer and coordinate with clinic personnel to ensure communication and transmission, typically on a formal, calendar-based schedule. Early data from these systems reported technical feasibility and patient acceptance.13,14 Data transfer was similar to in-person interrogation. Nevertheless, these systems are cumbersome to use (patients reported difficulty in manipulating the wand,14 raising compliance issues) and time-consuming. As a tool, they essentially substituted for scheduled calendar-based in-person encounters, with the lack of monitoring in interim periods risking overlook of interim asymptomatic events. Therefore, although superior to TTM,9 patient-activated systems may not provide an efficient method of follow up.
Automatic Remote Monitoring
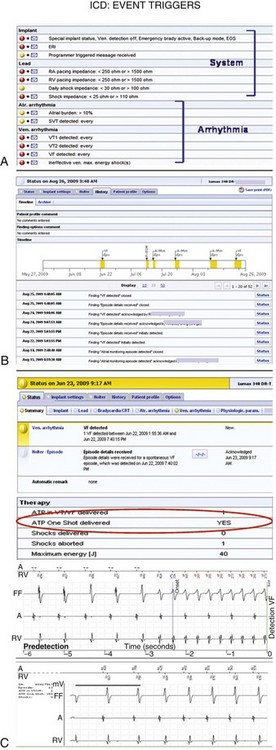
Current-generation remote monitoring systems implement an automatic transmission mechanism fully independent of patient or physician interaction. This relies on a CIED-initiated remote transmission using a Medical Implant Communication Service (402-405 MHz) or Industrial, Scientific and Medical (902-928 MHz) radiofrequency band allocated for implanted medical devices. An encrypted telemetric signal is sent from the CIED to a transceiver. The only requirement is that the patient be within a distance of approximately 6 feet (~2 m) from the transceiver. The transceiver (which may be a mobile unit with HM) uses telephone links, which may be landline and/or cellular based (see Fig. 32-1). The system can be programmed to download data at specific times and dates, usually when the patient is sleeping adjacent to the base unit. The transceiver automatically connects the phone link for data transfer. The treating physician can manually access the information and preprogram a customized set of alerts for each patient according to need (Fig. 32-2). If triggered, these alerts are transmitted promptly for physician review (color-coded according to urgency), enabling prompt clinical intervention if necessary. The amount and quality of transmitted information, including real-time intracardiac data, continue to improve and have reached the level of an in-office interrogation.
Automatic remote monitoring was pioneered by Biotronik (Home Monitoring, HM) and has been used in more than 50 countries (U.S. FDA approval in 200215). Other manufacturers are following (Table 32-2). This technology allows for automatic, patient-independent, wireless transmission of diagnostic ICD, CRT, and pacemaker data (see Fig. 32-1). Published data for technical efficacy for automatic remote monitoring are available only for the HM system.12,16 Reliability and early notification ability of this communication system were excellent. The wireless transmission ability is especially useful since almost 20% of U.S. households are currently estimated to have no landline facility.17 Greater than 90% of transmissions were received in less than 5 minutes (Fig. 32-3), and data integrity was 100% preserved.5,10,16,18 HM system operation is not energy costly and not susceptible to electromagnetic interference. The HM transceiver is a fully-mobile cellular unit. The novel quad-band transceiver is compatible with all major Global System for Mobile (GSM) cellular networks worldwide. It permits a unique callback function so that the medical professional can contact the patient worldwide via the transceiver, which is especially useful for travelers. Thus, automatic HM has the ability to maintain surveillance and rapidly bring to attention significant data, enabling clinically appropriate intervention.
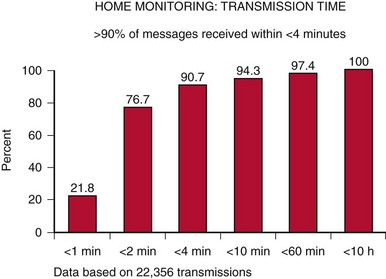
Figure 32-3 Transmission time with automatic remote monitoring.
Current Home Monitoring (HM) units are able to provide transmission speeds of less than 30 seconds.
(From Varma N, Stambler B, Chun S: Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol 28(suppl 1):133-136, 2005.)
TRUST Trial
The current variety of follow-up practices, with or without the assistance of different remote technologies, contrasts with a paucity of data to support any particular method or schedule. Although guidelines advocate 3 to 6 monthly clinic checks, the efficacy of this schedule with regard to patient safety, adherence, incidence of unscheduled encounters, and rate of problem detection remain untested. The TRUST (Lumos-T Safely Reduces Routine Office Device Follow-up) trial was undertaken to resolve some of these outstanding questions in a heart failure population receiving ICDs.19 TRUST has been the only prospective multicenter clinical study to assess and compare conventional follow-up and remote monitoring. The automatic remote monitoring mechanism tested was Biotronik Home Monitoring (HM).
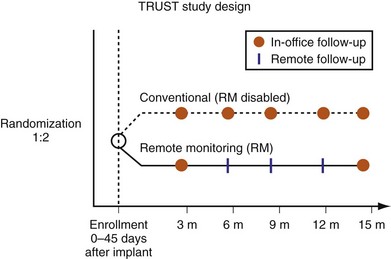
Representing U.S. implant recipient demographics, 1516 patients enrolled at 105 U.S. sites in a predominantly community-based setting. Patients were randomized to conventional care (HM off) or to remote monitoring (HM on) groups (Fig. 32-4). Eligible patients were enrolled from 0 to 45 days after successful device implantation and then randomly assigned in a 2 : 1 ratio to HM or conventional groups, respectively. All patients had scheduled postimplant follow-ups at 3, 6, 9, 12, and 15 months, with unscheduled (interim) evaluations as needed. Patients assigned to conventional care were evaluated in-clinic only. In the HM arm, patients had HM checks followed by office visits at 3 and 15 months. At 6, 9, and 12 months and for interim visits, cumulated transmission files were remotely retrieved from the secure website and evaluated. Thus, both groups adhered to 3-month follow-up. Investigators were permitted to evaluate patients additionally in-office following HM checks during the study. Between these periodic checks, early notification could automatically occur for compromised system integrity (battery, lead parameters, high-voltage circuitry) or arrhythmia occurrence (e.g., AF, ventricular arrhythmia). These were evaluated online and patients brought in for office visits if deemed necessary by the investigator. Scheduled and unscheduled clinic visits (including responses to HM event notifications) were tracked for each individual in both study arms. Unscheduled interrogations in interim periods resulted from physician or patient initiation. TRUST’s primary objectives were to compare total in-hospital device evaluations in HM compared to conventional care and to assess the safety of the remote follow-up method.
Home monitoring reduced total (scheduled and unscheduled) hospital encounters for device interrogation by 45% (Fig. 32-5).20 The number of in person encounters occurring at scheduled 3-month intervals was reduced by 60%. This was not a 75% reduction, expected if all 6-, 9-, and 12-month checks were performed online, because the protocol permitted reviewing physicians to follow online check with in-person evaluations if deemed necessary. However, the small number of such cases indicated physicians’ confidence in the extent and quality of transmitted data, even at a stage of gaining familiarity with a new technology. Thus, more than 85% of all HM-group 6-, 9-, and 12-month follow-ups were performed using HM only, indicating that HM provided sufficient assessment in these patients. HM maintained better continuity of follow-up compared with traditional methods. A small proportion of the total number of in-person evaluations also resulted from unscheduled encounters. This is an important observation: a concern exists in the implanting community that an automatic remote monitoring system that self-screens daily would result in an increased presentation of patients to the hospital. However, this did not occur. Extension of face-to-face encounters to yearly in remote monitoring was not accompanied by loss of safety (see Fig. 32-5). The incidence of death and stroke trended toward improvement with use of HM.
