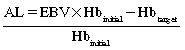14 Fluid and electrolytes
Anions: Ions that carry a negative charge and migrate to the anode (terminal) in an electric field.
Autologous: Originating within the same person, such as an autotransfusion.
Cations: Ions that carry a positive charge and migrate to the cathode (terminal) in an electric field.
Chvostek Sign: An abnormal spasm of the facial muscles elicited by light taps on the facial nerve that indicates hypocalcemia.
Colloids: Compounds such as red blood cells, albumin, or dextran that, because of size, are retained within a specific fluid compartment and increase the oncotic pressure of that compartment.
Cryoprecipitate: A preparation rich in factor VIII needed to restore normal coagulation in hemophilia. The preparation is collected from fresh human plasma that has been frozen and thawed.
Crystalloids: Balanced electrolyte solutions that are in isotonic solutions of water or dextrose and can move between the intravascular and interstitial compartments.
Edema: Accumulation of fluid in the interstitial spaces.
Hemolysis: A disruption of the integrity of the red cell membrane that causes release of cell contents to include hemoglobin.
Hemostasis: The arrest of bleeding by the interaction of the platelet with the blood vessel wall and the formation of the platelet plug.
Hypercalcemia: Increased plasma concentration of calcium (>5.6 mEq/L).
Hyperkalemia: Greater than 6 mEq/L blood concentration of potassium.
Hypermagnesemia: An increase in the plasma concentration of magnesium (>2.6 mEq/L).
Hypernatremia: An increase in sodium in the plasma of more than 145 mEq/L.
Hypertonic Solutions (Hyperosmotic): Solutions that have an osmolality greater than that of plasma.
Hypocalcemia: Reduced plasma concentration of calcium (<4.4 mEq/L).
Hypokalemia: Less than 3 mEq/L blood concentration of potassium.
Hypomagnesemia: A decrease in the plasma concentration of magnesium (<1.6 mEq/L).
Hyponatremia: A decrease of sodium in the plasma of less than 135 mEq/L.
Hypotonic Solutions (Hypoosmotic): Solutions that have an osmolality less than that of plasma.
Isotonic Solutions: Solutions that have the same osmolality as plasma.
Milliequivalent (mEq): Replaced with the SI units millimole (mmol); mEq/L has been replaced by mmol/L.
Osmolality: A physical property of a solution, one that is dependent on the number of dissolved particles in the solution.
Tetany: A condition characterized by cramps, muscle twitching, sharp flexion of the wrist and ankle joints, and convulsions.
Third Space: Losses of fluid and electrolytes from the extracellular fluid to a nonfunctional space, an acute sequestered space that accompanies surgery.
Trousseau Sign: A test for latent tetany in which carpal spasm is induced with inflation of a sphygmomanometer cuff on the upper arm to a pressure that exceeds systolic blood pressure for 3 minutes.
Body fluid balance
Water is the most abundant and essential component of the body. It represents approximately 50% to 60% of adult body weight and 75% to 77% of body weight in infants less than 1 month of age. By approximately 17 years of age, the adult composition is attained; and in a person weighing 154 lb (70 kg), the total body water is approximately 42 L. Because women have higher fat content in their bodies and because fat is essentially free of water, they have a lower water content than men do. Older adults and those with diabetes, hypertension, or obesity also have a lower proportion of water in their bodies.
The water formed during metabolism of ingested food is called endogenous water. Because metabolism varies with body temperature, the amount of exercise performed, and other factors, the amount of endogenous water available also varies on a daily basis. In a healthy adult who performs a moderate amount of exercise, an average of 300 to 350 mL of endogenous water is available daily. Intake is influenced by the thirst center located in the hypothalamus, which is stimulated by either a decrease in blood pressure or extracellular fluid, or an increase in serum osmolality. If the fluid volume inside the cells decreases, salivary secretion is reduced, thereby causing a dry mouth and the sensation of thirst. In normal circumstances, an individual then drinks and restores the fluid volume (Box 14-1).
Surgical patient considerations
The surgical patient experiences even greater fluid losses. Unless the patient is coming to the operating room for a surgical emergency, in most cases adults will be NPO for at least eight hours (Box 14-2). The goal of preoperative fluid therapy is to replace preexisting fluid deficits, normal intraoperative losses (maintenance requirements), and surgical wound losses (third spacing and blood loss).
