23 Focal axillary hyperhidrosis
Summary and Key Features
• Sweating is controlled by the sympathetic nervous system; however, eccrine glands are activated by acetylcholine
• Eccrine glands in patients with primary hyperhidrosis do not demonstrate any histopathologic changes or glandular hyperplasia
• Hyperhidrosis prevalence is reported to be 2.8%, though it may be underestimated owing to undiagnosed cases
• The hyperhidrosis disease severity scale (HDSS) is a validated scale that is used in selecting patients appropriate for therapy and assessing effectiveness of treatment
• The starch iodine test is a simple, colormetric test to detect the presence of active sweat glands and identify the surface area involved. In the event of a negative or equivocal result, the hair-bearing skin is a good estimate of the area necessary to treat
• The axillary procedure is well tolerated; no anesthesia is required as pain is minimal
• OnabotulinumtoxinA is FDA approved for treatment of axillary hyperhidrosis. When injected at the depth of the deep dermis and subcutaneous tissue, the chemodenervation is localized, reversible, and long lasting
• Currently the US standard dose listed in the package insert for onabotulinumtoxinA is 50 units per axilla
• An average of 10–15 injections (0.1–0.2 mL each) should be delivered to each axilla
• If symptoms persist after treatment, repeat the starch iodine test to detect any area that may have been missed. A small area of active sweating is easily touched up with 1–2 injections
• If treatment response is overall inadequate or short lived (e.g. 1–2 months), simply increase the dose of onabotulinumtoxinA to 100 units per axilla
• Retreatment with onabotulinumtoxinA will average every 6–7 months, but can vary depending on the patient’s response
Introduction
Hyperhidrosis
Hyperhidrosis may be classified as generalized or focal. Generalized hyperhidrosis typically occurs as the result of an underlying cause (secondary origin). Focal or localized hyperhidrosis may have a secondary origin; for example, lesions or tumors of the central or peripheral nervous system. However, more commonly, focal hyperhidrosis is idiopathic (primary origin) and referred to simply as ‘hyperhidrosis’ (HH). Diagnostic criteria for primary hyperhidrosis have been suggested by a consensus panel (Box 23.1). Evaluation and testing should be tailored by the history and review of systems. This chapter will focus on primary focal axillary hyperhidrosis, henceforth identified simply as axillary hyperhidrosis (AHH).
Epidemiology
The prevalence of HH was reported by Strutton et al to be 2.8%, though it may be underestimated due to undiagnosed cases. The prevalence is similar for men and women, although interestingly, Lear and colleagues reported that women are more likely to seek evaluation and treatment. Trigger factors include emotional stress, higher environmental temperatures, and stimulants such as caffeine. However, episodes of excessive sweating can occur without any known trigger. The body areas most often affected are the axilla, palms, soles, craniofacial, inframammary and groin (Table 23.1).
Table 23.1 Hyperhidrosis site prevalence in a patient population reported with respect to each site*
| Anatomical site | Prevalence (%) |
|---|---|
| Axilla | 73 |
| Palms | 45.9 |
| Soles | 41.1 |
| Face / scalp | 22.8 |
| Groin | 9.3 |
| Other sites (eg. Inframammary, Buttocks, etc.) | 9.6 |
* Data from Lear W, Kessler E, Solish N, et al 2007 An epidemiological study of hyperhidrosis. Dermatologic Surgery 33:S69–S75.
Quality of life
The hyperhidrosis disease severity scale (HDSS) is a validated scale that is used in selecting patients appropriate for therapy and assessing effectiveness of treatment. The HDSS is based on one question the patient can answer in the office: ‘Which best describes the impact of sweating on your daily activity?’ The answer is rated with a single value 1–4, with 3–4 corresponding to uncontrolled severe HH (Table 23.2). It is frequently used in clinical practice to assess the need for therapy and response to it.