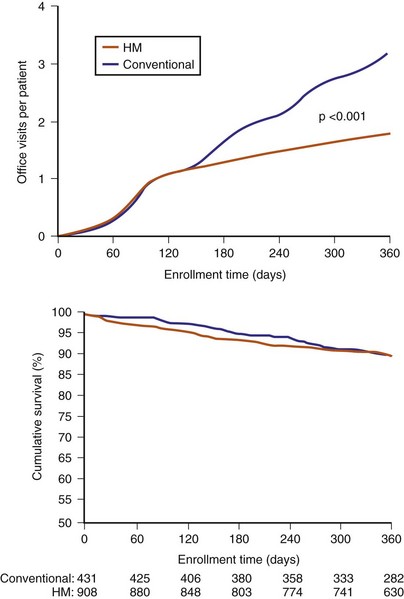
Figure 32-5 TRUST results.
(Compiled from Varma N, Epstein A, Irimpen A, et al: Efficacy and safety of automatic remote monitoring for ICD follow-up: the TRUST Trial. Circulation 122:325-332, 2010.)
TRUST’s secondary endpoint assessed the early-detection capability of HM, defined by time elapsed from event onset to physician evaluation. Evaluation in HM occurred on receipt of event notifications in response to detection of preprogrammed events or in-office interrogation (scheduled or unscheduled). In conventional care, event detection occurred at in-office interrogation (scheduled or unscheduled). HM enhanced problem discovery (even if asymptomatic) despite less frequent hospital evaluations. Detection was advanced by more than 30 days compared to conventional care20 (Fig. 32-6). (With 6 monthly scheduled conventional visits, HM would provide a correspondingly greater advance notification of at least 3-4 months.) Notably, this was not a test of transmission time (a technologic feature) but of “time to physician evaluation” (i.e., of clinical practice).
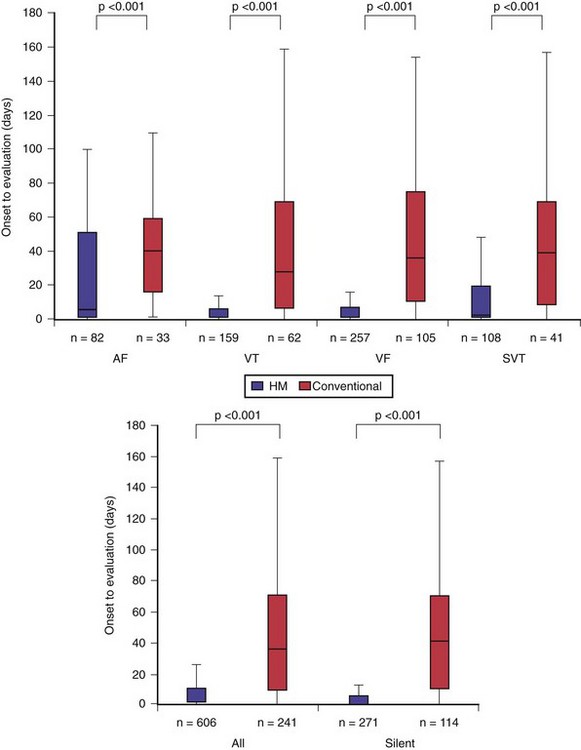
Figure 32-6 Early detection with home monitoring (TRUST secondary endpoints).
HM secured earlier physician evaluation of arrhythmias (top) and of silent events (bottom).
(Compiled from Varma N, Epstein A, Irimpen A, et al: Efficacy and safety of automatic remote monitoring for ICD follow-up: the TRUST Trial. Circulation 122:325-332, 2010.)
Another study, Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision (CONNECT), applied a separate automatic technology from a different manufacturer to a similar patient group.21 It reported broadly similar effects on total in-person evaluations, endorsing the results of TRUST. A significant shortening of hospital length of stay was observed as well, possibly because of a positive impact on patients’ health. Overall hospital costs were reduced by use of remote technology. However, only a minority of attempted alerts were transmitted successfully, severely limiting the utility of this proprietary technology for early detection.
In summary, TRUST demonstrated significant limitations of previously untested “conventional care” while, in contrast, showing the safety, efficacy, and early-warning capability of automatic remote monitoring. Automatic HM ensured follow-up continuity of a large patient volume, avoiding unnecessary in-hospital patient evaluation (thus reducing clinic load by almost 50%), but maintained near-continuous surveillance to rapidly identify problems. The trial results promote a paradigm shift in current clinical practice to exception-based hospital care.22
Implementation of Remote Monitoring
Patients receiving CIEDs span a wide spectrum: young versus old, sedentary versus competitive athletes, working versus retired, and those who may be completely healthy versus those with extensive comorbidities. Before implant, patients must receive education regarding the indications for the device and expected postimplant restrictions. Some may have limited access to telephone landlines or to transportation (e.g., remote areas) or may be physically limited, affecting choice of remote technology. Automatic remote monitoring may have particular utility in young children, who are susceptible to higher rates of lead or pulse generator failure than adults but are unable to describe specific symptoms.23 Inquiries about occupation and environment may yield important information regarding potential sources of electromagnetic interference. In-person monitoring is required after implantation before hospital discharge. Complications such as hematoma, lead dislocation, and perforation are generally seen within 24 hours after implantation. This assessment should document appropriate CIED function, establish patient-specific programming, document initial telemetry values, ensure the absence of operative complications, educate and emotionally support the patient and family, and provide a CIED identification card to the patient.
Device Clinic
The use of remote monitoring has important implications for device clinics, which have increasing patient volume but limited trained health care professionals.24 However, in-person follow-up at 2 to 12 weeks after implant as an outpatient is important.2 It permits assessment of wound healing and determination of chronic thresholds as well as setting of final pacing parameters.5,6 Problems such as lead perforations or system errors requiring revision and symptomatic reactions to implantation (e.g., pacemaker syndrome, diaphragmatic pacing, pocket infection) cluster in this early postimplant period and occur more frequently with dual-chamber or resynchronization units.5,6,25
After the in-person 3-month postimplant assessment, face-to-face checks may be scheduled once yearly with three interim monthly checks via remote monitoring, according to current guidelines.2 These checks mainly involve collection of routine measurements only (e.g., battery status, lead impedance, sensing function), which require no programming or device changes, or alteration of antiarrhythmic medications (see Table 32-1). Current-generation devices have automatic threshold assessment, which permits monitoring of threshold changes. If an actionable (or questionable) event is detected, the patient may be contacted to report for formal assessment. However, these represent a minority, because TRUST demonstrated that approximately 90% of these three monthly checks were “nonactionable.”
