CHAPTER 8 Fluoroscopic examinations of the pharynx, esophagus and stomach
Introduction
Roentgen’s first publication on the discovery of x-rays and the x-ray absorption characteristics of metals and salts was in 1895. The potential for fluoroscopic examination of the upper gastrointestinal tract with contrast medium was raised immediately after Roentgen’s seminal paper. In 1896, the German physician Wolf Becher utilized Roentgen’s work to develop the concept of using contrast medium to provide an image of the stomach (Thomas, 1998).
Walter Bradford Cannon, a physiologist at Harvard University, studied digestion in animals using bismuth and x-rays. Using heavy metal salts mixed with food, Cannon studied gastrointestinal motility, tracing the fluoroscopic appearance of contrast through the alimentary tract. Changing from bismuth to barium in 1924, Cannon adapted the study for humans (Sebastian, 1999).
The barium swallow
The barium swallow is a simple, safe and effective examination for patients with symptoms of dysphagia. It can provide a significant amount of information both on its own or complementary to endoscopy or esophageal motility studies (Esfandyari et al., 2002; Hansmann and Grenacher, 2006).
The clinical indications for a barium swallow are high (pharyngeal) or low (esophageal/esophago-gastric) dysphagia, the root cause being either neuromuscular or mechanical. Common causes for high and low dysphagia are given in Table 8.1.
| High (pharyngeal) dysphagia | Low (esophageal/gastro-esophageal) dysphagia |
|---|---|
CVA: cerebrovascular accident
The objective of the barium swallow is for the practitioner to:
Pharyngeal and esophageal swallow technique
Before commencement of formal imaging, the patient is given an initial swallow of barium, and its passage to the stomach is screened to check there is no mechanical obstruction. The patient is placed in the right anterior oblique or lateral position as this projects the esophagus away from the spine and trachea. In this position, there is a better chance of differentiating barium in the trachea originating from a tracheo-esophageal fistula from that which has been aspirated. Regardless of the cause of the barium in the trachea, the patient will cough if that reflex is present. Coughing will result in the barium coating the trachea which might subsequently make it difficult to determine the cause for the barium being present.
Imaging
As an optimal minimum, imaging patients with high dysphagia should include videofluoroscopy or rapid imaging sequences of the pharynx (e.g. at least 3–4 frames per second). Videofluoroscopic assessment of swallowing disorders is expored further in Chapter 7.
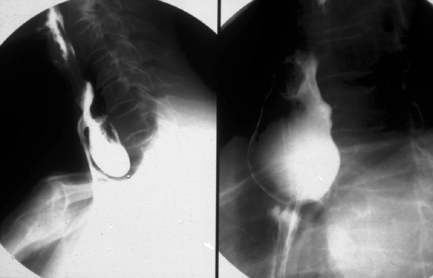
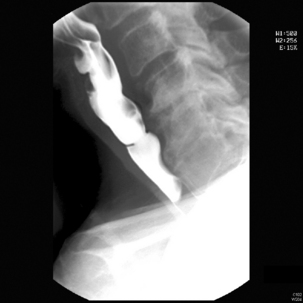
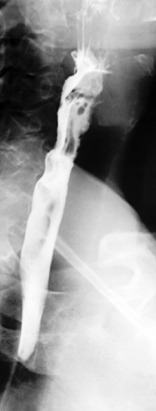
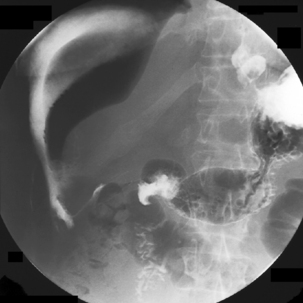
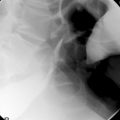
The pharynx is examined in the lateral, the left or right anterior oblique, and the anteroposterior projection. The alternate oblique need only be considered if an abnormality is suggested. If pharyngeal imaging options are limited, the lateral projection provides the greatest breadth of information and can demonstrate aspiration and pharyngeal abnormalities such as pharyngeal pouch, webs, pharyngeal neoplasm, prominence of the cricopharyngeus (Figures 8.1, 8.2, 8.3, 8.4 and 8.5) and osteophyte impression.