Table 23.2 Hyperhidrosis disease severity scale question:
Which best describes the impact of sweating on your daily activity?
| Score | Answer |
|---|---|
| 1 | My (underarm) sweating is never noticeable and never interferes with my daily activity. |
| 2 | My (underarm) sweating is tolerable but sometimes interferes with my daily activity. |
| 3 | My (underarm) sweating is barely tolerable and frequently interferes with my daily activity. |
| 4 | My (underarm) sweating is intolerable and always interferes with my daily activity. |
Clinical assessment of hyperhidrosis
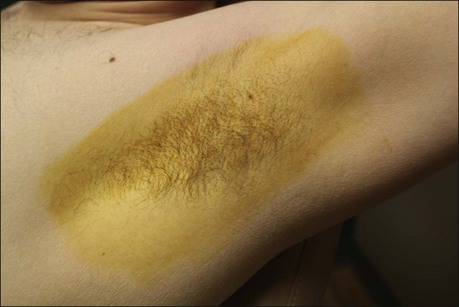
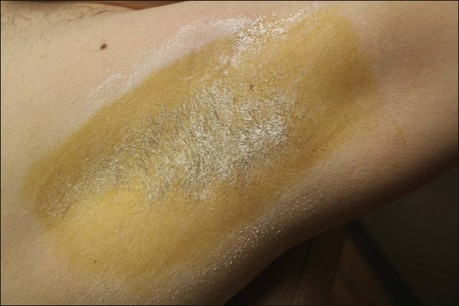
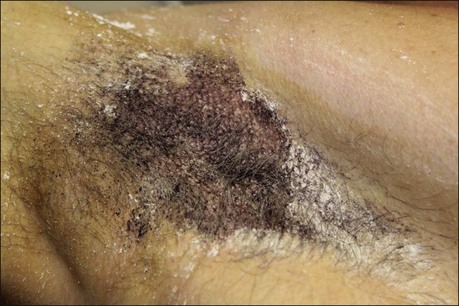
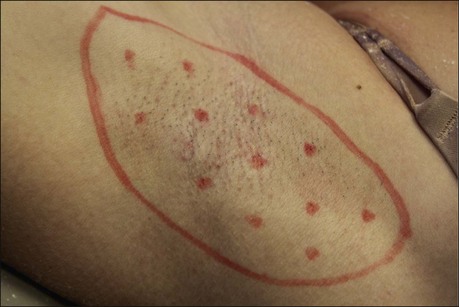
The starch iodine test is a simple way to detect the presence of sweat and identify the surface area involved, however it is not quantitative. The test is effective on both shaved and non-shaved skin. To perform, the skin to be tested is completely dried using a towel. An iodine solution (commonly Betadine®) is painted over the field and allowed to dry (Fig. 23.1). After the solution is thoroughly dry, a starch powder such as plain cooking corn starch is sprinkled on the surface. Various tools may be used to accomplish the distribution of starch such as loose gauze, cosmetic brushes or a fine-opening shaker (Fig. 23.2). Accurate colormetric results are achieved when the amount of powder is minimized. Moisture from sweat dissolves the iodine and starch followed by a chemical reaction producing a purple to black color. True positive reactions have a speckled appearance as moisture is released from duct openings (Fig. 23.3). For iodine-sensitive patients, Alizarin or Ponceau red dye and starch can be used instead. Regardless of which is used, a colorimetric outline of the sweating area is achieved.
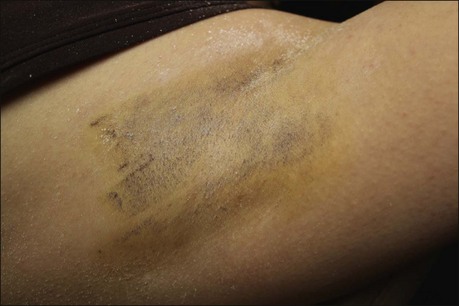
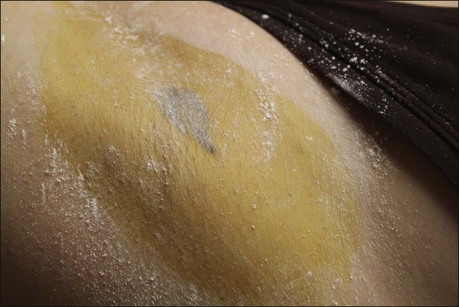
False positives occur if the skin has not been thoroughly dried of sweat or if iodine solution is not thoroughly dry prior to starch application. This appears as streaks or smears of dark pigment (Fig. 23.4). False negatives may occur if starch is applied too heavily (Box 23.2).