Evaluation of interim unscheduled checks is important. These may be patient or physician initiated. Remote technology may be used to triage patient inquiries, such as perceived device discharges. Currently, most patients call their physician/clinic immediately after perceiving a received shock, often prompting an emergency room (ER) or clinic visit. With remote monitoring, shock data and online electrogram (EGM) are available and may be used to determine optimal patient management confidently. An appropriate shock may require reassurance only, without the need for hospital visit. In contrast, a series of inappropriate shocks may be rapidly evaluated online and the patient asked to report to the hospital promptly. Using conventional monitoring methods, increased frequency of visits were mandated for generators approaching the elective replacement indicator (ERI), deviations in lead behavior (impedance, sensing, or threshold), and ICD components under advisory, which tend also to manifest with battery, high-voltage (HV) circuitry, or lead failure.26 In contrast, physicians following such patients with automatic remote monitoring may perform accelerated follow-up online and wait for affected devices to self-declare an issue rapidly, thus avoiding overloading device clinics with non-actionable inpatient encounters (Figs. 32-7 to 32-9). This “wait-and-see” management strategy emphasizes a monitoring philosophy of exception-based care. Problem discovery during unscheduled visits is significantly higher than with routine evaluations.6 In TRUST, patients evaluated in person on basis of physician- or patient- initiated encounters, or called in because of event notification requiring further assessment, had an actionability rate greater than 30%; that is, problem discovery rates were approximately threefold those for scheduled checks.7
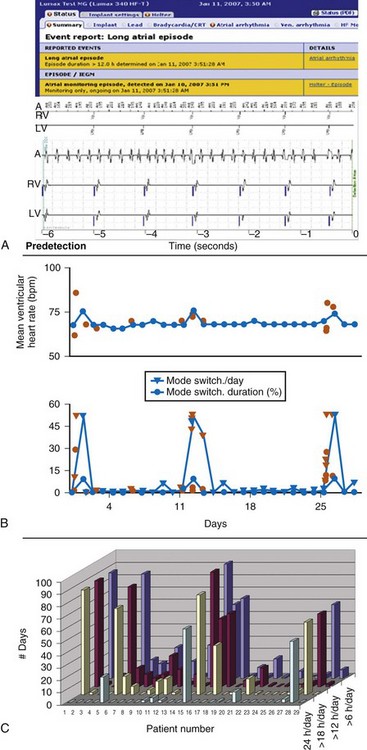
Figure 32-7 Atrial fibrillation (AF).
(B compiled from and C generated from central HM service center data. A from Varma N, Stambler B, Chun S: Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol 28(suppl 1):133-136, 2005.)
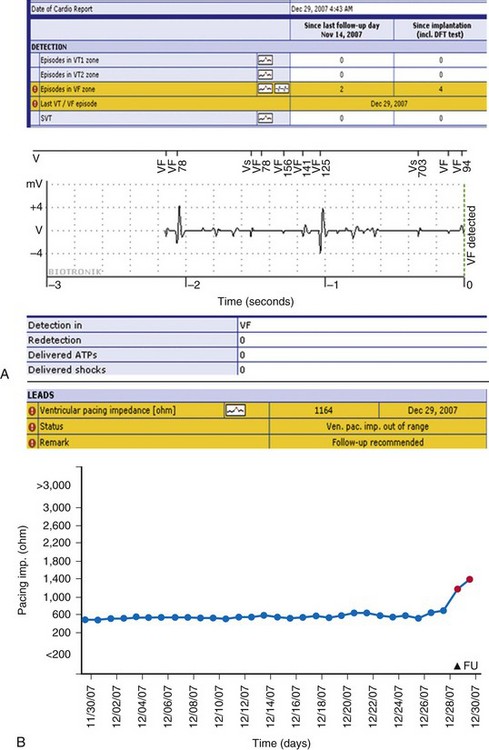
In this patient, events occurred silently during sleep at 4:43 am, 6 weeks after last clinic follow-up on Nov. 14. A, Two occurrences of detection in VF zone are noted (top) and flagged (exclamation mark). The accompanying wirelessly transmitted electrogram (EGM) demonstrated irregular sensed events. Marker channels indicated coupling intervals as short as 78 msec. The transmission terminated with VF detection (marked). The summary below shows that no therapy was delivered, indicating that this VF sensed event terminated spontaneously. B, The second event report shows a separate notification in response to the same event, indicating a lead impedance alert. Lead impedance trend was stable below 600 ohms in prior weeks, but then suddenly increased >500 ohms, triggering an event notification (red), although this absolute value was still within specifications. The resolution of the illustrated intracardial EGM transmission is 1/64 second in this first-generation device with wireless EGM transmission (Lumos). Internally, the device works with a resolution of 1 msec. The signal is compressed by means of a pattern-like analysis to make the EGM data fit into one SMS. In this case, the overall image together with markers provided sufficient information to enable a clinical decision, although EGM definition was modest. Current-generation devices transmit EGMs with improved resolution (1/128 second) and longer duration, including postdetection sequences. These devices use lossless compression; i.e., EGM is identical to the view on the programming device (e.g., in Fig. 32-11).
(Modified from Varma N: Remote monitoring for advisories: automatic early detection of silent lead failure. Pacing Clin Electrophysiol 32:525-527, 2009.)
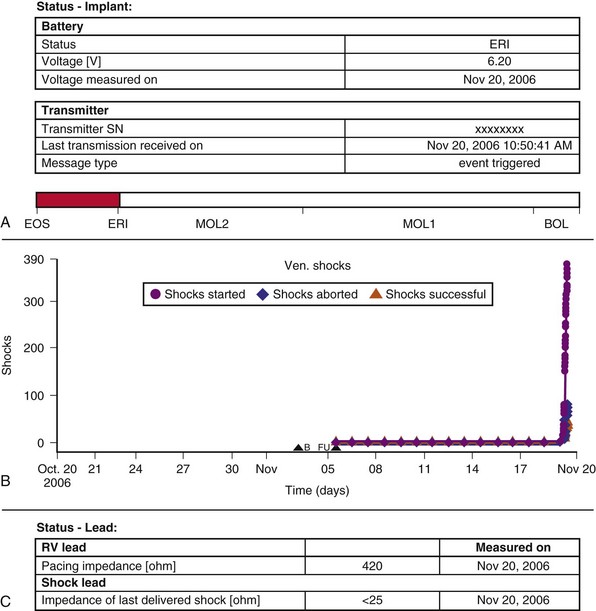
Figure 32-9 Generator malfunction.
(Compiled from Varma N, Michalski J, Epstein A, Schweikert R: Automatic remote monitoring of ICD lead and generator performance: the TRUST trial. Circ Arrhythm Electrophysiol 3:428-36, 2010.)
