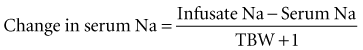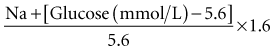113 Fluids and Electrolytes in Children
 Body Water Distribution in Children
Body Water Distribution in Children
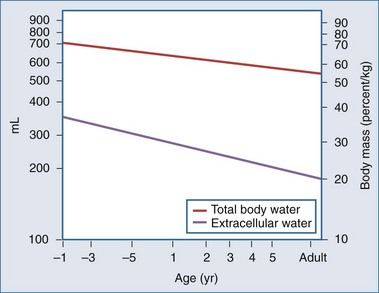
Body water content changes significantly with age in children.1,2 Total body water (TBW) is high in the fetus and preterm infant. During early fetal life, TBW represents 90% of total body weight, with 65% being in the extracellular fluid (ECF) compartment. By term, ECF and intracellular fluid (ICF) volume has fallen to 45% and 30% of TBW, respectively (Figure 113-1). The preterm infant has a relative expansion of both TBW and ECF volume expansion, and a diuresis in the first few days of postnatal life is a common finding. Fractional excretion of sodium is inversely correlated with age in the preterm, who is susceptible to both sodium loss and sodium and volume overload.3 In addition, glomerular filtration rate is lower than in the term infant, and the large surface area–to–body weight ratio leads to considerable evaporative losses.4–7 Further discussion of fluid and electrolyte physiology in the preterm infant is beyond the scope of this chapter.
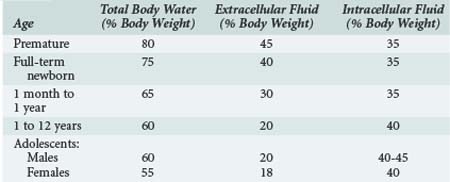
Significant changes occur in TBW over the first year of life, from 75% of body weight at birth to 65% at 6 months and 60% at 1 year (Table 113-1). Some of this is accounted for by an increase in body fat. By puberty, TBW is approximately 60% of body weight in males, with a slightly lower percentage in females. Extracellular fluid volume decreases over the first year of life to 30% of TBW and decreases with age thereafter, reaching adult values early in childhood. The relatively high ECF volume in infancy is largely due to the larger interstitial lymph space. In contrast, the ICF volume remains relatively constant during childhood.
 Fluid Homeostasis in Children
Fluid Homeostasis in Children
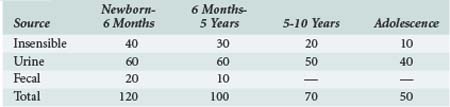
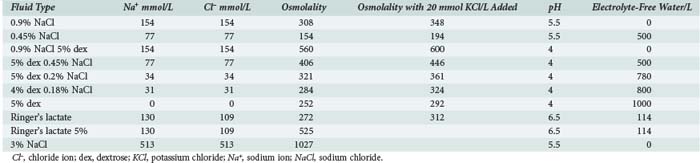
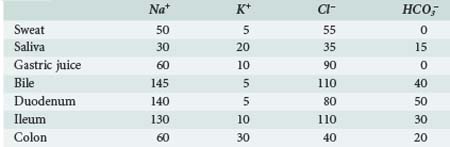
To achieve normal fluid homeostasis, fluid intake must balance losses. The latter consist of urine output plus insensible losses (evaporative from the skin surface and respiratory tract), with the addition of fluid loss in the stool, which in the absence of diarrhea should be minimal. Insensible losses are mainly in the form of electrolyte-free water (EFW) from the respiratory tract (15 mL/100 kcal/d). This loss is eliminated during positive-pressure ventilation. Sweat contains mainly water with a small amount of sodium, except in situations where sweat glands contain excessive amounts of sodium, such as in patients with cystic fibrosis. Evaporative losses also increase with elevations in body temperature; during thermal stress, water losses may increase to as much as 25 mL/100 kcal/d (Table 113-2).
Obligate water excretion in the urine is dependent upon solute load and the ability to concentrate and dilute urine. The average osmolar excretion in newborn infants receiving infant formula is 16 to 20 mOsm/kg/d.2 Infants are somewhat disadvantaged compared to the older child and adult in that they cannot maximally dilute (infant 200 mOsm/L versus adult 80 mOsm/L) and concentrate urine (infant 800 mOsm/L versus adult 1200 mOsm/L). In addition, the infant’s high metabolic rate and the solute load from enteral feeding formula means they require more water excretion per unit solute amount. High solute load and limited urine concentrating ability makes them prone to significant ECF contraction (dehydration) when there are excessive amounts of water loss. Typically this occurs in gastroenteritis, where reduced oral intake is combined with excessive water and electrolyte loss in the stool.
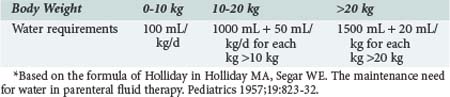
In the normal healthy individual, water intake is regulated by thirst stimulated via osmoreceptors in the hypothalamus. Infants and small children are unable to regulate their intake because they do not have access to water for the same reasons that apply in older children or adults in coma or with reduced levels of consciousness. When oral intake is replaced by parenteral fluids in children, the amount of fluid (i.e., water) given depends on body weight and energy expenditure. In 1957, Holliday8 published a formula that linked body weight to energy expenditure (Table 113-3). An allowance of 100 mL/100 kcal/d was made for insensible water loss, with 66.7 mL/100 kcal/d to replace urine output. Factoring in water of oxidation of 16.7 mL/100 kcal/d leaves a total of 100 mL/100 kcal/d for replacement of normal losses. The estimates for Na (3 mmol/100 kcal/d) and K (2 mmol/100 kcal/day) in maintenance fluids were calculated from the sodium and potassium concentration of cow’s milk and breast milk.
This paper by Holliday became the standard reference text for parenteral fluid administration in pediatrics. Although convenient and simple to use, the assumptions made about daily requirements for sodium, potassium, and EFW mandate the use of hypotonic intravenous (IV) solutions, which has been almost universal practice in pediatric medicine for almost 50 years (Table 113-4). However, nonphysiologic stimuli for antidiuretic hormone (ADH) secretion, which inhibits excretion of EFW (e.g., pain, anxiety, narcotics, positive-pressure ventilation), are common in critically ill patients. It is therefore not surprising that mild degrees of hyponatremia are a common finding in pediatric patients receiving parenteral fluid therapy. In a study by Gerigk9 of 103 children admitted to the hospital with acute medical illnesses, the median plasma Na value was 136 mmol/L, with plasma ADH levels that were higher than would be expected for that degree of hyponatremia. In 31 control patients (elective surgical admissions), median serum Na levels were 139 mmol/L, with lower ADH levels. We have made a similar observation in patients with hospital-acquired hyponatremia, who received twice as much EFW compared with a control group.10 The nonphysiologic secretion of ADH has been reported in association with many acute medical illnesses including meningitis, bronchiolitis, encephalitis, traumatic brain injury, and gastroenteritis.11–22 An increasing number of publications are now recommending the use of isotonic or near-isotonic fluids for standard maintenance in pediatrics to avoid administration of EFW, which is potentially hazardous in situations where ADH secretion is not inhibited.10,23–26 Hypotonic fluids should be reserved for patients with a demonstrated need for EFW (serum Na+ > 145 mmol/L).