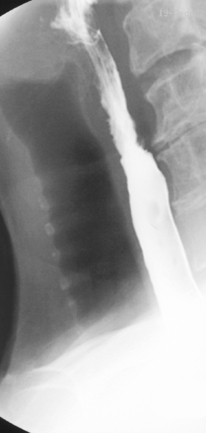
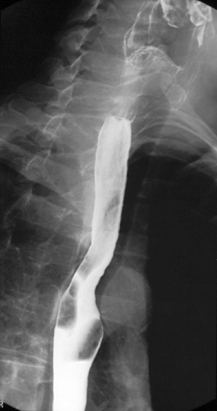
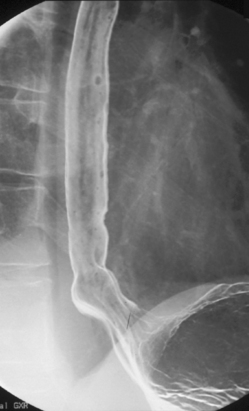
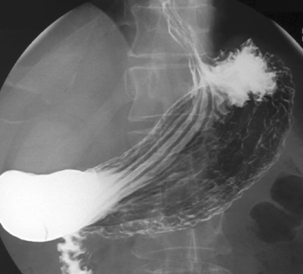
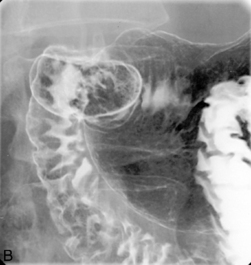
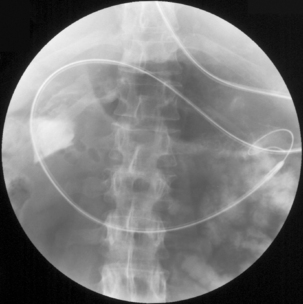
Imaging of the esophagus, esophago-gastric junction and gastric fundus must always be included whether symptoms suggest high or low dysphagia. Turning the patient towards the right anterior oblique position will demonstrate the esophagus away from the spine (Figures 8.6 and 8.7).
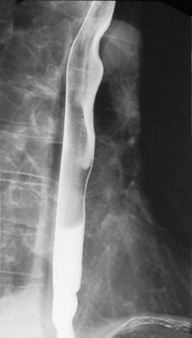
Demonstration of the esophagus can be achieved by asking the patient to drink the barium and keep swallowing as quickly as possible. Esophageal distension is maintained by the second swallow inhibiting the first and the third inhibiting the second etc. If the patient cannot cope with continuous swallowing, the alternative is to take a number of separate swallows, overlapping imaging of the distended esophagus.
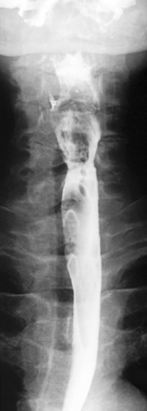
The importance of obtaining full distenson of the esophagus cannot be emphasized enough as plaque lesions can be missed (Figure 8.8). If full distension is not considered to have been achieved the swallow must be repeated.
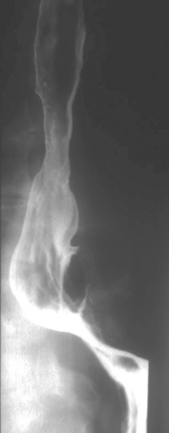
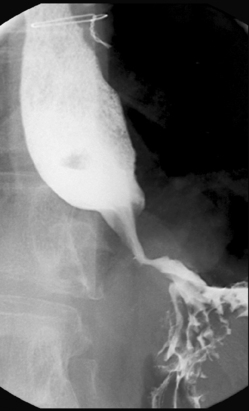
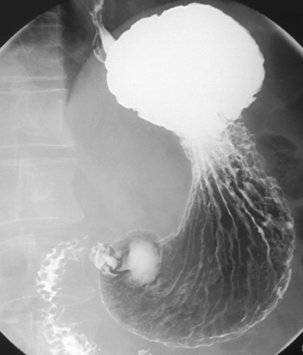
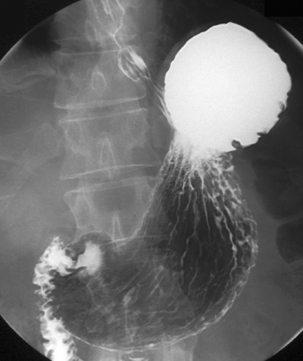
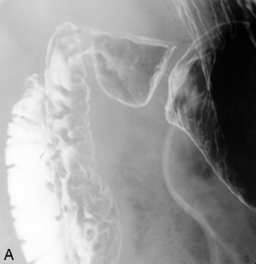
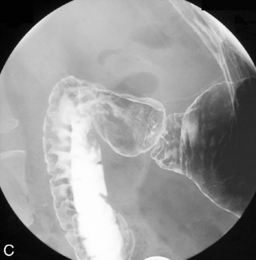
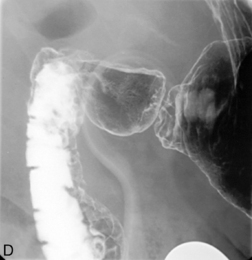
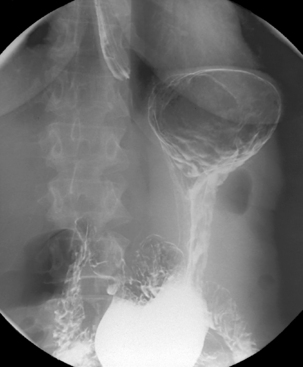
Separate images should be taken of the esophago-gastric junction (Figures 8.9, 8.10 and 8.11); the optimal degree of obliquity can be identified from the initial screened swallow. Additional images should be considered as appropriate if an abnormality is suggested.