BOX 14-2 Summary of Fasting Recommendations
From American Society of Anesthesiologists Committee on Standards and Practice Parameters: Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures, Anesthesiol 114:495-511, 2011.
NPO guidelines are enforced because of the risk of pulmonary aspiration. Over the past few years, fasting times have become more liberal after studies have shown that reduced fasting times lower residual gastric volumes. Furthermore, prolonged fasting can contribute to hypovolemia, hypoglycemia, and patient anxiety. Longer fasting times are generally enforced in patients who are at increased risk for aspiration (Box 14-3).
BOX 14-3 Patient Conditions at Increased Risk for Aspiration
• Increased intracranial pressure
• Decreased level of consciousness
From Nagelhout J, Plaus K: Nurse anesthesia, ed 4, St. Louis, 2010, Saunders; Barash P, et al: Clinical anesthesia, ed 6, Philadelphia, 2009, Lippincott Williams & Wilkins.
Lungs
The amount of fluid lost through the lungs varies with the humidity, temperature of inspired air, and the rate and depth of respiration. As a rule, 300 to 400 mL of water is lost daily. This loss of water via the respiratory tract is termed insensible loss of water, so named because one is not aware of this loss. The water content of inhaled gases decreases as the ambient temperature decreases. Consequently, the insensible water loss from the lungs is higher in cold environments. Therefore patients with respiratory dysfunction need a greater water intake to offset the increased insensible water loss when they are in cold environments. In addition, an increase in the respiration rate can increase the water loss to as much as 2000 mL, which is significant in patients with chronic obstructive pulmonary disease (see Chapter 48).
Distribution of body fluids
The fluids in the body can be divided into two compartments along with a potential third compartment or space. The two compartments are normally divided relative to the location of the cell membrane: intracellular (inside the cell) and extracellular (outside the cell). The intracellular fluid (ICF) is estimated to be approximately 40% of the body weight, or approximately 28 L of fluid, and represents approximately two thirds of the total body water. ICF provides a medium for all intracellular activities. The other compartment, the extracellular fluid (ECF), is approximately 20% of the body weight and ranges from 12 to 14 L of fluid. The fluid compartment includes the blood plasma or intravascular fluid, the interstitial fluid (ISF) that bathes the cells, the lymph, the cerebrospinal fluid (CSF), and the transcellular fluids. The transcellular fluids include the synovial fluid, peritoneal fluid, digestive fluids, and fluids of the eye and ear. The lymph, CSF, and the transcellular fluids normally constitute approximately 1% of the body mass. Blood constitutes approximately 4% of the body weight, and the interstitial fluid constitutes 15.7%.1
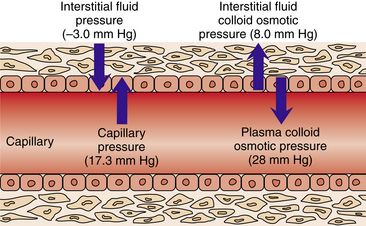
Fluid balance involves not only the total amount of body water but also the maintenance of a relatively constant distribution of that water in the different compartments. Circulation of fluid between compartments depends on the relative hydrostatic and osmotic pressures in each compartment. Hydrostatic pressure is the force that pushes fluid from one compartment to the other. If the hydrostatic pressure in the capillaries (blood pressure) exceeds the pressure in the interstitial space, fluid moves from the capillary into the interstitial space. Osmotic pressure is the “pull” of fluids into the compartment; it is a function of the number of dissolved molecules in the solution and is not influenced by weight or size of the molecule. Electrolytes are the major contributors to the osmotic pressure of the fluids.2
The extracellular fluid is regulated carefully by the kidneys to facilitate the cells being bathed in fluid that contains appropriate concentrations of electrolytes to include sodium, potassium, and nutrients. A patient with major abdominal surgery usually excretes large amounts of potassium during the first 48 hours postoperatively and for several days thereafter. As a result, the potassium is usually administered intravenously in the immediate postoperative period. The body has significant stores of potassium; therefore hypokalemia might not be evident for a number of days postoperatively. Potassium levels are generally monitored closely postoperatively, and replacement is administered intravenously when needed. It is important to note that plasma potassium measurements do not exactly predict total body potassium, because potassium is primarily an intracellular ion. From a clinical chemistry point of view, the international standard unit is the millimole (mmol), commonly called the milliequivalent (mEq). The clinical implications for the perianesthesia nurse is that patients who undergo major surgery should routinely have potassium levels checked and evaluated before surgery for determining whether they are receiving any non–potassium-sparing diuretics (see Chapter 13).