Automatic remote monitoring with daily screening may generate increased alert transmissions that may augment unscheduled office visits. This concern was assessed in TRUST. The number of patients generating an event notification was less than 50% at 12 months. Less than 2% of 350,000 potential “opportunity” days to trigger an event notification were used.27 These data are consistent with previous reports.11,28 Therefore, even though an event notification may be potentially triggered every day, in practice such messages occurred infrequently, resulting in a very low transmission load, indicative of the strong filtering exerted by a programmable messaging system. A significant advantage of the HM remote monitoring systems is that alerts may be customized online according to changing needs, without demanding patients to report for in-person reprogramming. For example, the physician may want to be alerted for ventricular tachycardia (VT) during adjustments of antiarrhythmic drug therapy, but not in all ICD recipients, or may need to eliminate AF notifications in a patient in whom attempts at restoration of sinus rhythm have been abandoned. Thus, diagnostic event messages may be tailored for each patient at the physician’s discretion (Figs. 32-10 and 32-11; see also Fig. 32-2).
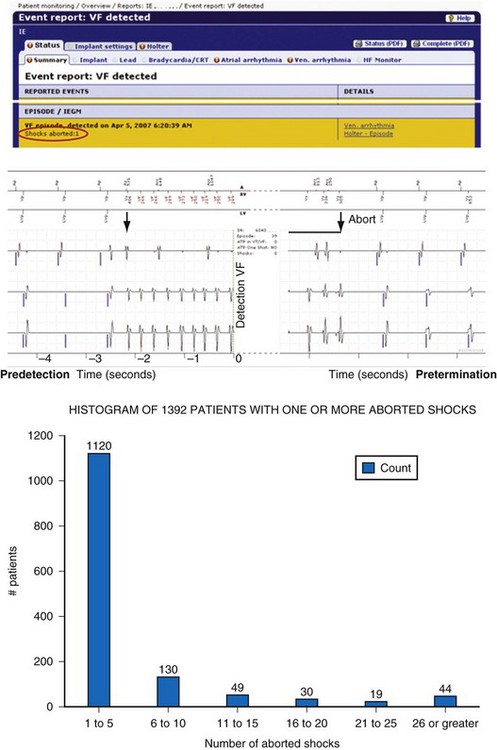
Figure 32-10 Example of remote monitoring surveillance.
(Modified from Varma N, Johnson MA: Prevalence of cancelled shock therapy and relationship to shock delivery in recipients of implantable cardioverter-defibrillators assessed by remote monitoring. Pacing Clin Electrophysiol 32[suppl 1]:42-46, 2009.)
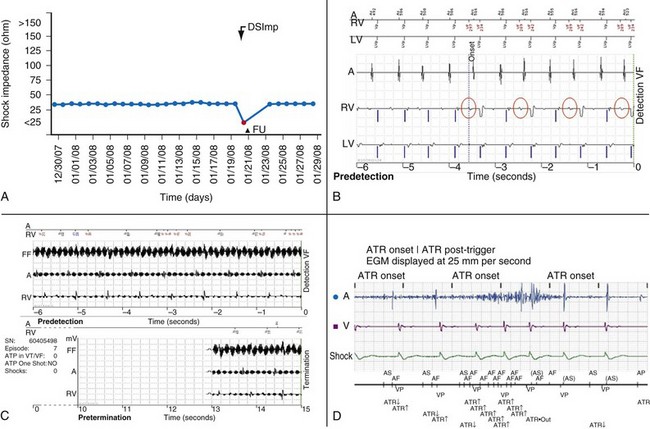
Figure 32-11 Monitoring lead function.
A, High-voltage impedance alert. B, T-wave oversensing triggering an event notification. This silent problem was corrected the following day with reprogramming before inappropriate therapy was delivered. C, Nonphysiologic signals. In this case, electromagnetic interference triggered an event notification. The patient was informed within 24 hours. Asymptomatic detections meeting ventricular fibrillation (VF) detection criteria resulted in immediate event notifications. Wireless electrogram transmission automatically accompany detections in VF zone, even if shock therapy is aborted.43 D, Atrial lead noise (Latitude) triggering an alert.
(Compiled from Varma N, Michalski J, Epstein A, Schweikert R: Automatic remote monitoring of ICD lead and generator performance: the TRUST trial. Circ Arrhythm Electrophysiol 3:428-36, 2010.)
As implanted devices from all manufacturers begin to incorporate automatic remote monitoring mechanisms, follow-up routines in device clinics may become more uniform. Several major institutions already depend on remote monitoring as an essential instrument for managing large patient volumes. TRUST demonstrated that almost 90% of scheduled checks, whether in person or remote, were nonactionable, indicating that most of the three routine monthly checks may be accomplished online safely. However, implementation requires a change in clinic and physician workflow patterns, with daily attention to remote websites to evaluate event notifications, and a structured approach to maintain consistent scheduled device interrogations. Website review may be conducted rapidly compared to in-person follow-up, which improves clinic efficiency and delivers prompt communication to patients when necessary. This is especially valuable for potentially dangerous asymptomatic events. Remote CIED monitoring systems have improved presentation as well as capacity of data retrieval. For example, reports graphically display trended parameters of atrial and ventricular pacing, heart rate, AF events, and patient activity or sensor-derived data (e.g., OptiVol). The rapid presentation of salient data facilitates prompt interpretation. Thus, in one HM study, a nurse expert in cardiac pacing consulted the website daily and reviewed problematic transmissions with a physician. During a mean follow-up of 227 ±128 days, 23,545 daily messages and 1665 alert events were received. The nurse and physician spent on average, respectively, 1 and 0.2 hr/week analyzing the data. The mean connection time per patient was 2 minutes and had decreased to 1.65 min/patient for the last 50 connections. The nurse submitted 133 transmissions from 56 patients to the physician. The authors concluded that automatic remote monitoring optimized medical treatment and device programming, at low cost for the health care system.16 Device clinics using remote monitoring were found to reduce physician time compared with conventional care (8 vs. 26 minutes) and also patient time (7 minutes vs. 3 hours).29 Thus, application of the TRUST follow-up protocol, which gained U.S. Food and Drug Administration (FDA) approval in May 2009,30 is likely to require fewer hospital personnel while improving patient surveillance.
Patient Aspects
Automatic remote home monitoring enabled routine follow-up evaluations without face-to-face encounters, but also permitted in-person assessment when required, and sooner. (In contrast, patient-activated remote systems failed to reduce cardiac-related resource utilization).31 This shift to exception-based assessment has key advantages for patients who, to maintain in-person follow up, have to take time off work, or need an accompanying person, or for whom access is limited (e.g., nursing home residents, geographically remote patients). Patients who travel extensively (including internationally) may benefit from the versatility of cellular service. Home monitoring enabled physician evaluation within 24 hours, including detection of asymptomatic events (vs. ≥5 months with patient-activated systems9). This ability for rapid self-declaration of fault detection enabling preemptive clinical action may be potentially lifesaving, especially for clinically silent problems, such as lead fracture, device dysfunction,32,33 and arrhythmias (see Figs 32-7 and 32-8). Current systems provide an opportunity for patient notification via the receiver (callback function, third-party arrhythmia service, live interaction) that may be reassuring for patients during remote follow-up.