 Perioperative Fluid Management
Perioperative Fluid Management
Standard practice in perioperative fluid management has been to replace intravascular volume loss with blood or colloid solutions and to use electrolyte solutions to provide for ongoing fluid requirements, replacement of losses from exposed serosal surfaces in open body cavities in thoracic and abdominal surgery, and losses from third-space fluid sequestration (Table 113-5). Extra fluid is also frequently administered to treat hypotension due to the vasodilating effects of anesthetic agents. The preferred electrolyte solution used by most anesthesiologists for intraoperative fluid administration is now Ringer’s lactate or isotonic saline because of concerns about the development of postoperative fluid retention and hyponatremia associated with elevated ADH levels.27–29 The potential for this is increased when hypotonic dextrose/saline solutions are used.28,30–32 This inability to excrete a sodium-free water load is amply illustrated in scoliosis surgery, where patients seem to be particularly at risk for the development of hyponatremia postoperatively.33 Two nonrandomized studies have shown that the degree of hyponatremia is less when isotonic or near-isotonic solutions are used.34,35 In a nonrandomized trial, Burrows35 compared Ringer’s lactate with 0.2% sodium chloride (NaCl) infusion in a group of children following scoliosis surgery. He found that the postoperative plasma Na level fell in both groups, but that the reduction was marked in those patients receiving the hypotonic fluid. Although at first glance, the explanation for this is EFW retention due to nonphysiologic stimulation of ADH secretion, it does not explain the reduction in plasma Na seen with Ringer’s lactate.
Further insights to explain this observation come from the study by Steele,36 where plasma and urine Na were measured in adult patients undergoing elective surgery, all of whom received Ringer’s lactate as their perioperative fluid. They found that the urine Na concentration was consistently above 150 mmol/L and as high as 350 mmol/L in some instances. This was associated with a significant positive water balance and a fall in the plasma Na, a process they termed postoperative desalination. In a similar study of children undergoing elective surgery, all of whom received Ringer’s lactate, we found similar levels of urinary Na loss (unpublished observations). We think that this desalination process is consistent with the kidney’s attempts to deal with a volume overload situation after the vasodilating effects of anesthetic agents are no longer present, but ADH is still being actively secreted. In this situation, it would be unwise to prescribe hypotonic fluids in the postoperative period and impose an extra burden of more EFW to be excreted by the kidney.
Further evidence has now emerged that supports the use of isotonic rather than hypotonic fluid in the perioperative period. A prospective observational study in patients admitted to the intensive care unit (ICU) postoperatively documented an increased risk of development of hyponatremia associated with use of hypotonic saline; water retention and increased sodium excretion are to blame.37 Two recent prospective randomized trials have compared the use of isotonic with hypotonic saline. Both have shown that the incidence of hyponatremia was significantly reduced with isotonic saline, and in neither study did the patients develop hypernatemia.38,39
 Disorders of Sodium Homeostasis
Disorders of Sodium Homeostasis
Hyponatremia
Hyponatremia (serum Na <136 mmol/L) is the commonest electrolyte disorder seen in a hospitalized population and implies an expansion of the ICF compartment. It is caused by either water gain (e.g., use of hypotonic fluids) or salt loss (e.g., gastroenteritis) (Table 113-6).
| Water Gain |
Acute hyponatremia, defined as a fall in plasma Na to less than 130 mmol/L within 48 hrs, leads to rapid movement of water from the ECF to the ICF compartment and can cause cerebral edema, with catastrophic outcomes reported in children.30,40,41 Clinical findings are those of raised intracranial pressure (nausea, vomiting, headache), frequently undiagnosed until the onset of seizures. This is usually followed by apnea, indicating that brainstem coning has occurred. Symptomatic hyponatremia rarely occurs below a serum Na level of 125 mmol/L, but when it does, it constitutes a medical emergency. The primary objective is to raise serum Na to above this level to prevent brainstem herniation. This can be most effectively achieved with the use of hypertonic saline.42 Once this threshold has been reached, the serum Na can be allowed to correct by fluid restriction with or without the use of furosemide. IV mannitol has also been used successfully in the emergency treatment of acute symptomatic hyponatremia.43
Chronic hyponatremia is a common finding in patients with heart failure and renal failure and is associated with increased TBW and salt retention. It is not associated with cerebral edema, but correction of chronic hyponatremia with isotonic or hypertonic saline has been associated with central pontine demyelination.44–46
Hypernatremia
Hypernatremia is defined as a serum Na greater than 145 mmol/L and is caused by either water deficit or salt gain (Table 113-7). The former is seen in infants with severe gastroenteritis with a loss of water in excess of sodium, sometimes compounded by increased solute intake from incorrect mixing of infant formula. The absence of ADH secretion causing diabetes insipidus is seen in patients with pituitary tumors, traumatic brain injury, and central nervous system (CNS) infections.47–50 Water loss in critically ill children may also be associated with the use of loop diuretics or mannitol. Hypernatremia secondary to salt gain is seen with the excessive use of isotonic or hypertonic saline solutions or with the administration of IV bicarbonate.
