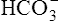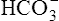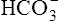Chapter 21
Filtration, Urine Formation, and Fluid Regulation
After reading this chapter you will be able to:
• Describe the anatomy of the nephron
• Differentiate between the terms filtrate and urine and reabsorption and secretion
• Explain how glomerular filtration rate and urine output are related
• Explain how autoregulation of the glomerular filtration rate and renal blood flow are related
• Explain how the nephron processes the tubular filtrate to excrete a concentrated or dilute urine
• Describe why it is important that the kidney’s countercurrent multiplier mechanism maintains a high osmotic pressure deep in the medulla
• Explain how aldosterone, natriuretic peptides, and antidiuretic hormone influence extracellular fluid volume
• Describe the mechanisms whereby various classes of diuretic drugs work
Functional Anatomy of the Kidneys
Gross Anatomy
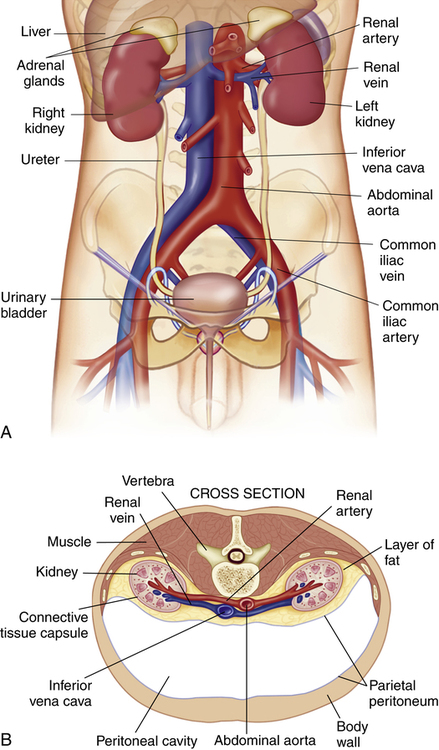
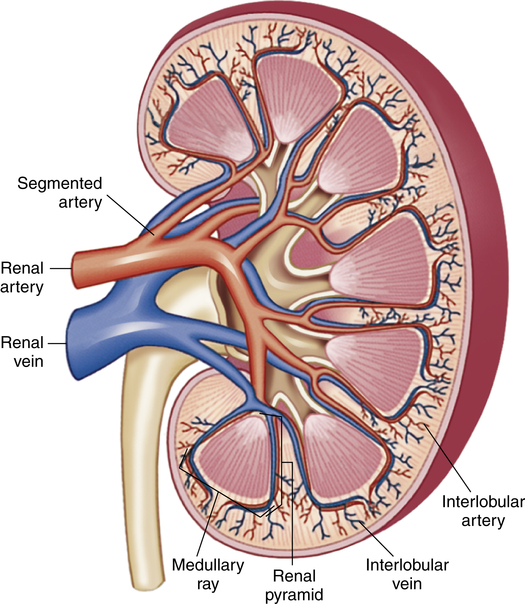
The kidneys are paired, bean-shaped organs that are located on the posterior abdominal wall behind the peritoneal membrane; hence, the kidneys are called retroperitoneal organs (Figure 21-1, A). A fat pad surrounding the renal capsule protects the kidneys from mechanical shock (Figure 21-1, B). Blood vessels and nerves enter the medial concave border of the kidney at the hilum (Figure 21-2). The kidney contains an outer cortex and inner medulla (see Figure 21-2). A ureter from each kidney carries urine to the bladder.