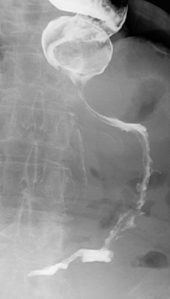
With the patient in the anteroposterior position, the table is tilted to horizontal. Coating the fundus with barium can be achieved by turning the patient to the left and then prone and finally to the right lateral position, tilting the table head up to a point that the barium drains out of the fundus to be replaced by the gas. Lateral (Figure 8.12) and then left anterior oblique (Figure 8.13) images of the upper body/lower fundus are taken at this point; reflux and motility are checked as described in the barium meal section below.
Images are taken of the fundus with the patient and table in the erect position (Table 8.2), then to complete the examination an overview can be taken of the stomach demonstrating gastric emptying. When the patient’s clinical condition severely limits movement, the barium swallow can still be considered. The table need only be tilted up from the horizontal position as far as the patient will tolerate.
Table 8.2 A quick reference guide to a standard barium swallow technique
| Pharyngeal swallow technique | Esophageal swallow technique |
|---|---|
| Erect imaging | Erect imaging |
In patients with a poor swallow or gag reflex, examination of the lower esophagus can be achieved by the trans-nasal insertion of a fine bore tube into the distal esophagus or site of obstruction, followed by the infusion of a contrast medium which, if necessary, can be aspirated through the tube at the end of the examination (see Chapter 11).
The double contrast barium meal (DCBM)
The demand for the double contrast barium meal has been significantly reduced in recent years. This is mainly due to the increased use of endoscopy and availability of endoscopists. Nurses, and more recently, radiographer endoscopists have become established and widely accepted, working as independent practitioners (Swarbrick et al., 2005).
Helicobacter pylori: the decline in peptic ulcer disease can be attributed to the discovery of Helicobacter (H.) pylori by Marshall and Warren (1984). Except in patients taking non-steroidal anti-inflammatory drugs (NSAIDs), all those with peptic ulcers have H. pylori infection. This can be tested for and treated if present, without the need for either endoscopy or DCBM.
For patients with dyspepsia who are seropositive, non-invasive testing for H. pylori in conjunction with anti-H. pylori therapy is considered the most cost effective management strategy (Ofman et al., 1997) and cures peptic ulcer disease (Ladabaum et al., 2002).
Makris et al., (2003) conclude that, although a management strategy based on the urea breath test (UBT) is the most costly, it is the most effective in patients over 45 years and neither endoscopy nor the double contrast barium meal were considered cost effective approaches to the managment of dyspepsia in patients under 45 years.
So where does this leave the double contrast barium meal? If gastroscopy fails or is declined by the patient, the DCBM is currently the best of the limited number of test options for the examination of the stomach and duodenum. Knowing how to undertake the DCBM will also give an insight on how to obtain the optimum imaging when the clinical condition of the patient significantly limits the examination (Levine and Rubesin, 1995).
Double contrast barium meal technique
The patient is starved for 4 hours to make sure the stomach is empty.