| Water Loss |
A rise in serum Na is associated with movement of water from the ICF to the ECF compartment and development of a hyperosmolar state. Brain cells adapt with an increase in electrolytes and “ideogenic” osmoles (inositol, taurine), which tends to mitigate the fluid shift with partial restoration of intracellular osmolality and brain cell volume.51–53 Levels of Na over 155 mmol/L are frequently associated with abnormal CNS findings, and there is an increased risk of subdural hemorrhage and infarction in infants with hypernatremic dehydration and serum Na levels higher than 160 mmol/L.54–57 There is also the added danger of development of brain edema during the attempt to correct these hyperosmolar states rapidly, using solutions that are hypo-osmolar compared to the ICF compartment.58–63 Published recommendations suggest that the rate of correction of serum Na should be less than 0.5 mmol/L/h using the following formula for correction, which estimates the effect of 1 L of any infusate on serum Na:
In severe hypernatremia (serum Na > 170 mmol/L), it is recommended that the maximum Na concentration not be corrected to below 150 mmol/L in the first 48 to 72 hrs.64
The epidemiology of hypernatremia in children has changed recently from gastroenteritis with dehydration as the principal cause to one of a hospital-acquired problem in association with either excess salt administration or a free-water deficit. In a study by Moritz of children with a serum Na above 150 mmol/L, the problem was hospital acquired in 60%, and the mortality was 11%.65 In a similar series of adult patients, the ICU mortality rate for patients with plasma Na levels above 150 mmol/L was 30%.66
 Management of Acute Water and Sodium Deficits in Children
Management of Acute Water and Sodium Deficits in Children
Water and Electrolyte Deficits in Diabetic Ketoacidosis
Diabetic ketoacidosis (DKA) is characterized by losses of water and electrolytes due to hyperglycemia-induced osmotic diuresis. The high osmolality of the ECF results in shift of water from the ICF compartment. Studies performed in adult humans with type 1 diabetes where insulin therapy has been withheld have shown fluid deficits of 5 to 10 L together with up to 20% loss of total body sodium and potassium.67 At the time of presentation, patients are ECF contracted, and clinical estimates of the deficit are usually in the range of 7% to 10%, although shock with hemodynamic compromise is a rare event in DKA in children. The hyperglycemia in DKA results in a hyperosmolar state, but serum Na concentration is an unreliable measure of the degree of ECF contraction, owing to the dilutional effect of fluid shift from the ICF to the ECF compartment. The effective osmolality (2 [Na + K] + glucose, all in mmol/L) at the time of presentation is frequently in the range of 300 to 350 mOsm/L. An elevated hematocrit may be a useful marker of severe ECF contraction. Urea is not an effective osmole because it moves freely across the cell membrane and is therefore not included in the calculation. An estimate of true ECF deficit can be made by “correcting” the measured serum Na for the increase in ECF water using the formula developed by Katz68:
The ECF contraction is associated with a reduction in GFR which results in reduced glucose and ketone clearance from the blood and worsening DKA. Studies in humans have shown that IV fluid administration alone results in substantial falls in blood glucose before insulin has been given; this is due to the increase in GFR.69 Serum K is also frequently elevated at the time of presentation70 but falls rapidly as GFR increases and insulin re-primes the Na+/K+-ATPase cell membrane pump.71
Cerebral edema as a complication of diabetic ketoacidosis (CE-DKA) was first described by Dillon in 1936.72 Although originally reported in adults,73–76 it is much more common in children and accounts for the majority of morbidity and mortality associated with DKA in this age group.77 The reported occurrence rate in the pediatric literature varies between 0.2% and 1%.78–81 However, this is likely to be an underestimate, as it is based on retrospective reviews relying on the clinical diagnosis of increased intracranial pressure. The incidence is also reported to be higher in new-onset diabetes and in younger children.78,81,82 Series of brain imaging studies in children with DKA have shown decreased ventricular size either early (<12 hours) in the treatment course83 or even before therapy has commenced.84 The ultimate consequence of this, namely brainstem herniation, has been reported to be 5.8% (9/153) in one series of all children presenting with DKA.85 The total adverse outcome rate (death or permanent neurologic injury) in CE-DKA is as high as 40% to 50% in some series, with few intact survivors where brainstem herniation has occurred.78,86,87 For these reasons, children with severe DKA (pH < 7.2) should be admitted to the ICU for close monitoring of CNS status during the first 24 hours of correction of the fluid deficit. Symptoms such as diminished level of consciousness, headache, or vomiting are signs of impending cerebral edema.
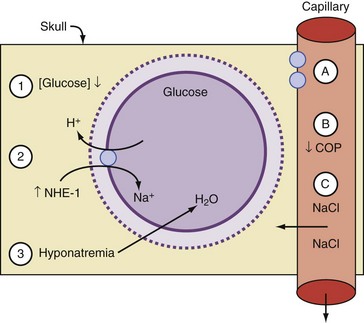
Many theories have been advanced to explain brain swelling in association with DKA, including overzealous rehydration with hypotonic IV fluids, rapid reduction of blood glucose with insulin, activation of the sodium/hydrogen ion (Na+/H+) transporter system, change in oncotic pressure, increased permeability of the blood-brain barrier, and changes in cerebral blood flow.78,81,85,86,88 Most of these have been developed from individual case reports or small case series. Although the precise cause is not fully understood, there is general agreement that the pathogenesis of CE-DKA involves an osmolar shift that results in fluid accumulation in the ICF compartment and cell swelling.
Although the cause of cerebral edema in DKA is a subject of much controversy,89 several case series have focused on fluid rehydration. The standard approach formerly was to give a bolus of between 10 and 30 mL/kg of fluid at the time of presentation, often with a bolus of insulin (0.1 units/kg). IV fluids were then administered depending on the clinical diagnosis of the degree of dehydration. This was done by calculating the fluid deficit and replacing this over 24 to 36 hours using a hypotonic fluid, generally 0.45% NaCl with added potassium. In the late 1980s and early 90s, a number of groups began to question the wisdom of this approach86,90,91 based on experiences with CE-DKA. They noted that in many cases of CE-DKA, the measured serum Na failed to rise during fluid resuscitation as expected, indicating a failure to protect against a rapid fall in the effective osmolality. As a result, they advocated a more conservative therapeutic approach, limiting the total fluid to under 4 L/m2/d and increasing the tonicity of IV fluids. Using this approach, Harris et al. reported a decrease in the incidence of symptomatic cerebral edema but not its elimination.90 In a second series by the same authors using the same approach, mannitol was administered for increasing obtundation in only 4/231 episodes, and there were no adverse outcomes in the total series.91 However, the practice of aggressive fluid resuscitation still persists. Roberts92 has recently reported a case series of 11 children who developed CE-DKA, most of whom received large amounts of IV fluid (>40 mL/kg in the first 4 hours). All received mannitol and, with one exception, recovered.
The issue of changes in serum osmolality as a risk factor has been identified in several series (Figure 113-2). A rapid reduction in effective osmolality is associated with either a fall in blood glucose or serum Na, or both, due to the rapid administration of IV fluid and possibly by bolus-dose insulin. Insulin administration is also known to activate the Na+/H+ ion exchanger, increasing the ICF Na concentration.88 Water follows the osmotic gradient back into the ICF compartment. Hale,93 in a retrospective series, found that CE-DKA developed in children when there was a progressive fall in serum Na and osmolality compared to patients without brain swelling, where effective osmolality did not change. In a large series that included age-matched controls, other identified risk factors for development of cerebral edema were a low PaCO2 and a high urea at the time of presentation.81 These are probably reflective of the severity of the acidosis and ECF contraction. The only treatment variable that was associated with CE-DKA in this series was the use of bicarbonate therapy.