Concerns that remote follow-up would promote a loss of contact between patient and physician have not been borne out when tested. Earlier remote monitoring versions demonstrated a high level of satisfaction among patients, who found their physicians to be just as available when required.13 TRUST demonstrated that scheduled evaluations were better maintained with remote technology compared to in-person interactions (which demonstrated a significant loss of adherence). This occurred without increase in ER utilization or number of patient-initiated evaluations, indicative of patient confidence in relying on remote monitoring for their follow-up. On trial conclusion, 98% of patients assigned to remote follow-up mode elected to retain it.34 Similarly, in a separate study, almost 99% of patients expressed complete or high satisfaction with remote interrogation.35
The follow-up scheme described is directed to device management only and is not intended to replace patient consultations with internists, cardiologists, or heart failure specialists, which may otherwise occur. The established follow-up framework may require interim adjustments according to individual situations (e.g., stability of patient’s medical condition) (see Box 32-1). CIEDs may need to be temporarily reprogrammed before and after a medical investigation or surgical procedure, to prevent potential damage (e.g., from cautery). The frequency of system checking may need to be temporarily increased for recurrence of arrhythmic events, assessment of response to treatment, or recently modified parameters for sensing, pacing, or therapy. This may need to be accompanied by in-person follow-up. For example, more frequent monitoring of arrhythmias may be accomplished by remote checks, but treatment of heart failure decompensation may require inpatient assessment. In either case, when the clinical condition has stabilized, the monitoring schedule may revert to protocol. Patients who are terminally ill may opt for deactivation of their CIED.
 Special Situations
Special Situations
Arrhythmias
The most common event notifications received during automatic remote surveillance are for AF and nonsustained ventricular arrhythmias27 (see Figs. 32-7 and 32-10). These may drive important clinical decisions. Atrial fibrillation in heart failure patients may be associated with increased morbidity and mortality.36,37 This is likely to be multifactorial, involving increased risk of heart failure by adversely affecting ventricular hemodynamics as well as stroke and facilitation of ventricular arrhythmias.38 Detection is important; a first episode of AF in a patient with risk factors prompts anticoagulation initiation, and AF conducting with rapid ventricular rates may elicit inappropriate shock therapy. However, diagnosis is challenging because of the evanescent and largely asymptomatic nature of this arrhythmia. Automatic remote monitoring in patients with CIEDs may resolve this problem and aid diagnosis and response to treatment.12,39 In the future, analysis of automatically archived data may enable accurate arrhythmia characterization, to provide insights into AF patterns, including absolute AF burden, degree of temporal dispersion, and progression to persistent arrhythmia (see Fig. 32-7, bottom). This characterization may aid understanding of risks posed and responses to treatment. For example, AF presence and duration (0 vs. >5 minutes or 24 hours) detected by continuous AF monitoring combined with CHADS-2 score–enhanced thromboembolic risk assessment.40 Early detection of asymptomatic AF by HM allows prompt clinical reaction, including antiarrhythmic therapy, cardioversion, and timely introduction of anticoagulation therapy. Computer modeling studies suggest that this could reduce potential stroke risk by 9% in patients with long-lasting episodes compared to standard follow-up.41 Ongoing large-scale prospective trials are examining these questions further.
The principal reason for ICD implantation is to treat ventricular arrhythmia. However, inappropriate shocks occur with appreciable frequency, especially in the current era, when more ICDs are implanted prophylactically than for secondary prevention.42 Remote monitoring has an important role in this problem by early identification of atrial arrhythmias or hardware/sensing problems, to enable intervention to minimize inappropriate shocks and maximize appropriate therapy (see Fig. 32-11). Recurrent nonsustained ventricular arrhythmias occur in a sizable minority of the ICD population. These are significant because they may contribute to early battery depletion and may also presage delivered shocks43 (see Fig. 32-10). Emerging data indicate that shock therapy may have deleterious long-term consequences.44,45 Early recognition by remote monitoring of nonsustained events may provide an opportunity for preemptive intervention and improve patient outcomes.
Heart Failure
Recipients of ICD therapy typically have heart failure, which is a dynamic condition. Hospitalizations for acute decompensated heart failure (ADHF) continue to increase46 and are associated with increased 1-year mortality47,48 and significant expense for health care services. ADHF development is complex, involving several processes, which may include hemodynamic, neurohumoral, electrophysiologic, and vascular abnormalities that converge to manifest with fluid congestion. Therapeutic strategies aimed at interrupting this train of events are potentially valuable. Symptoms are unreliable as early warning signs in this cascade. Implantable devices, including leadless automatic physiologic monitors, may serve this purpose.49 Some of the upstream factors triggering heart failure deterioration may include atrial and ventricular arrhythmias, occurrence of right ventricular (RV) pacing,50 or lack of biventricular pacing in patients with implantable devices. These data are routinely quantified in device diagnostics.
Figure 32-12 illustrates several asymptomatic parameters tracked over time to monitor the progress of clinical deterioration: AF, loss of CRT pacing, and fluid accumulation. These parameters also show the time taken from an inciting event (likely AF in this case) to clinical presentation. CRT’s mortality benefit51 diminishes when it drops to less than 92% because of AF or significant ventricular ectopy.52 Thus, AF with rapid ventricular rates may have promoted loss of CRT pacing, a possible contributor to ultimate decompensation. Figure 32-12 also demonstrates a positive detection of excess fluid, derived by decreased electrical impedance between the RV lead and generator. This measure correlated inversely with pulmonary capillary wedge pressures in hospitalized patients in one study,53 and it began decreasing approximately 2 weeks before hospitalization for ADHF. However, assessment in recent large scale trials (e.g., SENSE-HF) has indicated that this has an indifferent predictive value. Impedance vectors employing a left ventricular (LV) lead may improve this yield.54 Direct hemodynamic assessments (e.g., pulmonary artery or left atrial pressure) may reduce heart failure hospitalizations.55–57
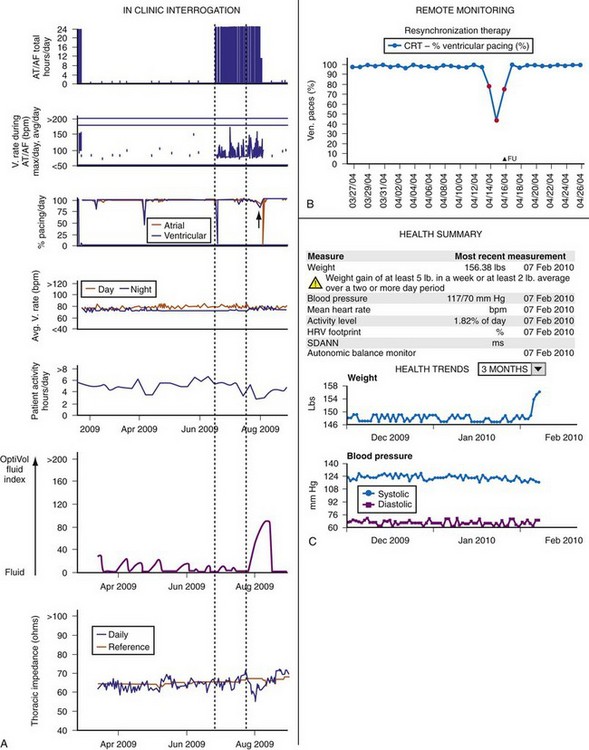
Figure 32-12 Heart failure diagnostics.
(A modified from Varma N: Automatic remote monitoring of implantable cardiac devices in heart failure: the TRUST trial. J Innov Card Rhythm Manage 1:22-29, 2010.)