Box 23.2
Key features of a successful starch iodine test
2. Sweat must be thoroughly dried from skin.
3. Iodine in castor oil or a Betadine® swab is used to apply a layer of iodine to a wide area of skin.
4. Iodine must dry before applying starch powder.
5. Standard cooking corn starch gives superb results compared with other starch products.
6. Sprinkle starch powder, rubbing may create smear artifacts.
7. Minimizing the amount of starch powder gives most accurate results.
8. In the event of a negative or equivocal result, the hair-bearing skin is a good estimate of the area necessary to treat.
Axillary hyperhidrosis
To concentrate the BoNT-A into the affected area and optimize treatment, the area of involvement should be identified by a starch iodine test (as previously reviewed). Injection points may be marked with a pen to ensure adequate treatment (Fig. 23.5). The axilla does not need to be shaved prior to performing a starch iodine test or before injecting BoNT-A. The BoNT-A is injected into the deep dermis at the dermal subcutaneous level. Each injection is placed 1–2 cm apart. Thin axillary skin allows for visualization of a wheal at each site (Fig. 23.6). An average of 10–15 injections per axilla are required, but will depend on the size of the axilla and hyperhidrodic area. In the event that a starch iodine test cannot be performed prior to treatment or the test is equivocal, the physician should treat the hair-bearing areas using the same technique (Box 23.3).
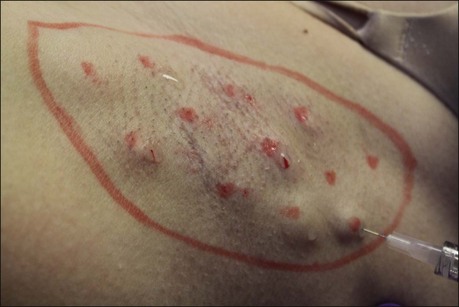
Figure 23.6 Depth of injection is at the dermal–subcutaneous junction. In the axilla, a small wheal can be visualized.
Box 23.3
Key features of Botox® treatment of axillae
1. Rule out contraindications of botulinum toxin therapy.
2. Identify involved surface area using starch iodine test or other colormetric test.
3. Axillary pain is minimal; anesthesia is predominantly unnecessary.
4. Reconstitute 100 units (one standard vial) of onabotulinumtoxinA with 4 mL of sterile saline.
5. Fifty units of onabotulinumtoxinA (2 mL) are injected per axilla.
6. Depending on the size of the axilla, an average of 15 injection points per axilla are used.
7. Injections are delivered at deep dermal to superficial subcutaneous depth.
Should symptoms fail to improve within 2 weeks, the patient should return to the office for a repeat starch iodine test to identify any persistently ‘active’ eccrine glands (Fig. 23.7). The skin should be injected with 3–5 units of BoNT-A for each centimeter surface area identified.
Alam M, Dover JS, Arndt KA. Pain associated with injection of botulinum A exotoxin reconstituted using isotonic sodium chloride with and without preservative: a double-blind, randomized controlled trial. Archives of Dermatology. 2002;138:510–514.
Kowalski J, Ravelo A, Glaser D, et al 2004 Quality-of-life effect of botulinum toxin type A on patients with primary axillary hyperhidrosis: Results from a North American Clinical Study Population. Poster 196. American Academy of Dermatology 62nd Annual Meeting.
Birklein F, Eisenbarth G, Erbguth F, et al. Botulinum toxin type B blocks sudomotor function effectively: a 6 month follow up. Journal of Investigative Dermatology. 2003;121:1312–1316.
Cheshire WP, Freeman R. Disorders of sweating. Seminars in Neurology. 2003;23:399–406.
Dressler D, Adib Saberi F, Benecke R. Botulinum toxin type B for treatment of axillar hyperhidrosis. Journal of Neurology. 2002;249:1729–1732.
Frasson E, Brigo F, Acler M, et al. Botulinum toxin type A vs type B for axillary hyperhidrosis in a case series of patients observed for 6 months. Archives of Dermatology. 2011;147:122–123.
Glaser D, Kowalski J, Eadie N, et al, Hyperhidrosis disease severity scale (HDSS): Validity and reliability results from three studies. Poster presented at the 62nd Annual Meeting of the American Academy of Dermatology, 6–10 February. Washington DC, p 198, 2004.