Nephron Anatomy
The nephron is the basic functional unit of the kidney. This structure is responsible for filtering the blood plasma, eliminating only the unwanted substances, and returning the remainder to the circulation. These unwanted substances include urea (a by-product of amino acid metabolism), creatinine (from muscle cells), uric acids (from nucleic acids), bilirubin (from hemoglobin breakdown), and metabolites of assorted hormones; in addition, the kidneys eliminate various toxins and foreign substances, whether produced by the body or ingested.1
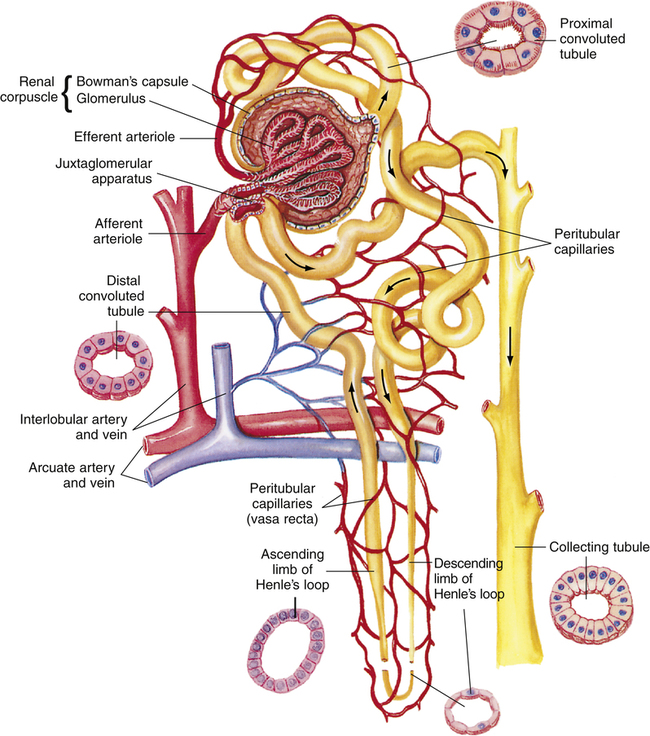
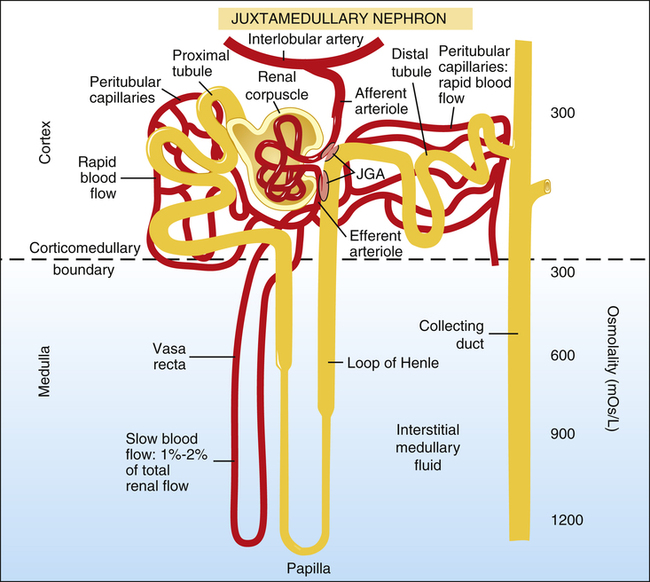
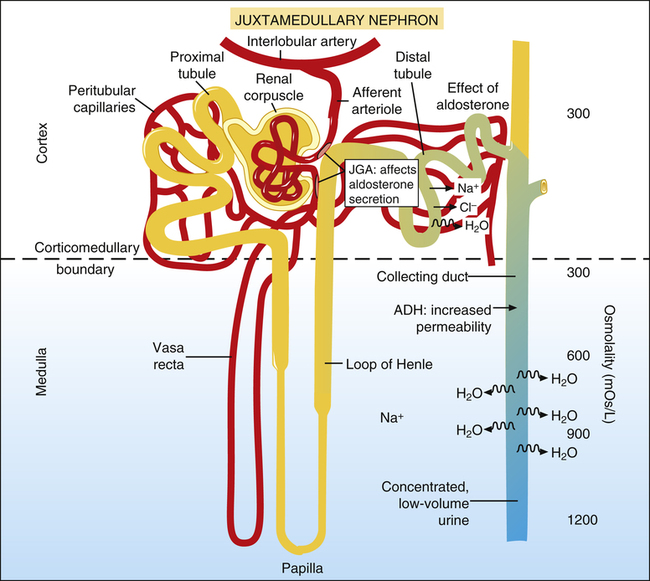
Each kidney contains between 0.4 million and 1.2 million nephrons.2 Because the kidney cannot regenerate new nephrons, normal aging and disease reduce their number such that a normal 80-year-old individual has about 40% fewer functioning nephrons than he or she did at age 40.1 The nephron is composed of the following structures, listed in the order in which fluid flows through them: (1) Bowman’s capsule, (2) proximal convoluted tubule, (3) loop of Henle, (4) distal convoluted tubule, and (5) collecting duct (Figure 21-3).