Although there is no clear consensus as to the most appropriate fluid resuscitation in DKA to prevent cerebral edema, most would agree that large amounts of hypotonic fluids are not appropriate.86,91,93–96 The practice of using an IV bolus dose of insulin at the initiation of DKA treatment is now far less common and has largely been replaced by a more conservative rate of continuous infusion. Although the role of insulin in the development of CE-DKA remains speculative, bolus insulin at the start of therapy does not appear to provide a therapeutic benefit, and we believe its use should be avoided on sound theoretical grounds.
In the absence of a single unifying hypothesis as to the cause of cerebral edema in DKA, it is not possible to provide a definitive treatment approach that will predictably prevent CE-DKA. It remains likely that the pathogenesis of CE-DKA is multifactorial in nature and includes both patient and treatment-related factors. The objective of treatment should be gradual reduction in serum osmolality, which can be achieved by conservative fluid resuscitation and avoidance of hypotonic fluids in the initial resuscitation period.97 A general rule is that failure of the serum Na to rise during IV fluid replacement indicates too rapid a rate of infusion. Our own approach is to use no more than 7.5 to 10 mL/kg over the first hour of treatment of normal saline (0.9% NaCl), with a reduction to 3.5 to 5 mL/kg/h thereafter.95 In addition, insulin is given as a continuous infusion at the rate of 0.1 unit/kg/h, with the dose adjusted to avoid a drop in blood glucose concentration greater than about 5 mmol/h. It remains to be seen whether this approach will lower the risk of cerebral edema associated with the onset and management of DKA in children. This is consistent with the approach of Harris and others advocated more than 10 years ago.90 A recent retrospective study from our center demonstrates that the use of isotonic saline, with the associated rise in serum sodium as the glucose falls, protects against the development of cerebral edema.98
Children presenting with DKA require close monitoring for alteration in level of consciousness and other signs of increased ICP such as headache and vomiting. This level of care is best provided in an ICU setting. In the event cerebral edema is suspected, serum osmolarity should immediately be raised by the administration of mannitol, 1 gm/kg IV,92 or 2 to 3 mL/kg of 3% saline99 and a decrease in the IV fluid and insulin infusion. This should be done without waiting for a computed tomography (CT) scan, which may fail to demonstrate cerebral edema.
Fluid and Electrolyte Deficits in Gastroenteritis
Acute gastroenteritis is the commonest form of disturbance of fluid and electrolyte homeostasis seen in childhood. Infants with diarrhea are particularly vulnerable to significant losses of fluid, sodium, chloride, and bicarbonate from the small intestine and present with what is frequently classified as hypotonic, isotonic, or hypertonic dehydration based on the serum Na level. This terminology is technically incorrect; only in the hypertonic form is there loss of fluid from the ICF compartment, and these patients are truly dehydrated. Patients with diarrheal illnesses associated with fluid loss with normal or reduced serum Na have loss of TBW and ECF with normal or increased ICF volume.100 Infants with hypernatremic dehydration are the ones at greatest risk of an adverse neurologic event (see earlier), but seizures from severe hyponatremia have been reported in infants presenting with acute gastroenteritis due to oral salt-free fluids being given as replacement.11,101,102 Assessment of the degree of ECF deficit is usually made on clinical grounds using the time-honored clinical signs of capillary refill time, dry mucous membranes, skin turgor, and so on.103 However, these are open to subjective interpretation, and there may be a tendency to overestimate the degree of ECF contraction in less severely ill children. In a study by Mackenzie,104 the fluid deficit in children with gastroenteritis and mild to moderate “dehydration” was overestimated, which resulted in overuse of IV fluids. Skin turgor, increased capillary refill time, high urea, low pH, and increased base deficit all correlated with the degree of ECF contraction but not the presence of thirst or oliguria. Other studies have shown that a reduced bicarbonate is the most common electrolyte abnormality associated with significant ECF contraction in gastroenteritis.105,106
Patients with gastroenteritis whose serum is isotonic and hypotonic should be managed with isotonic saline, and those who are hypertonic should receive solutions that contain EFW. An observational study has found that ADH levels are frequently elevated in these patients.107 A randomized controlled trial of IV fluid rehydration in children with gastroenteritis has shown that the use of isotonic saline protected against the development of hyponatremia without the development of hypernatremia when compared with hypotonic.22 Infants with severe hypernatremia should have their free-water deficit corrected slowly because of the dangers of rapid fluid shift to the ICF compartment (see earlier). There is an increasing trend to rapidly rehydrate these patients with IV solutions in the emergency department prior to discharging them home,108,109 but a more simple and effective technique is to use oral rehydration therapy (ORT), which has a proven efficacy in clinical trials of patients with acute gastroenteritis. These solutions contain Na concentrations of between 45 and 90 mmol/L.109–112
 Chloride
Chloride
Hyperchloremia is seen in association with respiratory alkalosis, hypernatremic dehydration, and administration of isotonic saline. Large amounts of isotonic saline used during fluid resuscitation can result in a hyperchloremic metabolic acidosis.113 If the serum Cl is not measured, an increased base deficit could be wrongly interpreted as indicating inadequate volume resuscitation in shock.114
Plasma chloride measurements are an integral part of the calculation of the anion gap, which is important for the diagnosis of metabolic acidosis.115 This is the difference between the measured cations (Na+) and anions (Cl− + HCO3−), which is normally in the range of 12 to 16. The anion gap is increased when unmeasured anions are present, such as lactate and the accumulation of β-hydroxybutyrate in DKA. A normal or reduced anion-gap acidosis is seen in association with hyperchloremia from saline administration or other situations where there is an increase in serum Cl.113,116,117
 Potassium
Potassium
Hypokalemia
Hypokalemia in children is commonly seen with gastroenteritis and diarrhea where ECF contraction leads to stimulation of aldosterone secretion. There is also total body potassium depletion in DKA, although the initial measured level is high due to the acidosis.70 Adolescents with anorexia nervosa can present with profound degrees of hypokalemia, and it is a known cause of sudden death in this syndrome.118 In the critical care setting, hypokalemia is most commonly associated with diuretic use, nasogastric suction, hypomagnesemia, and metabolic alkalosis. In acute metabolic alkalosis, each 0.1-unit rise in pH results in a fall of between 0.2 and 0.4 mmol/L in the serum K.119 In chronic metabolic alkalosis, K is exchanged for hydrogen ion in the distal nephron. Increased K output in the urine is also associated with renal tubular defects (Bartter’s syndrome, renal tubular acidosis) and the use of drugs such as amphotericin, ticarcillin, carbenicillin, and steroids.120
Hyperkalemia
Acute hyperkalemia represents a medical emergency; serum levels in excess of 6 mmol/L can result in cardiac arrest and sudden death, particularly in the post cardiopulmonary bypass setting. Frequently the only clinical manifestation is the finding of tall, peaked T waves and widening of the QRS complex on the ECG tracing, but the absence of these findings does not exclude the diagnosis. Patients with borderline high levels of serum K can develop life-threatening hyperkalemia with the development of an acidosis. Because it is the extracellular K level which is harmful, emergency measures should be directed at increasing the transmembrane flux from ECF to the ICF compartment. These include the use of bicarbonate to correct acidemia, β-agonist therapy, and use of glucose/insulin.119,121 IV calcium chloride will help protect the heart against the development of cardiac rhythm disturbances. These are temporizing measures while steps are taken to increase K removal from the body either by using sodium/potassium exchange resins (rectally or via NG tube) or acute dialysis.