Glaser DA, Hebert AA, Pariser DM, et al. Facial hyperhidrosis: best practice recommendations and special considerations. Cutis. 2007;79:29–32.
Glogau RG. Topically applied botulinum toxin type A for the treatment of primary axillary hyperhidrosis: results of a randomized, blinded, vehicle-controlled study. Dermatologic Surgery. 2007;33:S76–S80.
Goldsmith L. Biology of ecrine and apocrine sweat glands. In: Freedberg IM, Fitzpatrick TB. Fitzpatrick’s dermatology in general medicine. 5th edn. New York: McGraw-Hill, Health Professions Division; 1999:157–164.
Hamm H, Naumann MK, Kowalski JW, et al. Primary focal hyperhidrosis: disease characteristics and functional impairment. Dermatology. 2006;212:343–353.
Heckmann M, Breit S, Ceballos-Baumann A, et al. Side-controlled intradermal injection of botulinum toxin A in recalcitrant axillary hyperhidrosis. Journal of the American Academy of Dermatology. 1999;41:987–990.
Hornberger J, Grimes K, Naumann M, et al. Recognition, diagnosis, and treatment of primary focal hyperhidrosis. Journal of the American Academy of Dermatology. 2004;51:274–286.
Huh CH, Han KH, Seo KI, et al. Botulinum toxin treatment for a compensatory hyperhidrosis subsequent to an upper thoracic sympathectomy. Journal of Dermatological Treatment. 2002;13:91–93.
Hund M, Rickert S, Kinkelin I, et al. Does wrist nerve block influence the result of botulinum toxin A treatment in palmar hyperhidrosis? Journal of the American Academy of Dermatology. 2004;50:61–62.
Kavanagh GM, Oh C, Shams K. BOTOX delivery by iontophoresis. British Journal of Dermatology. 2004;151:1093–1095.
Lear W, Kessler E, Solish N, et al. An epidemiological study of hyperhidrosis. Dermatologic Surgery. 2007;33:S69–S75.
Lowe N, Campanati A, Bodokh I, et al. The place of botulinum toxin type A in the treatment of focal hyperhidrosis. British Journal of Dermatology. 2004;151:1115–1122.
Lowe NJ, Glaser DA, Eadie N, et al. Botulinum toxin type A in the treatment of primary axillary hyperhidrosis: a 52-week multicenter double-blind, randomized, placebo-controlled study of efficacy and safety. Journal of the American Academy of Dermatology. 2007;56:604–611.
Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. British Medical Journal. 2001;323:596–599.
Nelson L, Bachoo P, Holmes J. Botulinum toxin type B: a new therapy for axillary hyperhidrosis. British Journal of Plastic Surgery. 2005;58:228–232.
Reisfeld R, Nguyen R, Pnini A. Endoscopic thoracic sympathectomy for treatment of essential hyperhidrosis syndrome: experience with 650 patients. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques. 2000;10:5–10.
Saadia D, Voustianiouk A, Wang AK, et al. Botulinum toxin type A in primary palmar hyperhidrosis: randomized, single-blind, two-dose study. Neurology. 2001;57:2095–2099.
Sarifakioglu N, Sarifakioglu E. Evaluating effects of preservative-containing saline solution on pain perception during botulinum toxin type-a injections at different locations: a prospective, single-blinded, randomized controlled trial. Aesthetic Plastic Surgery. 2005;29:113–115.
Schlereth T, Mouka I, Eisenbarth G, et al. Botulinum toxin A (Botox) and sweating-dose efficacy and comparison to other BoNT preparations. Autonomic Neuroscience. 2005;117:120–126.
Strutton DR, Kowalski JW, Glaser DA, et al. US prevalence of hyperhidrosis and impact on individuals with axillary hyperhidrosis: results from a national survey. Journal of the American Academy of Dermatology. 2004;51:241–248.
Wollina U, Karamfilov T. Botulinum toxin A for palmar hyperhidrosis. Journal of the European Academy of Dermatology and Venereology. 2001;15:555–558.
Wollina U, Karamfilov T, Konrad H. High-dose botulinum toxin type A therapy for axillary hyperhidrosis markedly prolongs the relapse-free interval. Journal of the American Academy of Dermatology. 2002;46:536–540.