Edema
A delicate balance of pressures keeps fluids passing between compartments (Fig. 14-1). A dynamic equilibrium exists between the plasma and the interstitial fluid because proteins are too large to cross the capillary barrier, which creates a colloid osmotic pressure between the two components. The hydrostatic pressures of the blood and the interstitial fluid tend to oppose each other, which is called the effective filtration pressure. Similarly, the colloid osmotic (or oncotic) pressure is the opposition between the blood and the interstitial fluid. The final common pathway is that these pressures result in a pulling in opposite directions when in appropriate physiologic equilibrium that does not allow fluid to accumulate into the interstitial spaces. Edema then results when either of the two pressures are in dysfunction.
Electrolytes
Sodium
The body fluids are maintained in an isotonic state with regulation of the concentration of sodium and its most abundant anion, chloride. Concentration of sodium and chloride in the fluids is maintained primarily with loss or retention of water. Loss of salt is accompanied by loss of water and retention of salt by retention of water. Water moves into areas where salt is in higher concentration, which is why patients are often placed on a low-salt diet in an effort to reduce fluid overload on the heart and other major organs. However, patients who receive magnesium sulfate can also have impaired fluid excretion.
Hypernatremia is most often caused by a loss of body fluids resulting in excess sodium. Elective surgery should be postponed until sodium levels greater than 150 mEq/L are corrected. Correction of water deficit should take place over 48 hours with hypotonic solutions. Rapid correction can result in seizures, cerebral edema, and coma.3
Potassium
Potassium depletion can be accompanied by changes in plasma potassium concentration. True depletion develops only with a net loss of potassium, whereas a decrease in plasma potassium, hypokalemia, can occur with a shift of potassium from the ECF to the ICF. Decreased intake can cause a mild deficit because the mechanisms for potassium conservation are not as efficient as those for sodium. Severe depletion results from abnormal losses rather than decreased intake. Most common causes of severe potassium loss are usually associated with diuretics (see Chapter 13), vomiting, acute blood loss, gastrointestinal surgery, and nasogastric suctioning. Cardiac arrhythmias, polyuria, confusion, and weakness of skeletal muscle are commonly observed in mild hypokalemia. Hallucinations, diminished reflexes, ST segment depression, widened QRS, flattened T waves, and cardiac arrest result from severe depletion. Oral replacement with potassium chloride is in the range of 60 to 80 mEq/day. Peripheral intravenous (IV) potassium should not exceed 8 mEq/h so as not to irritate veins. Central IV potassium can be infused at 10 to 20 mEq/h. The administration of 0.5 mEq/kg of potassium chloride usually raises the serum potassium concentration by 0.6 mEq/L. If the patient is receiving catecholamine drugs, the increase is approximately 0.1 mEq/L; if the patient is receiving beta-adrenergic antagonists, the serum concentration increases by approximately 0.9 mEq/L. It is important to note that correction of hypomagnesemia may be needed to avoid the increased loss of potassium by the kidneys.
Hyperkalemia is often associated with situations in which cells are injured or destroyed. Examples include chronic and acute renal failure, crush injuries, burn victims, and newborns who receive relatively large transfusions. The administration of succinylcholine, a depolarizing skeletal muscle relaxant, can produce also produce hyperkalemia (discussed in Chapter 23). Accidental lethal doses of supplemental IV potassium have been administered to patients with rapid intravenous infusion in the PACU. Cases have been recorded in which death occurred within 5 minutes of the rapid injection of just 25 mEq of potassium; therefore under no circumstance should potassium chloride be given via IV push.3
Calcium
Calcium is one of the major extracellular cations. It is deposited in the bone tissue as crystalline salts composed primarily of calcium and phosphate; the remainder is in the plasma, ISF, and soft tissues. The major fraction of calcium that accounts for its physiologic effects is the ionizable calcium in plasma, of which the normal plasma concentration is maintained between 4.0 and 5.6 mg/dL. The remainder is bound to protein and other substances in nonionizable form, with normal serum calcium levels rending 8.5 to 10.5 mg/dL. Calcium has an important function in neuromuscular transmission, skeletal muscle contraction, blood coagulation, and exocytosis necessary for release of neurotransmitters and autacoids (serotonin, histamine, kinins). In addition, the balance of the appropriate calcium concentration is controlled by the parathyroid hormone, calcitonin, and vitamin D. Calcium also has a reciprocal relationship with phosphate ions.