 Calcium
Calcium
Hypocalcemia is seen in neonates with birth asphyxia, preterm infants, term newborns in the first week of life, and infants of diabetic mothers. It is an invariable finding in newborn infants with DiGeorge syndrome, where it is seen in association with conotruncal congenital heart defects, typically truncus arteriosus and interrupted aortic arch. The majority of these infants have microdeletions of the long arm of chromosome 22 (22q minus syndrome) and immunodeficiency. For this reason, all transfused blood products must be irradiated. Hypocalcemia is a common finding in critically ill older children, with a reported incidence of 49% in one study.122 Causes include cardiopulmonary bypass, use of citrated blood and blood products, albumin transfusions, burns, sepsis, use of loop diuretics, and aminoglycosides. Hyperphosphatemia, seen in tumor lysis syndrome and renal failure, can also result in hypocalcemia.
Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823-832.
Hoorn EJ, Geary D, Robb M, Halperin ML, Bohn D. Acute hyponatremia related to intravenous fluid administration in hospitalized children: an observational study. Pediatrics. 2004;113:1279-1284.
Duke T, Molyneux EM. Intravenous fluids for seriously ill children: time to reconsider. Lancet. 2003;362:1320-1323.
Neville KA, Verge CF, Rosenberg AR, O’Meara MW, Walker JL. Isotonic is better than hypotonic saline for intravenous rehydration of children with gastroenteritis: a prospective randomised study. Arch Dis Child. 2006;91:226-232.
Eulmesekian PG, Perez A, Minces PG, Bohn D. Hospital-acquired hyponatremia in postoperative pediatric patients: prospective observational study. Pediatr Crit Care Med. 2010;11:479-483.
Steele A, Gowrishankar M, Abrahamson S, Mazer CD, Feldman RD, Halperin ML. Postoperative hyponatremia despite near-isotonic saline infusion: a phenomenon of desalination. Ann Intern Med. 1997;126:20-25.
Montanana PA, Modesto I, Alapont V, Ocon AP, Lopez PO, Lopez Prats JL, et al. The use of isotonic fluid as maintenance therapy prevents iatrogenic hyponatremia in pediatrics: a randomized, controlled open study. Pediatr Crit Care Med. 2008;9:589-597.
Neville KA, Sandeman DJ, Rubinstein A, Henry GM, McGlynn M, Walker JL. Prevention of hyponatremia during maintenance intravenous fluid administration: a prospective randomized study of fluid type versus fluid rate. J Pediatr. 2010;156:313-319. e1-2
Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee W, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009;10:118-133.
1 Simpson J, Stephenson T. Regulation of extracellular fluid volume in neonates. Early Hum Dev. 1993 Oct;34(3):179-190.
2 Boineau FG, Lewy JE. Estimation of parenteral fluid requirements. Pediatr Clin North Am. 1990 Apr;37(2):257-264.
3 Herin P, Aperia A. Neonatal kidney, fluids, and electrolytes. Curr Opin Pediatr. 1994 Apr;6(2):154-157.
4 Arant BS. Developmental patterns of renal functional maturation compared in the neonate. J Pediatr. 1978;92:705-712.
5 Haycock GB, Aperia A. Salt and the newborn kidney. Pediatr Nephrol. 1991 Jan;5(1):65-70.
6 Aperia A, Herin P, Lundin S, Melin P, Zetterstrom R. Regulation of renal water excretion in newborn full-term infants. Acta Paediatr Scand. 1984 Nov;73(6):717-721.
7 Aperia A, Broberger O, Herin P, Thodenius K, Zetterstrom R. Postnatal control of water and electrolyte homeostasis in pre-term and full-term infants. Acta Paediatr Scand Suppl. 1983;305:61-65.
8 Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics. 1957;19:823-832.
9 Gerigk M, Gnehm HE, Rascher W. Arginine vasopressin and renin in acutely ill children: implication for fluid therapy. Acta Paediatr. 1996;85(5):550-553.
10 Hoorn EJ, Geary D, Robb M, Halperin ML, Bohn D. Acute hyponatremia related to intravenous fluid administration in hospitalized children: an observational study. Pediatrics. 2004 May;113(5):1279-1284.
11 Bhalla P, Eaton FE, Coulter JB, Amegavie FL, Sills JA, Abernethy LJ. Hyponatraemic seizures and excessive intake of hypotonic fluids in young children. BMJ. 1999;319(7224):1554-1557.
12 Hanna S, Tibby SM, Durward A, Murdoch IA. Incidence of hyponatraemia and hyponatraemic seizures in severe respiratory syncytial virus bronchiolitis. Acta Paediatr. 2003 Apr;92(4):430-434.
13 Singh BS, Patwari AK, Deb M. Serum sodium and osmolal changes in tuberculous meningitis. Indian Pediatr. 1994;31(11):1345-1350.
14 Padilla G, Leake JA, Castro R, Ervin MG, Ross MG, Leake RD. Vasopressin levels and pediatric head trauma. Pediatrics. 1989 May;83(5):700-705.
15 Poddar U, Singhi S, Ganguli NK, Sialy R. Water electrolyte homeostasis in acute bronchiolitis. Indian Pediatr. 1995 Jan;32(1):59-65.
16 Shann F, Germer S. Hyponatraemia associated with pneumonia or bacterial meningitis. Arch Dis Child. 1985;60(10):963-966.