Data from these studies applied to remote follow-up may propel development of leadless stand-alone devices acting as automatic physiologic monitors. Other derived features include heart rate variability (HRV), calculated by continuous measurement of atrial-to-atrial depolarization intervals during predominant sinus rhythm. Both the absolute value and its change over time are important markers of clinical stability.58 A decrease in HRV preceded clinical decompensation by 16 days in one large study.59 These analyses may be performed best by data collected remotely.
Therefore, device-based physiologic information and diagnostics support the concept that several interdependent cardiovascular factors may change several days to weeks before patients present with volume overload necessitating hospitalization. All the features reported merit testing in clinical trials.60 To be effective, features need to be specific and have a high predictive value for ADHF requiring hospitalization and enable implementation of a treatment algorithm that may prevent hospitalization. In this regard, preliminary data are modest. For example, fluid sensors demonstrated 60% sensitivity with 60% positive predictive value for detecting hospitalization. The false-positive rate was 38%.61 However, early discovery of out-of-bounds parameters may enable earlier and more effective intervention. In this regard, remote monitoring such as HM, which automatically establishes trends with prompt notification of deviations, has considerable value.62 Remote care strategies require prospective evaluation of change-detection algorithms, as in ongoing trials.63
 Lead and Device Performance
Lead and Device Performance
The last several years have witnessed a series of well-publicized problems associated with CIED performance, which continue. Although the absolute incidence usually has been low, the issues have highlighted the lack of a robust monitoring mechanism. Assessing system performance is challenging in view of increasing volume and device complexity, but it is an important responsibility for both physician and industry, recognized by recent position statements.2–4 The Fidelis lead advisory illustrates that innovative designs may degrade rather than enhance performance32,33 (see Fig. 32-8). Critical system problems cluster around battery depletion, HV circuitry failure, and lead failure.26 These are often clinically asymptomatic and could present with sudden death in the event of lack of appropriate therapy, as with lead failure in a pacemaker-dependent patient or undersensing of ventricular arrhythmias in an ICD patient. Alternatively, problems could present with extreme patient morbidity, as with lead failure causing multiple inappropriate ICD therapy from oversensing of noise. Premature battery failure may be catastrophic (see Fig. 32-9). Early detection of these complications is desirable to ensure patient safety.
The demand for stringent performance evaluation contrasts with the techniques employed to date. Methods based on voluntary return of products are vulnerable to reporting bias, resulting in incomplete and nonvalidated data.64 ICD generator malfunction is acknowledged to be low (0.003 per patient-year was reported from a meta-analysis of device registries), but this is still an order of magnitude higher than for pacemakers, emphasizing the need for monitoring.65 Lead malfunction is more frequent, but rates reported vary sharply among independent studies: 0.6% at 1 year66 and 2.5% to 15% at 5 years.67,68 Malfunction rates reported vary even for a single component in different studies.69 This likely reflects inconsistent methods of data collection and lack of uniform definitions of normal performance versus malfunction. Thus, in one study, problems were discovered during routine face-to-face follow-up, and reprogramming changes without surgical intervention were included in the “failure” rate.67 In contrast, in another study, 76% of lead malfunction came to clinical attention because of inappropriate ICD therapies, and the need for surgical revision defined failure.68 Both reports may underestimate the true incidence of lead failure if malfunctions are asymptomatic or intermittent or result in death (only a minority of devices are interrogated postmortem).
Automatic remote monitoring resolves many of these problems. In TRUST the detection method (HM) for system performance was independent of symptoms, follow-up schedule, or patient in-clinic attendance. Additionally, TRUST used a uniform definition for out-of-range behavior and prespecified the recording of clinical actions requiring surgical intervention. The results demonstrated very high system reliability of the ICD system used. Malfunction, when it occurred, was often asymptomatic but could be handled with reprogramming (see Fig. 32-11) and required system revision infrequently. Importantly, remote monitoring detected more patients with device-related issues than calendar-based in-person interrogations.70 Thus, conventional follow-up may underreport device malfunctions, although monitoring guidelines indicate that patients may be followed either in person or remotely.2 HM permitted prompt evaluation of life-threatening problems, including disintegration of HV circuitry and battery or lead failure persisting asymptomatically33,71,72 (see Figs. 32-8, 32-9, and 32-11). Transient asymptomatic sensing issues (e.g., nonphysiologic electrical signals) could be diagnosed rapidly and managed conservatively by reprogramming, thus avoiding inappropriate therapies.70,73 The study creates a device management model in which near-continuous surveillance is combined with an automatic ability for self-declaration of system problems. The timeliness and quality of transmitted information enable it to be used as a rigorous mechanism for monitoring system performance, directing prompt therapeutic interventions when required. Automatic archiving of longitudinally collected data with high temporal (24-hour) definition and detection of sudden parameter deviations from baseline trends facilitates assessment of system performance. These features meet the Heart Rhythm Society’s call for a technology providing continuous surveillance, rapid problem identification and notification, and system performance monitoring.3,4
 Economic and Regulatory Considerations
Economic and Regulatory Considerations
Organizational costs may be reduced by preventing unnecessary hospital utilization by nonactionable scheduled or unscheduled visits (e.g., ER attendance due to phantom shocks). Cost benefits are favorable for patients living at greater distances and for facilities that use remote monitoring for large proportions of their patients, and these accrue with time.16,74,75 Although physicians regard prompt notification of life-threatening events as valuable, it remains to be proven whether early diagnosis and timely intervention of serious medical events via remote monitoring reduces hospital admissions and associated costs. However, attaching a value to the economic benefits of remote monitoring also depends on health care models. For example, the cost savings observed in some countries may not be easily transferable to other countries, or indeed within a single country from one health care system to another.
Reimbursement tends to spur adoption and implementation of new technologies. Reimbursement guidelines for remote interrogation and in-person programming evaluations were established in January 2009 in the United States (http://www.ama-assn.org/) with CPT (current procedural terminology) codes for monitoring and technical support (which need not be permanent). A single fee is provided for all remote interrogations performed within a 90-day period even if some individual patients may generate several transmissions. However, remote interrogations that prompt in-person programming evaluations may be billed as well. The programming evaluation, which can also be done at least yearly, includes an interrogation of capture, sensing, sensor, rhythms, and overall function through the programmer. Permanent alteration of programmed parameters is not necessary to qualify the in-person evaluation as a programming evaluation.
Integration of Services
Device data retrieved by remote monitoring may be of potential value to several health care providers involved with a patient’s care. This may variously be the implanting or referring physician or heart failure specialist. A communication system is necessary to direct appropriate data to the responsible physician to enable this process. Multidisciplinary efforts aid patient care when applied to a CRT optimization clinic.76 Access to Internet-based information systems may provide a framework for such collaborative models, such as electrophysiologists monitoring device function (arrhythmias, paced burden) and heart failure experts assessing hemodynamic parameters. This may be facilitated by remote monitoring interfacing with electronic medical records, thus immediately placing all relevant data in a single, central database accessible by all treating physicians.