Bowman’s Capsule
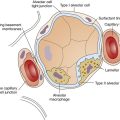
The beginning of the proximal tubule is called Bowman’s capsule (a hollow sphere composed of epithelium, deeply indented to form a double-walled pouch). A small afferent arteriole enters this spherical pouch and branches diffusely to form a dense tuft of capillaries called the glomerulus. The glomerulus is in intimate contact with the inner capsular wall. Glomerular capillaries contain thousands of minute pores, or fenestrations, rendering them highly permeable to all plasma constituents except large protein molecules. Blood leaves the glomerulus by way of the efferent arteriole, formed by the convergence of the glomerular capillaries. The glomerulus and its surrounding double-walled capsule are collectively called the renal corpuscle (see Figure 21-3). This entire structure is located in the renal cortex. The epithelial layer forming the inner pouch of Bowman’s capsule (the visceral layer) and the fenestrated glomerular capillary endothelium form the glomerular filtration membrane. The first step of urine formation occurs when glomerular hydrostatic pressure (about 60 mm Hg)1 forces plasma through the glomerular filtration membrane. The resulting filtrate enters Bowman’s capsule.
Proximal Convoluted Tubule
The tubule draining the filtrate from Bowman’s capsule follows a curved, twisted path and is called the proximal convoluted tubule. Its terminal end approaches the medullary border, where it forms the beginning of the loop of Henle. (It is called the proximal tubule because it is located before the loop of Henle.) The tubule’s epithelium has a brushlike border of numerous fine microvilli facing its luminal side (see Figure 21-3). These microvilli greatly increase the luminal wall surface area.
Loop of Henle
The loop of Henle consists of a straight descending limb, a sharp hairpin turn, and an ascending limb (see Figure 21-3). About 70% to 80% of the glomeruli are located in the outer cortex (cortical nephrons) and have short loops of Henle that never reach the medulla.1 The remaining nephrons located deeper in the cortex near the medullary border are called juxtamedullary nephrons. These nephrons have long loops dipping deep into the medulla.
Renal Vasculature
Because the kidneys must eventually filter all the blood plasma, they receive an extremely high blood flow relative to their mass (about 20% of the cardiac output).1 Renal arteries enter the hilum and eventually subdivide to form afferent arterioles entering Bowman’s capsule (see Figure 21-3).
The kidneys have two capillary beds: the glomerulus and peritubular capillaries. The peritubular capillaries are branches of the efferent arteriole, which was formed by the convergence of the glomerular capillaries. The peritubular capillaries closely surround proximal and distal convoluted tubules. The vasa recta are a branch of the peritubular circulation that surrounds the loop of Henle (see Figure 21-3).
Because of the moderately high resistance of the efferent arteriole, the glomerular capillaries are a high-pressure bed, and the peritubular capillaries are a low-pressure bed. As a result, glomerular capillaries act as filtration vessels, and peritubular capillaries act as absorption vessels. The vasa recta receive only 1% to 2% of the renal blood flow.1 Medullary blood flow is quite sluggish, in contrast to rapid cortical blood flow.
Juxtaglomerular Apparatus
Near their glomerular entry and exit points, afferent and efferent arterioles contain cuffs of smooth muscle that control vessel diameter. At these points, the distal convoluted tubule abuts both arterioles.1 The densely packed distal tubular cells in this area are called the macula densa; the smooth muscle cuffs of the afferent and efferent arterioles are called juxtaglomerular cells. The macula densa and juxtaglomerular cells are collectively known as the juxtaglomerular apparatus (see Figure 21-3). When systemic blood pressure decreases, cells of the juxtaglomerular apparatus secrete renin, an enzyme that activates angiotensin, which ultimately leads to widespread systemic arteriole constriction.
