Flail Chest
After reading this chapter, you will be able to:
• List the anatomic alterations of the lungs associated with a flail chest.
• Describe the causes of a flail chest.
• List the cardiopulmonary clinical manifestations associated with a flail chest.
• Describe the general management of a flail chest.
• Describe the clinical strategies and rationales of the SOAPs presented in the case study.
• Define key terms and complete self-assessment questions at the end of the chapter and on Evolve.
Anatomic Alterations of the Lungs
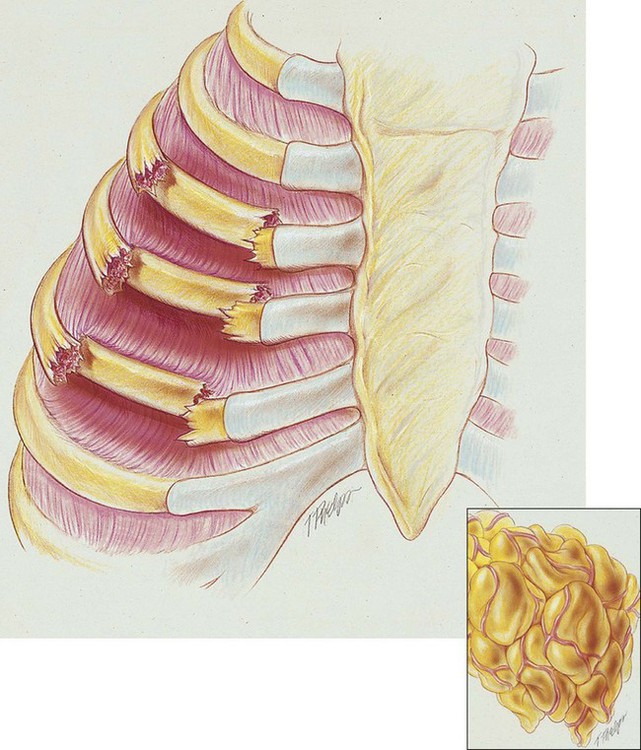
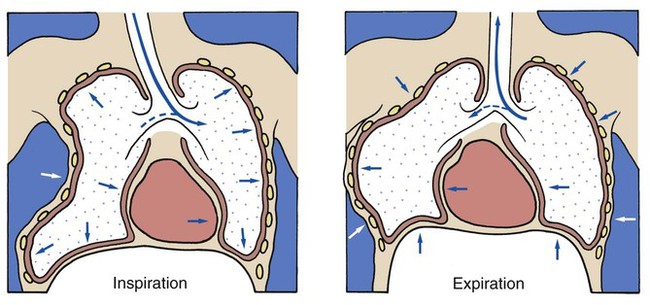
A flail chest is the result of double fractures of at least three or more adjacent ribs, which causes the thoracic cage to become unstable—to flail (see Figure 21-1). The affected ribs cave in (flail) during inspiration as a result of the subatmospheric intrapleural pressure. This compresses and restricts the underlying lung area and promotes a number of pathologies, including atelectasis and lung collapse. In addition, the lung also may be contused under the fractured ribs.
General Management of Flail Chest
Respiratory Care Treatment Protocols
Oxygen Therapy Protocol
Oxygen therapy is used to treat hypoxia, decrease the work of breathing, and decrease myocardial work. It should be noted, however, that the hypoxemia that develops in flail chest is most commonly caused by the alveolar atelectasis and capillary shunting associated with the disorder. Hypoxemia caused by capillary shunting is often refractory to oxygen therapy (see Oxygen Therapy Protocol, Protocol 9-1).
CASE STUDY
Flail Chest
Admitting History and Physical Examination
Respiratory Assessment and Plan
S N/A—patient is intubated, put on mechanical ventilator, sedated, and paralyzed (Norcuron).
O No spontaneous respirations. No paradoxic movement of chest wall on ventilator. BP 110/70, HR 100 regular, RR 12 on vent. On 100% O2, pH 7.48, Paco2 30, 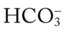 23, and Pao2 360. CXR: Bilateral rib fractures, left lung contused, no pneumothorax, no hemothorax.
23, and Pao2 360. CXR: Bilateral rib fractures, left lung contused, no pneumothorax, no hemothorax.
• Bilateral flail chest (history, paradoxic chest movement, CXR)
• Acute alveolar hyperventilation with overoxygenation (ABG)
P Mechanical Ventilation Protocol: Decrease VT to correct acute alveolar hyperventilation and maintain patient on controlled ventilation per protocol until chest wall is stable. Wean oxygen per Ventilator Protocol (decreased to Fio2 0.4). Alert charge nurse (to request increased sedation and muscle paralysis) if the patient begins to inhale above preset mechanical ventilation rate. Routine ABG monitoring and continuous Sao2 monitoring. Careful chest assessment and auscultation to monitor for secondary pneumothorax and pneumonia.
Respiratory Assessment and Plan
S N/A—intubated, sedated, and paralyzed.
O Afebrile. HR 160 regular, BP 142/82, RR 12 (on vent). Right chest tube shows air leak. Crackles bilaterally. CXR: Fractures appear in line; bilateral dense infiltrates. ABG on an Fio2 of 0.7: pH 7.37, Paco2 38, 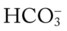 23, and Pao2 58. Sputum thick, yellow.
23, and Pao2 58. Sputum thick, yellow.
• Persistent bilateral flail chest (CXR)
• Bilateral dense infiltrates suggest atelectasis vs pulmonary edema vs ARDS vs pneumonia (CXR)
• Adequate alveolar ventilation with moderate hypoxemia on present ventilator settings; oxygenation continues to worsen (ABG)
• Thick, yellow bronchial secretions (sputum)
P Mechanical Ventilation Protocol and Lung Expansion Therapy Protocol. Increase PEEP to 12 cm H2O. Oxygen therapy per protocol (increase Fio2 to 0.8). Institute Bronchopulmonary Hygiene Therapy Protocol and Aerosolized Medication Protocol (in-line med. neb. with 2.0 mL premixed albuterol, followed by direct instillation of acetylcysteine q4h, and suction prn. Obtain sputum for Gram stain and culture). Assist physician in the replacement of the Swan-Ganz catheter to optimize fluid therapy. Continue Sao2 monitoring.
Discussion
This complicated case demonstrates the care of the traumatized patient with multiorgan failure. In this case the second organ system affected was the cardiovascular system, probably secondary to fluid overload. Initial therapy included chest wall rest and internal fixation with mechanical ventilation. By the time of the second assessment, the more classic clinical manifestations of pulmonary parenchymal change secondary to flail chest had developed. The clinical scenarios of Atelectasis (see Figure 9-8) and/or Alveolar Consolidation (see Figure 9-9) were well established, with oxygen-refractory pulmonary capillary shunting clearly in evidence.