With a horizontal fluoroscopy table and the patient supported upon their elbow on their left side, the effervescent powder followed by the citric acid activating agent are given to the patient along with a high density barium such as 340 g EZ HD (E Z Em) diluted with 65 ml H2O. The patient then, without sitting up, turns to lie on their back, thus keeping the barium within the gastric fundus. At this stage, an intravenous hypotonic agent is given (see Chapter 3).
From the right side raised lateral position the patient is turned into the right anterior oblique position (RAO) (Figure 8.14) to demonstrate the gas filled gastric antrum before barium passes into the duodenum overlapping and possibly obscuring parts of the stomach.
Maintaining the barium within the fundus, the patient is placed in the supine position (Figure 8.15), gas will then fill the body and upper body, although as the antrum is projecting posteriorly in this position it is not always gas filled or seen to its fullest extent.
Turning the patient onto their right side and tilting the table head up will enable the barium to drain out of the fundus and be replaced by gas distension. A left side raised lateral image of the fundus is recorded (see Figure 8.12). The barium passes to the gastric antrum and on into the duodenum.
Controlling the patient’s movement, they are then turned towards the left anterior oblique under fluoroscopic control. The practitioner is watching for the movement of barium and gas to demonstrate the fundus and upper body distal to the esophago-gastric junction. The optimum position is immediately before the barium gas interchange occurs (see Figure 8.13). Depending on the shape and size of the stomach, altering the table position may or may not be of value.
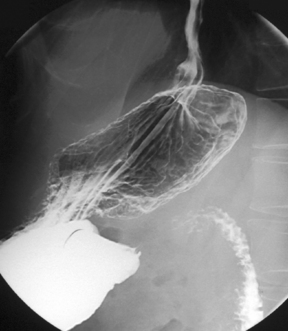
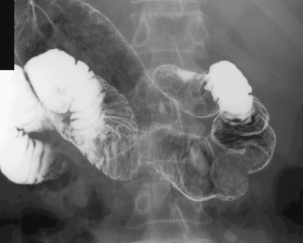
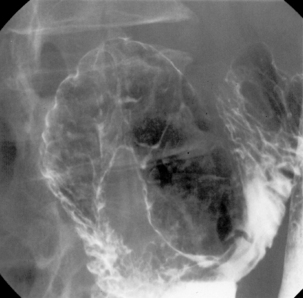
With the patient supine, the table is returned to the horizontal position where barium is confirmed to be in the duodenum. Tilting the table up enough to drain barium out of the duodenal bulb, the patient is turned to the left lateral position and the table returned to the horizontal with barium in the third/fourth part of the duodenum and gas refills the antrum. With a pad held in the midline of the epigastrium, the patient is turned prone onto the pad. Pad compression of the stomach against the spine minimizes the passage of barium and optimizes the viewing of the duodenal loop (Figure 8.16).
Returning the patient onto their left side, magnified views are taken of the duodenal bulb in varying degrees of obliquity starting with the RAO (Figure 8.17A–D). Should the gas be expelled and the bulb collapse, it only requires turning the patient onto their left side once more to enable the bulb to reinflate.
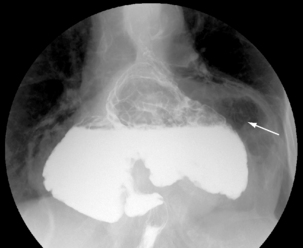
The patient and table are tilted to the erect position, being aware that the patient may feel they are falling forwards during travel through the last few degrees to the vertical. Gas will migrate to the fundus so that an erect image can be acquired of stomach and, in particular, the distended dome of the fundus (Figure 8.18). In the erect position, an RAO is taken of the duodenal bulb (Figure 8.19), in particular to demonstrate the apex of the bulb. The LAO may be of value, but imaging is often limited by overlapping gastric or duodenal anatomy.
Reflux. Anyone can be made to reflux and, in checking for its presence, it should not be forced. Options to check for reflux could include tilting the table head down with the patient slightly in the LAO position, bringing the barium in the fundus in contact with the esophago-gastric junction. Peristalsis as a result of the patient going through the act of swallowing should demonstrate reflux should it be present. An alternative method is to raise the patient’s legs and ask them to cough.