17 Moritz ML, Ayus JC. La Crosse encephalitis in children. N Engl J Med. 2001 Jul 12;345(2):148-149.
18 Fajardo JE, Stafford EM, Bass JW, Roscelli JD, Sato AK, Claybaugh JR. Inappropriate antidiuretic hormone in children with viral meningitis. Pediatr Neurol. 1989 Jan-Feb;5(1):37-40.
19 Kaplan SL, Feigin RD. The syndrome of inappropriate secretion of antidiuretic hormone in children with bacterial meningitis. J Pediatr. 1978 May;92(5):758-761.
20 Narotam PK, Kemp M, Buck R, et al. Hyponatremic natriuretic syndrome in tuberculous meningitis: the probable role of atrial natriuretic peptide. Neurosurgery. 1994;34:982-988.
21 Singhi SC, Singhi PD, Srinivas B, Narakesri HP, Ganguli NK, Sialy R, et al. Fluid restriction does not improve the outcome of acute meningitis. Pediatr Infect Dis J. 1995;14(6):495-503.
22 Neville KA, Verge CF, Rosenberg AR, O’Meara MW, Walker JL. Isotonic is better than hypotonic saline for intravenous rehydration of children with gastroenteritis: a prospective randomised study. Arch Dis Child. 2006 Mar;91(3):226-232.
23 Moritz ML, Ayus JC. Prevention of hospital-acquired hyponatremia: a case for using isotonic saline. Pediatrics. 2003 Feb;111(2):227-230.
24 Shafiee MA, Bohn D, Hoorn EJ, Halperin ML. How to select optimal maintenance intravenous fluid therapy. QJM. 2003 Aug;96(8):601-610.
25 Duke T, Mokela D, Frank D, Michael A, Paulo T, Mgone J, et al. Management of meningitis in children with oral fluid restriction or intravenous fluid at maintenance volumes: a randomised trial. Ann Trop Paediatr. 2002 Jun;22(2):145-157.
26 Duke T, Molyneux EM. Intravenous fluids for seriously ill children: time to reconsider. Lancet. 2003 Oct 18;362(9392):1320-1323.
27 Cochrane JP, Forsling ML, Gow NM, Le Quesne LP. Arginine vasopressin release following surgical operations. Br J Surg. 1981 Mar;68(3):209-213.
28 Judd BA, Haycock GB, Dalton RN, Chantler C. Antidiuretic hormone following surgery in children. Acta Paediatr Scand. 1990 Apr;79(4):461-466.
29 Judd BA, Haycock GB, Dalton N, Chantler C. Hyponatraemia in premature babies and following surgery in older children. Acta Paediatr Scand. 1987;76(3):385-393.
30 Arieff AI. Postoperative hyponatraemic encephalopathy following elective surgery in children. Paediatr Anaesth. 1998;8(1):1-4.
31 Arieff AI, Ayus JC, Fraser CL. Hyponatraemia and death or permanent brain damage in healthy children. BMJ. 1992;304(6836):1218-1222.
32 Paut O, Remond C, Lagier P, Fortier G, Camboulives J. Severe hyponatremic encephalopathy after pediatric surgery: report of seven cases and recommendations for management and prevention. Ann Fr Anesth Reanim. 2000 Jun;19(6):467-473.
33 Cowley DM, Pabari M, Sinton TJ, Johnson S, Carroll G, Ryan WE. Pathogenesis of postoperative hyponatraemia following correction of scoliosis in children. Aust N Z J Surg. 1988;58(6):485-489.
34 Brazel PW, McPhee IB. Inappropriate secretion of antidiuretic hormone in postoperative scoliosis patients: the role of fluid management. Spine. 1996 Mar 15;21(6):724-727.
35 Burrows FA, Shutack JG, Crone RK. Inappropriate secretion of antidiuretic hormone in a postsurgical pediatric population. Crit Care Med. 1983;11(7):527-531.
36 Steele A, Gowrishankar M, Abrahamson S, Mazer CD, Feldman RD, Halperin ML. Postoperative hyponatremia despite near-isotonic saline infusion: a phenomenon of desalination. Ann Intern Med. 1997;126:20-25.
37 Eulmesekian PG, Perez A, Minces PG, Bohn D. Hospital-acquired hyponatremia in postoperative pediatric patients: Prospective observational study. Pediatr Crit Care Med. 11(4), 2010 Jan 29.
38 Montanana PA, Modesto I, Alapont V, Ocon AP, Lopez PO, Lopez Prats JL, et al. The use of isotonic fluid as maintenance therapy prevents iatrogenic hyponatremia in pediatrics: a randomized, controlled open study. Pediatr Crit Care Med. 2008 Nov;9(6):589-597.
39 Neville KA, Sandeman DJ, Rubinstein A, Henry GM, McGlynn M, Walker JL. Prevention of hyponatremia during maintenance intravenous fluid administration: a prospective randomized study of fluid type versus fluid rate. J Pediatr. 2010 Feb;156(2):313-319. e1-2
40 Halberthal M, Halperin ML, Bohn D. Acute hyponatraemia in children admitted to hospital: retrospective analysis of factors contributing to its development and resolution. BMJ. 2001;322(7289):780-782.
41 Playfor S. Fatal iatrogenic hyponatraemia. Arch Dis Child. 2003 Jul;88(7):646-647.
42 Sarnaik AP, Meert K, Hackbarth R, Fleischmann L. Management of hyponatremic seizures in children with hypertonic saline: a safe and effective strategy. Crit Care Med. 1991;19:758-762.
43 Porzio P, Halberthal M, Bohn D, Halperin ML. Treatment of acute hyponatremia: ensuring the excretion of a predictable amount of electrolyte-free water. Crit Care Med. 2000;28(6):1905-1910.
44 Kumar S, Berl T. Sodium. Lancet. 1998;352(9123):220-228.
45 Choe WJ, Cho BK, Kim IO, Shin HY, Wang KC. Extrapontine myelinolysis caused by electrolyte imbalance during the management of suprasellar germ cell tumors. Report of two cases. Childs Nerv Syst. 1998;14(4-5):155-158.
46 Chercover DJ, Norman MG. Central pontine myelinolysis in a 6-month-old infant with rapidly corrected hyponatremia. Ann Neurol. 1984 Aug;16(2):261-262.
47 Maghnie M. Diabetes insipidus. Horm Res. 2003;59(Suppl. 1):42-54.
48 Maghnie M, Cosi G, Genovese E, Manca-Bitti ML, Cohen A, Zecca S, et al. Central diabetes insipidus in children and young adults. N Engl J Med. 2000 Oct 5;343(14):998-1007.
49 Baskin DS, Wilson CB. Surgical management of craniopharyngiomas. A review of 74 cases. J Neurosurg. 1986 Jul;65(1):22-27.