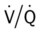 ratio decreases. This leads to intrapulmonary shunting and venous admixture (
ratio decreases. This leads to intrapulmonary shunting and venous admixture (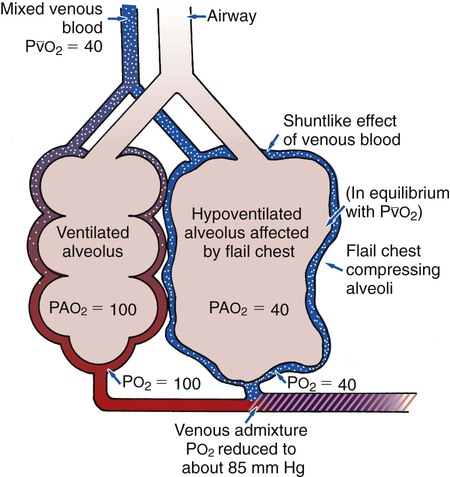
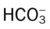

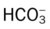
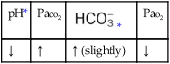
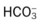 values will be lower than expected for a particular Pa
values will be lower than expected for a particular Pa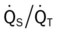
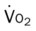
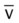 )
)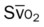

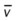 )O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio;
)O2, Arterial-venous oxygen difference; DO2, total oxygen delivery; O2ER, oxygen extraction ratio; 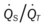 , pulmonary shunt fraction;
, pulmonary shunt fraction; 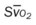 , mixed venous oxygen saturation;
, mixed venous oxygen saturation; 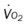 , oxygen consumption.
, oxygen consumption.
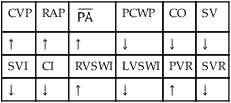
 , mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.
, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSWI, right ventricular stroke work index; SV, stroke volume; SVI, stroke volume index; SVR, systemic vascular resistance.
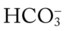 23, and Pa
23, and Pa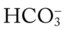 23, and Pa
23, and Pa