Motility. A simple test to assess motility is to have the patient in the prone position with their head turned to the side. Ingesting a mouthful of barium in one swallow and observing its passage will enable the stripping of the primary and secondary peristaltic wave to be observed. It is important the patient does not swallow twice in succession as the second swallow will inhibit assessment of the first. A method of inhibiting a second swallow is for the patient to open their mouth immediately after the first swallow (Table 8.3).
Table 8.3 Quick reference guide to the double contrast barium meal
| Patient lies on their left to ingest contrast |
| Supine |
RAO, right anterior oblique; LAO, left anterior oblique
The acute esophagus, stomach and duodenum
If an iatrogenic or pathological esophageal perforation is being questioned, a low-osmolar water-soluble contrast medium, such as oral Gastromiro (Bracco UK Ltd), should be used or, if infused via a fine bore tube, Ultravist 240 (Scherring Health Care Ltd). The use of the cheaper hyperosmolar contrast medium ‘Gastrografin’ is dangerous, particularly if the patient is at risk of aspirating, as it can possibly induce severe bronchial irritation and pulmonary edema (Morcos, 2003).
Every effort should be made to place the contrast medium dependent to all aspects of the esophageal lumen. If an anastomotic leak is being queried, post esophagectomy, imaging should first be undertaken with a low osmolar water-soluble contrast agent. If no leak is demonstrated, barium should be used (Tanomkiat and Galassi, 2000).
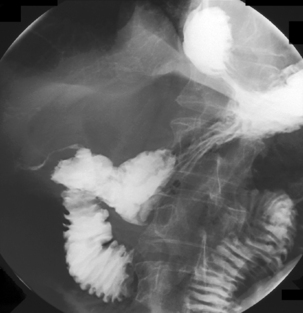
If gastric or duodenal perforation is being considered (Figures 8.21 and 8.22), barium preparations should never be used due to the risk of leakage into the abdominal cavity. Complications of barium sedimenting within the peritoneum can include peritonitis, abscess formation and barium granuloma. If a nasogastric drainage tube is present and sited so that all the ports are in the stomach, dilute hyperosmolar contrast medium can be considered as long as there has been no evidence of the patient being at risk from aspiration.
Hypotonic duodenography
This examination can be used to identify stricturing, mucosal abnormality and filling defects. It can be performed using an 8 French gauge fine bore tube or by the ingestion of barium and an effervescent; both techniques are performed in conjunction with the injection of an intravenous hypotonic agent (see Chapter 3). In both cases, the duodenum is coated with barium and then distended with gas.
Esfandyari T., Potter J.W., Vaezi M.F. Dysphagia: a cost analysis of the diagnostic approach. Am. J. Gastroenterol. 2002;97(11):2733-2737.
Hansmann J., Grenacher L. Radiological imaging of the upper gastrointestinal tract, part 1. The oesophagus. Radiologe. 2006;46(12):1077-1088.
Ladabaum U., Chev W.D., Scheiman J.M., et al. Reappraisal of non-invasive managment strategies for uninvestigated dyspepsia: a cost minimization analysis. Aliment. Pharmacol. Ther.. 2002;16(8):1450-1491.
Levine M.S., Rubesin S.E. The Helicobacter pylori revolution: radiologic perspective. Radiology. 1995;195:593-596.
Makris N., Barkun A., Crott R., et al. Cost-effectiveness of the alternative approaches in the management of dyspepsia. Int. J. Tech. Assess. Health Care. 2003;19(3):446-464.
Marshall B.J., Warren J.R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323(8390):1311-1315.
Morcos S.K. Review article: effects of radiographic contrast media on the lung. Br. J. Radiol.. 2003;76(905):290-295.
Ofman J.J., Etchason J., Fullerton S., et al. Management strategies for helicobacter pylori-seropositive patients with dyspepsia: clinical and economic consequences. Ann. Intern. Med.. 1997;126(4):280-291.
Sebastian A. Barium swallow. A dictionary of the history of medicine. Parthenon Publishing, 1999;103. Google 12th Jan 2007
Swarbrick E., Harnden R., Hodson R., et al. Non medical endoscopists. A report of the working party of the British Society of Gastroenterologists. 2005 http://www.bsg.org.uk.
Tanomkiat W., Galassi W. Barium sulphate as a contrast medium for evaluation of postoperative anastomotic leaks. Acta Radiologica. 2000;41(5):482-485.
Thomas A.. An occasional newsletter, No.10 The radiology history & heritage charitable trust. 1998 http://www.rhhct.org.uk/news/10.htmlt.