Regulations
In the United States there is a legal requirement for postmarket registration and tracking of CIEDs.77 Data registration and maintenance are responsibilities shared among implanting physician, center, and manufacturer. These mandates necessitate documentation of model and serial numbers of CIED components as well as patient demographics and communication details. CIED revisions and replacements need to be similarly tracked. Access to these data becomes critical if a patient presents emergently or when a field safety corrective action is issued by regulatory agencies. A database that directly communicates with the registration system would automatically state the CIED follow-up physician and update patient demographics and contact information.
Repositories such as the National Cardiovascular Device Registry (NCDR) are important to permit analysis of utilization, programming, and monitoring of system performance and to enhance understanding of treatment effects in different subgroups and disease processes.78 Automatic remote monitoring has a critical role. The ability to collect detailed device and patient data, with component function and clinical parameters assessed daily and automatically, sets a precedent for longitudinal evaluation to follow patient condition and establish norms for lead and generator performance. Automatic data upload and trending reduce the margin of error associated with manual data entry in an era of advancing complexity. These analyses are important not only to practicing physicians but also to private and public insurance agencies, regulatory agencies, professional bodies, and ministries of health that enable provision of these services.
Medicolegal Issues
Several medicolegal issues must be addressed. Foremost, technologies can now deliver important information within seconds,33 but response from medical teams may take longer. This may create issues regarding delayed response to a spontaneous transmission of a life-threatening event. Responsibilities of all the parties involved in remote monitoring for specific aspects of the process have been outlined in a recent expert consensus document.2 Patients need to be informed of the purpose, use, and limitations of the technology, and that although the incoming data are verified regularly, remote monitoring is not an emergency information system. Patient inclusion in the responsibility of acquisition, management, and therapy implementation of remotely acquired data may be an appropriate step. Transmitted data, especially with systems providing worldwide cellular links, permit medical decisions to be made across state and international boundaries. Issues of confidentiality and medical responsibility will require further definition. (See Chapter 35 for ethical issues involving CIEDs.)
1 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51:e1-e62.
2 Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907-925.
3 Carlson MD, Wilkoff BL, Maisel WH, et al. Recommendations from the Heart Rhythm Society Task Force on Device Performance Policies and Guidelines Endorsed by the American College of Cardiology Foundation (ACCF) and the American Heart Association (AHA) and the International Coalition of Pacing and Electrophysiology Organizations (COPE). Heart Rhythm. 2006;3:1250-1273.
4 Maisel WH, Hauser RG, Hammill SC, et al. Recommendations from the Heart Rhythm Society Task Force on Lead Performance Policies and Guidelines: developed in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009;6:869-885.
5 Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;95(suppl 3):3-9.
6 Senges-Becker JC, Klostermann M, Becker R, et al. What is the “optimal” follow-up schedule for ICD patients? Europace. 2005;7:319-326.
7 Varma N, TRUST Investigators. Problem discovery rates of scheduled vs unscheduled and in-patient vs remote evaluations during post-implant ICD monitoring: actionability vs futility in the TRUST Trial. Heart Rhythm. 2010;7:S301.
8 Furman S, Escher DJ. Transtelephone pacemaker monitoring: five years later. Ann Thorac Surg. 1975;20:326-338.
9 Crossley GH, Chen J, Choucair W, et al. Clinical benefits of remote versus transtelephonic monitoring of implanted pacemakers. J Am Coll Cardiol. 2009;54:2012-2019.
10 Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30(suppl 1):2-12.
11 Nielsen JC, Kottkamp H, Zabel M, et al. Automatic home monitoring of implantable cardioverter defibrillators. Europace. 2008;10:729-735.
12 Varma N, Stambler B, Chun S. Detection of atrial fibrillation by implanted devices with wireless data transmission capability. Pacing Clin Electrophysiol. 2005;28(suppl 1):133-136.
13 Joseph GK, Wilkoff BL, Dresing T, et al. Remote interrogation and monitoring of implantable cardioverter defibrillators. J Interv Card Electrophysiol. 2004;11:161-166.
14 Schoenfeld MH, Compton SJ, Mead RH, et al. Remote monitoring of implantable cardioverter defibrillators: a prospective analysis. Pacing Clin Electrophysiol. 2004;27:757-763.
15 US Food and Drug Administration (FDA). Home Monitoring approval. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm113561.htm, 2001.
16 Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164-170.
17 Blumber S, Luje J. Wireless substitution: early release of estimates from the National Health Interview Survey (NHIS). National Center for Health Statistics http://www.cdc.gov/nchs/nhis.htm
18 Heidbuchel H, Lioen P, Foulon S, et al. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10:351-357.
19 Varma N. Rationale and design of a prospective study of the efficacy of a remote monitoring system used in implantable cardioverter defibrillator follow-up: the Lumos-T Reduces Routine Office Device Follow-Up Study (TRUST). Am Heart J. 2007;154:1029-1034.
20 Varma N, Epstein A, Irimpen A, et al. Efficacy and safety of automatic remote monitoring for ICD follow-up: the TRUST trial. Circulation. 2010;122:325-332.
21 Crossley G, Boyle A, Vitense H, et al. The Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision (CONNECT) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181-1189.
22 Varma N. Automatic remote monitoring of implantable cardiac devices in heart failure: the TRUST trial. J Innov Card Rhythm Manage. 2010;1:22-29.
23 Zartner P, Handke R, Photiadis J, et al. Performance of an autonomous telemonitoring system in children and young adults with congenital heart diseases. Pacing Clin Electrophysiol. 2008;31:1291-1299.
24 Fye WB. Cardiology workforce: there’s already a shortage, and it’s getting worse!. J Am Coll Cardiol. 2002;39:2077-2079.
25 Lee DS, Krahn AD, Healey JS, et al. Evaluation of early complications related to de novo cardioverter-defibrillator implantation: insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55:774-782.
26 Hauser RG, Kallinen L. Deaths associated with implantable cardioverter-defibrillator failure and deactivation reported in the United States Food and Drug Administration Manufacturer and User Facility Device Experience Database. Heart Rhythm. 2004;1:399-405.
27 Varma N, Epstein A, Irimpen A, et al. Event notifications by remote monitoring systems performing automatic daily checks: load, characteristics and clinical utility. Eur Heart J. 2009;30:1909.
28 Theuns DA, Rivero-Ayerza M, Knops P, et al. Analysis of 57,148 transmissions by remote monitoring of implantable cardioverter defibrillators. Pacing Clin Electrophysiol. 2009;32(suppl 1):63-65.
29 Raatikainen MJ, Uusimaa P, van Ginneken MM, et al. Remote monitoring of implantable cardioverter defibrillator patients: a safe, time-saving, and cost-effective means for follow-up. Europace. 2008;10:1145-1151.
30 US Food and Drug Administration (FDA). Home Monitoring approval. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm166550.htm, 2009. P050023/S020
31 Al-Khatib SM, Piccini JP, Knight D, et al. Remote monitoring of implantable cardioverter defibrillators versus quarterly device interrogations in clinic: results from a randomized pilot clinical trial. J Cardiovasc Electrophysiol. 2010;21:545-550.
32 Medtronic. Physician advisory letter: Urgent medical device information, Sprint Fidelis lead, patient management recommendations. http://www.medtronic.com/product-advisories/physician/sprint-fidelis/PROD-ADV-PHYS-OCT.htm, 2007.