Magnesium
Hypermagnesemia is a rare clinical phenomenon. The most common cause of hypermagnesemia is the parenteral administration of magnesium as a treatment for pregnancy-induced hypertension. Symptoms of hypermagnesemia include sedation, myocardial depression, relaxed skeletal muscles and, when severe, paralysis of the muscles of ventilation. Treatment of the life-threatening hypermagnesemia is with calcium gluconate (1 g) given intravenously followed by a loop diuretic and increased fluid loading to produce diuresis in an effort to enhance the excretion of the excess magnesium. Monitoring for vasodilation and negative inotropic effects is critical.2
Perioperative blood and fluid replacement
Because of many factors (e.g., NPO, insensible fluid loss, surgical stresses of hemostatic function), fluid status, medical and surgical history, and medication regimens should be assessed. If problems with hemostasis are envisioned, coagulation function should be assessed before surgery to ensure appropriate intraoperative and postoperative coagulation. A patient can lose up to 75% of RBC volume if the total blood volume is maintained with the administration of colloid or crystalloid solutions. If the red cells are not replaced, the result is a loss in oxygen-carrying capacity, because RBCs carry approximately 90% of the oxygen in the blood. In the situation of massive transfusions, many complications can arise in the PACU, such as dilutional coagulopathy, acidosis, electrolyte abnormalities, and other long-term consequences as described in Chapter 29.
Crystalloid and colloid administration
Crystalloid fluids are electrolyte solutions dissolved in water or dextrose and water. These electrolytes are impermeable to the cellular membrane, and dextrose crosses cell membranes; however, crystalloids are freely permeable to the vascular membranes. Crystalloid solutions help to determine the total osmotic pressure or osmolality that helps to balance water between the extracellular and the intracellular compartments. Osmolality reflects the number of dissolved particles in solutions. An isotonic solution, such as normal saline, has the same osmolality as plasma, whereas a hypertonic solution has an elevated concentration of particles and a hypotonic solution has fewer dissolved particles than plasma does. Administering hypertonic solutions, such as 3% saline, promotes movement of water from the cells into the plasma and shrinks the brain, whereas hypotonic solutions (e.g., D5W) expand the brain (Fig. 14-2).
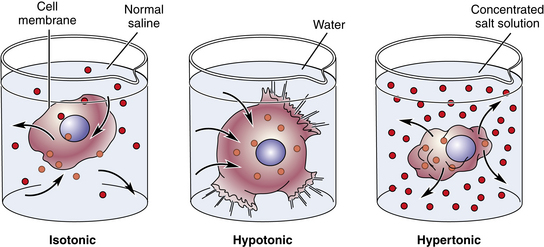
FIG. 14-2 Effects of osmosis—isotonic, hypotonic, and hypertonic solutions.
(From Herlihy B: The human body in health and illness, ed 4, St. Louis, 2011, Saunders.)