50 Baylis PH, Cheetham T. Diabetes insipidus. Arch Dis Child. 1998 Jul;79(1):84-89.
51 Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000 May 25;342(21):1581-1589.
52 Lien YH, Shapiro JI, Chan L. Effects of hypernatremia on organic brain osmoles. J Clin Invest. 1990 May;85(5):1427-1435.
53 Trachtman H. Cell volume regulation: a review of cerebral adaptive mechanisms and implications for clinical treatment of osmolal disturbances: II. Pediatr Nephrol. 1992 Jan;6(1):104-112.
54 Finberg L, Luttrell C, Redd H. Pathogenesis of lesions in the nervous system in hypernatremic states. II. Experimental studies of gross anatomic changes and alterations of chemical composition of the tissues. Pediatrics. 1959 Jan;23(1 Part 1):46-53.
55 Finberg L. Hypernatremic (hypertonic) dehydration in infants. N Engl J Med. 1973 Jul 26;289(4):196-198.
56 Macaulay D, Watson M. Hypernatraemia in infants as a cause of brain damage. Arch Dis Child. 1967 Oct;42(225):485-491.
57 Luttrell CN, Finberg L, Drawdy LP. Hemorrhagic encephalopathy induced by hypernatremia. II. Experimental observations on hyperosmolarity in cats. Arch Neurol. 1959 Aug;1:153-160.
58 Finberg L. Dehydration in infancy and childhood. Pediatr Rev. 2002 Aug;23(8):277-282.
59 Banister A, Matin-Siddiqi SA, Hatcher GW. Treatment of hypernatraemic dehydration in infancy. Arch Dis Child. 1975 Mar;50(3):179-186.
60 Bruck E, Abal G, Aceto TJr. Pathogenesis and pathophysiology of hypertonic dehydration with diarrhea. A clinical study of 59 infants with observations of respiratory and renal water metabolism. Am J Dis Child. 1968 Feb;115(2):122-144.
61 Dunn K, Butt W. Extreme sodium derangement in a paediatric inpatient population. J Paediatr Child Health. 1997;33(1):26-30.
62 Morris-Jones PH, Houston IB, Evans RC. Prognosis of the neurological complications of acute hypernatraemia. Lancet. 1967 Dec 30;2(7531):1385-1389.
63 Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med. 2000 May 18;342(20):1493-1499.
64 Moritz ML, Ayus JC. Disorders of water metabolism in children: hyponatremia and hypernatremia. Pediatr Rev. 2002 Nov;23(11):371-380.
65 Moritz ML, Ayus JC. The changing pattern of hypernatremia in hospitalized children. Pediatrics. 1999 Sep;104(3 Pt 1):435-439.
66 Polderman KH, Schreuder WO, Strack van Schijndel RJ, Thijs LG. Hypernatremia in the intensive care unit: an indicator of quality of care? Crit Care Med. 1999 Jun;27(6):1105-1108.
67 Atchley D, Loeb R, Richards D. On diabetic acidosis. A detailed study of electrolyte balances following withdrawal and reestablishment of therapy. J Clin Invest. 1933;12:297-326.
68 Katz MA. Hyperglycemia-induced hyponatremia—calculation of expected serum sodium depression. N Engl J Med. 1973 Oct 18;289(16):843-844.
69 Waldhausl W, Kleinberger G, Korn A, Dudczak R, Bratusch-Marrain P, Nowotny P. Severe hyperglycemia: effects of rehydration on endocrine derangements and blood glucose concentration. Diabetes. 1979 Jun;28(6):577-584.
70 Rutledge J, Couch R. Initial fluid management of diabetic ketoacidosis in children. Am J Emerg Med. 2000;18:658-660.
71 Carlotti AP, Bohn D, Halperin ML. Importance of timing of risk factors for cerebral oedema during therapy for diabetic ketoacidosis. Arch Dis Child. 2003 Feb;88(2):170-173.
72 Dillon E, Riggs HE, Dyer WE. Cerebral lesions in uncomplicated fatal diabetic acidosis. Am J Med Sci. 1936;192:360-365.
73 Metzger AL, Rubenstein AH. Reversible cerebral oedema complicating diabetic ketoacidosis. BMJ. 1970 Sep 26;3(725):746-747.
74 Young E, Bradley RF. Cerebral edema with irreversible coma in severe diabetic ketoacidosis. N Engl J Med. 1967 Mar 23;276(12):665-669.
75 Taubin H, Matz R. Cerebral edema, diabetes insipidus, and sudden death during the treatment of diabetic ketoacidosis. Diabetes. 1968;17(2):108-109.
76 Clements RSJr, Morrison AD, Blumenthal SA, Winegrad AI. Raised CSF pressure during treatment of diabetic ketosis. Lancet. 1972 Feb 5;1(7745):322.
77 Edge JA, Ford-Adams ME, Dunger DB. Causes of death in children with insulin dependent diabetes 1990-96. Arch Dis Child. 1999 Oct;81(4):318-323.
78 Edge JA, Hawkins MM, Winter DL, Dunger DB. The risk and outcome of cerebral oedema developing during diabetic ketoacidosis. Arch Dis Child. 2001 Jul;85(1):16-22.
79 Bello FA, Sotos JF. Cerebral oedema in diabetic ketoacidosis in children [letter]. Lancet. 1990;336(8706):64.
80 Mel JM, Werther GA. Incidence and outcome of diabetic cerebral oedema in childhood: are there predictors? J Paediatr Child Health. 1995;31(1):17-20.
81 Glaser N, Barnett P, McCaslin I, Nelson D, Trainor J, Louie J, et al. Risk factors for cerebral edema in children with diabetic ketoacidosis. The Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. N Engl J Med. 2001 Jan 25;344(4):264-269.
82 Rosenbloom AL. Intracerebral crises during treatment of diabetic ketoacidosis. Diabetes Care. 1990;13(1):22-33.
83 Krane EJ, Rockoff MA, Wallman JK, Wolfsdorf JI. Subclinical brain swelling in children during treatment of diabetic ketoacidosis. N Engl J Med. 1985;312(18):1147-1151.
84 Hoffman WH, Steinhart CM, el Gammal T, Steele S, Cuadrado AR, Morse PK. Cranial CT in children and adolescents with diabetic ketoacidosis. AJNR Am J Neuroradiol. 1988;9(4):733-739.
85 Mahoney CP, Vlcek BW, DelAguila M. Risk factors for developing brain herniation during diabetic ketoacidosis. Pediatr Neurol. 1999;21(4):721-727.
86 Duck SC, Wyatt DT. Factors associated with brain herniation in the treatment of diabetic ketoacidosis. J Pediatr. 1988;113(1 Pt 1):10-14.