33 Varma N. Remote monitoring for advisories: automatic early detection of silent lead failure. Pacing Clin Electrophysiol. 2009;32:525-527.
34 Varma N, Stambler BS. Patient aspects of remote monitoring. Circulation. 2011;123(7):e247.
35 Ricci RP, Morichelli L, Quarta L, et al. Long-term patient acceptance of and satisfaction with implanted device remote monitoring. Europace. 2010;12:674-679.
36 Ehrlich JR, Hohnloser SH. Milestones in the management of atrial fibrillation. Heart Rhythm. 2009;6:S62-S67.
37 Bunch TJ, Day JD, Olshansky B, et al. Newly detected atrial fibrillation in patients with an implantable cardioverter-defibrillator is a strong risk marker of increased mortality. Heart Rhythm. 2009;6:2-8.
38 Stein KM, Euler DE, Mehra R, et al. Do atrial tachyarrhythmias beget ventricular tachyarrhythmias in defibrillator recipients? J Am Coll Cardiol. 2002;40:335-340.
39 Ricci RP, Morichelli L, Santini M. Remote control of implanted devices through home monitoring technology improves detection and clinical management of atrial fibrillation. Europace. 2009;11:54-61.
40 Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20:241-248.
41 Ricci RP, Morichelli L, Gargaro A, et al. Home monitoring in patients with implantable cardiac devices: is there a potential reduction of stroke risk? Results from a computer model tested through monte carlo simulations. J Cardiovasc Electrophysiol. 2009;20:1244-1251.
42 Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350:2151-2158.
43 Varma N, Johnson MA. Prevalence of cancelled shock therapy and relationship to shock delivery in recipients of implantable cardioverter-defibrillators assessed by remote monitoring. Pacing Clin Electrophysiol. 2009;32(suppl 1):42-46.
44 Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009-1017.
45 Sweeney MO, Sherfesee L, Degroot PJ, et al. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:353-360.
46 Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69-e171.
47 Pulignano G, Del Sindaco D, Tavazzi L, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF Registry). Am Heart J. 2002;143:45-55.
48 Blackledge HM, Tomlinson J, Squire IB. Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12,220 index admissions in Leicestershire 1993-2001. Heart. 2003;89:615-620.
49 Varma N, Wilkoff BL. Device features for heart failure. Heart Fail Clin North Am. 7, 2011.
50 Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288:3115-3123.
51 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539-1549.
52 Koplan BA, Kaplan AJ, Weiner S, et al. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53:355-360.
53 Yu CM, Wang L, Chau E, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112:841-848.
54 Khoury DS, Naware M, Siou J, et al. Ambulatory monitoring of congestive heart failure by multiple bioelectric impedance vectors. J Am Coll Cardiol. 2009;53:1075-1081.
55 Bourge RC, Abraham WT, Adamson PB, et al. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073-1079.
56 Hoppe UC, Vanderheyden M, Sievert H, et al. Chronic monitoring of pulmonary artery pressure in patients with severe heart failure: multicentre experience of the monitoring Pulmonary Artery Pressure by Implantable device Responding to Ultrasonic Signal (PAPIRUS) II study. Heart. 2009;95:1091-1097.
57 Ritzema J, Melton IC, Richards AM, et al. Direct left atrial pressure monitoring in ambulatory heart failure patients: initial experience with a new permanent implantable device. Circulation. 2007;116:2952-2959.
58 Adamson PB, Kleckner KJ, VanHout WL, et al. Cardiac resynchronization therapy improves heart rate variability in patients with symptomatic heart failure. Circulation. 2003;108:266-269.
59 Adamson PB, Smith AL, Abraham WT, et al. Continuous autonomic assessment in patients with symptomatic heart failure: prognostic value of heart rate variability measured by an implanted cardiac resynchronization device. Circulation. 2004;110:2389-2394.
60 Adamson PB, Conti JB, Smith AL, et al. Reducing Events in Patients with Chronic Heart Failure (ReduceHF) study design: continuous hemodynamic monitoring with an implantable defibrillator. Clin Cardiol. 2007;30:567-575.
61 Vollmann D, Nagele H, Schauerte P, et al. Clinical utility of intrathoracic impedance monitoring to alert patients with an implanted device of deteriorating chronic heart failure. Eur Heart J. 2007;28:1835-1840.
62 Ellery S, Pakrashi T, Paul V, Sack S. Predicting mortality and rehospitalization in heart failure patients with home monitoring: the Home CARE pilot study. Clin Res Cardiol. 2006;95(suppl 3):29-35.
63 Arya A, Block M, Kautzner J, et al. Influence of home monitoring on the clinical status of heart failure patients: design and rationale of the IN-TIME study. Eur J Heart Fail. 2008;10:1143-1148.
64 Hauser RG, Hayes DL, Epstein AE, et al. Multicenter experience with failed and recalled implantable cardioverter-defibrillator pulse generators. Heart Rhythm. 2006;3:640-644.
65 Maisel WH. Pacemaker and ICD generator reliability: meta-analysis of device registries. JAMA. 2006;295:1929-1934.
66 Borleffs CJ, van Erven L, van Bommel RJ, et al. Risk of failure of transvenous implantable cardioverter-defibrillator leads. Circ Arrhythm Electrophysiol. 2009;2:411-416.
67 Kleemann T, Becker T, Doenges K, et al. Annual rate of transvenous defibrillation lead defects in implantable cardioverter-defibrillators over a period of >10 years. Circulation. 2007;115:2474-2480.
68 Eckstein J, Koller MT, Zabel M, et al. Necessity for surgical revision of defibrillator leads implanted long-term: causes and management. Circulation. 2008;117:2727-2733.
69 Hauser RG, Hayes DL. Increasing hazard of Sprint Fidelis implantable cardioverter-defibrillator lead failure. Heart Rhythm. 2009;6:605-610.
70 Varma N, Michalski J, Epstein A, Schweikert R. Automatic remote monitoring of ICD lead and generator performance: the TRUST trial. Circ Arrhythm Electrophysiol. 2010;3:428-436.
71 Varma N, Cunningham D, Falk R. Central venous access resulting in selective failure of ICD defibrillation capacity. Pacing Clin Electrophysiol. 2001;24:394-395.
72 Hauser RG, Hayes DL, Almquist AK, et al. Unexpected ICD pulse generator failure due to electronic circuit damage caused by electrical overstress. Pacing Clin Electrophysiol. 2001;24:1046-1054.
73 Spencker S, Coban N, Koch L, et al. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483-488.
74 Elsner CH, Sommer P, Piorkowski C, et al. A prospective multicenter comparison trial of home monitoring against regular follow-up in MADIT II patients: additional visits and cost impact. Comput Cardiol. 2006;33:241-244.
75 Fauchier L, Sadoul N, Kouakam C, et al. Potential cost savings by telemedicine-assisted long-term care of implantable cardioverter defibrillator recipients. Pacing Clin Electrophysiol. 2005;28(suppl 1):255-259.
76 Mullens W, Grimm RA, Verga T, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765-773.
77 Furman S. Safe Medical Devices Act of 1990. Pacing Clin Electrophysiol. 1991;14:387-388.
78 Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6:1397-1401.