Colloids are solutions that contain natural or synthetic molecules that are usually impermeable to the vascular membrane. As a result, they remain predominately in the intravascular space. By doing so, colloids determine the colloid oncotic pressure that helps to balance the water distribution between the intravascular and interstitial spaces. Albumin is the prototype natural colloid and accounts for approximately two thirds of the plasma oncotic pressure. Dextrans 40 and 70, along with hetastarch 6% with an osmolarity of 310 mOsm/L, are the major synthetic colloids in clinical use. The advantages of colloid use are that the solution tends to remain in the intravascular compartment for up to 24 hours, thus causing less peripheral edema and rapidly restoring the circulating volume. In addition, smaller volumes of colloids compared with crystalloids can be used for fluid resuscitation, and the colloids restore the patient volume status sooner and create a sustained increase in plasma volume. Finally, because colloids increase the plasma colloid oncotic pressure, they prevent pulmonary edema. Some of the disadvantages of colloid use include the expense, the potential to cause coagulation problems and anaphylactic reactions, and the interference with blood-typing and cross-matching procedures. Although colloids improve the circulating volume, after 24 hours they redistribute into the third space and can exacerbate edema.5
Blood component therapy
Another type of autologous blood transfusion is the use of acute isovolemic hemodilution during the operative procedure. In this situation, a portion of the allowable blood loss is collected with a large-bore intravenous cannula. Usually an equal amount of crystalloid is administered to dilute and subsequently reduce the number of RBCs during the operation. Near the end of the operation, the autologous blood is reinfused to provide more RBCs and fresh platelets.
Types of blood component therapy
Fresh frozen plasma
Fresh frozen plasma (FFP) is used for the treatment of bleeding or documented coagulation problems. It should not be used for volume expansion or for replacement of large deficiencies in coagulation factors. FFP is separated from whole blood usually within 8 hours of collection. It can be stored for up to 1 year. It is important to note that various plasma products are available that do not meet the preparation requirements for FFP, including plasma, liquid plasma, thawed plasma, and plasma frozen within 24 hours after phlebotomy.
Transfusion reactions
If a hemolytic transfusion reaction is suspected, the PACU nurse should stop the transfusion immediately and attach normal saline solution to the intravenous catheter. The attending physician and the blood bank should be notified, and a specimen of blood should be drawn and sent to the blood bank along with the blood unit and administration set. A specimen of urine should be obtained to send to the laboratory for evaluation for hemoglobin content. Finally, the other units of blood for that patient should be rechecked. See Chapter 29 for further discussion of complications resulting from transfusion of blood products.
Summary
The objective of this chapter was to provide a broad view of the complex topic of body fluids and electrolytes, and their maintenance and treatment in the perioperative setting. The major electrolytes were presented along with discussion and examples of fluid replacement and fluid loss. Coagulation is presented in regard to the normal cascade of the various factors and the various laboratory tests that can be used for assessing coagulation from both the intrinsic and extrinsic components. Finally, one of the most important assessment parameters for the perioperative nurse in the PACU is for hypovolemia as a result of blood loss or overall fluid deficit, cardiovascular instability, and electrolyte imbalances.
1. Hall J. Guyton and Hall textbook of medical physiology, ed 12. Philadelphia: Saunders; 2011.
2. Rose B, Post T. Clinical physiology of acid-base and electrolyte disorders. ed 5. New York: McGraw-Hill; 2001.
3. Stoelting R, Hillier C. Pharmacology and physiology in anesthetic practice, ed 4. Philadelphia: Lippincott Williams & Wilkins; 2005.
4. Brunton L, et al. Goodman and Gilman’s the pharmacological basis of therapeutics, ed 12. New York: McGraw-Hill; 2011.
5. Nagelhout J, Plaus K. Nurse anesthesia, ed 4. St. Louis: Saunders; 2010.
Aitkenhead A, et al. Textbook of anaesthesia, ed 5. Edinburgh: Churchill Livingstone; 2007.
Alspach J. Core curriculum for critical care nursing, ed 6. Philadelphia: Saunders; 2006.
Atlee J. Complications in anesthesia, ed 2. Philadelphia: Saunders; 2007.
Barrett KE, et al. Ganong’s review of medical physiology, ed 23. New York: McGraw-Hill; 2010.
Evers A, Maze M. Anesthetic pharmacology: physiologic principles and clinical practice. Philadelphia: Churchill Livingstone; 2004.
Fauci AS, et al. Harrison’s principles of internal medicine, ed 17. New York: McGraw Hill; 2008.
Fleisher LA. Anesthesia and uncommon diseases, ed 5. Philadelphia: Saunders; 2007.
Gallager C, Issenberg B. Simulation in anesthesia. Philadelphia: Saunders; 2007.
Townsend C, et al. Sabiston textbook of surgery: the biological basis of modern surgical practice. ed 18. Philadelphia: Saunders; 2008.
Valchanov K, et al. Anaesthetic and perioperative complications. Cambridge: Cambridge University Press; 2011.