87 Edge JA. Cerebral oedema during treatment of diabetic ketoacidosis: are we any nearer finding a cause? Diabetes Metab Res Rev. 2000 Sep-Oct;16(5):316-324.
88 Van der Meulen JA, Klip A, Grinstein S. Possible mechanism for cerebral oedema in diabetic ketoacidosis. Lancet. 1987 Aug 8;2(8554):306-308.
89 Rosenbloom AL. Therapeutic controversy: cerebral edema in diabetic ketoacidosis. J Clin Endocrinol Metab. 2000 Feb;85(2):507-508.
90 Harris GD, Fiordalisi I, Harris WL, Mosovich LL, Finberg L. Minimizing the risk of brain herniation during treatment of diabetic ketoacidemia: a retrospective and prospective study. J Pediatr. 1990 Jul;117(1 Pt 1):22-31.
91 Harris GD, Fiordalisi I. Physiologic management of diabetic ketoacidemia. A 5-year prospective pediatric experience in 231 episodes. Arch Pediatr Adolesc Med. 1994;148(10):1046-1052.
92 Roberts MD, Slover R, Chase HP. Diabetic ketoacidosis with intracerebral complications. Pediatric Diabetes. 2001;2:109-114.
93 Hale PM, Rezvani I, Braunstein AW, Lipman TH, Martinez N, Garibaldi L. Factors predicting cerebral edema in young children with diabetic ketoacidosis and new onset type I diabetes. Acta Paediatr. 1997;86(6):626-631.
94 Felner EI, White PC. Improving management of diabetic ketoacidosis in children. Pediatrics. 2001 Sep;108(3):735-740.
95 Bohn D, Daneman D. Diabetic ketoacidosis and cerebral edema. Curr Opin Pediatr. 2002 Jun;14(3):287-291.
96 Finberg L. Therapeutic controversy: Appropriate therapy can prevent cerebral edema in diabetic ketoacidosis. J Clin Endocrinol Metab. 2000;85(2):508-509.
97 Wolfsdorf J, Craig ME, Daneman D, Dunger D, Edge J, Lee W, et al. Diabetic ketoacidosis in children and adolescents with diabetes. Pediatr Diabetes. 2009 Sep;10(Suppl. 12):118-133.
98 Hoorn EJ, Carlotti AP, Costa LA, MacMahon B, Bohn G, Zietse R, et al. Preventing a drop in effective plasma osmolality to minimize the likelihood of cerebral edema during treatment of children with diabetic ketoacidosis. J Pediatr. 2007 May;150(5):467-473.
99 Curtis JR, Bohn D, Daneman D. Use of hypertonic saline in the treatment of cerebral edema in diabetic ketoacidosis (DKA). Pediatr Diabetes. 2001;2:191-194.
100 Mange K, Matsuura D, Cizman B, Soto H, Ziyadeh FN, Goldfarb S, et al. Language guiding therapy: the case of dehydration versus volume depletion. Ann Intern Med. 1997 Nov 1;127(9):848-853.
101 Keating JP, Schears GJ, Dodge PR. Oral water intoxication in infants. An American epidemic. Am J Dis Child. 1991;145(9):985-990.
102 Wattad A, Chiang ML, Hill LL. Hyponatremia in hospitalized children. Clin Pediatr (Phila). 1992;31(3):153-157.
103 Gorelick MH, Shaw KN, Murphy KO. Validity and reliability of clinical signs in the diagnosis of dehydration in children. Pediatrics. 1997 May;99(5):E6.
104 Mackenzie A, Barnes G, Shann F. Clinical signs of dehydration in children. Lancet. 1989 Sep 9;2(8663):605-607.
105 Rothrock SG, Green SM, McArthur CL, DelDuca K. Detection of electrolyte abnormalities in children presenting to the emergency department: a multicenter, prospective analysis. Detection of Electrolyte Abnormalities in Children Observational National Study (DEACONS) Investigators. Acad Emerg Med. 1997 Nov;4(11):1025-1031.
106 Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care. 1997 Jun;13(3):179-182.
107 Neville KA, Verge CF, O’Meara MW, Walker JL. High antidiuretic hormone levels and hyponatremia in children with gastroenteritis. Pediatrics. 2005 Dec;116(6):1401-1407.
108 Kanaan U, Dell KM, Hoagland J, O’Riordan MA, Furman L. Accelerated intravenous rehydration. Clin Pediatr (Phila). 2003 May;42(4):317-324.
109 Liebelt EL. Clinical and laboratory evaluation and management of children with vomiting, diarrhea, and dehydration. Curr Opin Pediatr. 1998 Oct;10(5):461-469.
110 Meyers A. Outpatient oral rehydration. Ann Emerg Med. 1997 Apr;29(4):552-553.
111 Meyers A. Fluid and electrolyte therapy for children. Curr Opin Pediatr. 1994 Jun;6(3):303-309.
112 Hahn S, Kim Y, Garner P. Reduced osmolarity oral rehydration solution for treating dehydration due to diarrhoea in children: systematic review. BMJ. 2001 Jul 14;323(7304):81-85.
113 Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002 Feb;30(2):300-305.
114 Skellett S, Mayer A, Durward A, Tibby SM, Murdoch IA. Chasing the base deficit: hyperchloraemic acidosis following 0.9% saline fluid resuscitation. Arch Dis Child. 2000 Dec;83(6):514-516.
115 Koch SM, Taylor RW. Chloride ion in intensive care medicine. Crit Care Med. 1992 Feb;20(2):227-240.
116 Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999 May;90(5):1265-1270.
117 Durward A, Skellett S, Mayer A, Taylor D, Tibby SM, Murdoch IA. The value of the chloride: sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. 2001 May;27(5):828-835.
118 Vannacci A, Baronti R, Masini E, Ravaldi C, Ricca V. Anorexia nervosa and the risk of sudden death. Am J Med. 2002 Mar;112(4):327-328.
119 Khilnani P. Electrolyte problems in critically ill children. Indian J Pediatr. 1993 Jan-Feb;60(1):89-101.
120 Linshaw MA. Potassium homeostasis and hypokalemia. Pediatr Clin North Am. 1987;34:649-682.
121 McClure RJ, Prasad VK, Brocklebank JT. Treatment of hyperkalaemia using intravenous and nebulised salbutamol. Arch Dis Child. 1994 Feb;70(2):126-128.
122 Cardenas-Rivero N, Chernow B, Stoiko MA, Nussbaum SR, Todres ID. Hypocalcemia in critically ill children. J Pediatr. 1989 Jun;114(6):946-951.