Basic Theory of Nephron Function
The nephron accomplishes its function in the following way: First, it filters about 20% of the renal blood plasma through the glomerular membrane into the proximal tubules1; next, it reabsorbs most of the filtrate’s water and electrolytes and all of the glucose back into the peritubular capillaries. The remaining filtrate (water and waste products) stays in the tubules and is eliminated in the urine. In addition, the nephron actively secretes certain substances directly into the tubular filtrate; urine contains both filtered and secreted substances.
Formation of Glomerular Filtrate
Filtration Pressure
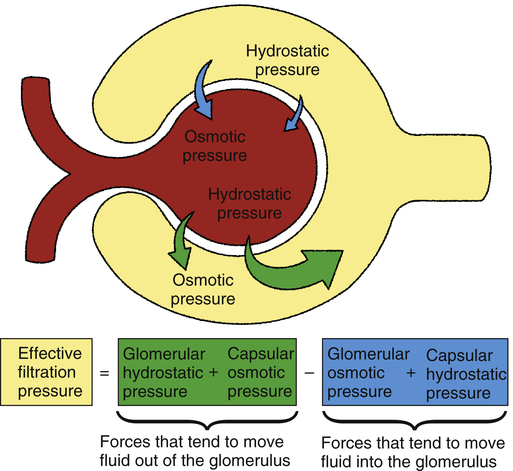
Filtration is the movement of water and solutes from the plasma in the glomerulus, across the glomerular membrane, and into Bowman’s capsule. Filtration occurs because of a pressure gradient between glomerular capillary blood and the capsular filtrate. The main factor establishing this pressure gradient is the hydrostatic pressure of the glomerular blood (Figure 21-4). This pressure is about 60 mm Hg, much higher than capillary pressures anywhere else in the body.1 The major cause of this high pressure is the high resistance of the efferent arteriole.
Figure 21-4 illustrates the various factors that affect glomerular filtration pressure. Blood osmotic pressure and capsular hydrostatic pressure oppose filtration. The net filtration pressure equals the sum of forces pushing fluid out of the glomerular capillaries minus the sum of forces pushing fluid back into the glomerular capillaries, as the following equation shows:1
| 60 mm Hg | − | (18 mm Hg + 32 mm Hg) | = | 10 mm Hg | |
| Glomerular hydrostatic pressure | Capsular hydrostatic pressure | Glomerular osmotic pressure | Net filtration pressure | ||
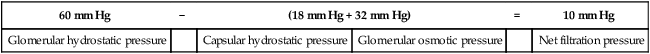
Glomerular filtration occurs much more rapidly than filtration in other tissue capillaries because (1) the glomerular membrane is about 100 to 500 times more permeable than other capillaries1 and (2) glomerular hydrostatic pressure is much higher than in other capillaries. The normal filtration pressure of 10 mm Hg produces a glomerular filtration rate (GFR) of about 125 mL per minute.1 This represents about 20% of the glomerular blood flow, or a filtration fraction of about 20%.1 At a GFR of 125 mL per minute, about 180 L of filtrate is produced daily. Of this filtrate, about 99% is reabsorbed into the blood for a total urine output of slightly greater than 1.5 L per day.
Filtrate Constituents
Glomerular filtrate is quite similar in composition to interstitial fluids of body tissues. It contains no red blood cells and only about 0.03% protein (about 1⁄240 of the protein in plasma).1 Glomerular filtrate is the same as plasma except it contains almost no proteins.
Threshold Substances
Threshold substances in the filtrate are totally reabsorbed unless they are present in amounts greater than normal.1 All actively reabsorbed substances have a maximum rate at which they can be reabsorbed; this is called the tubular transport maximum. If the tubular transport maximum is exceeded, these substances are excreted in the urine. Substances with a threshold tubular transport maximum include glucose, amino acids, phosphates, and sulfates. For example, urine normally contains no glucose, unless the plasma glucose concentration is extremely high. In such an instance, large amounts of glucose are filtered into the tubular fluid, exceeding the tubular reabsorption capacity.
Electrolytes
Sodium, potassium, chloride, and bicarbonate are almost totally reabsorbed from the tubules. Table 21-1 shows the reabsorption percentage for these electrolytes.
TABLE 21-1
Net Daily Reabsorptive Work Performed by the Kidneys∗
| Substance | Filtered | Excreted | Net Reabsorption (%) |
| Water | 180 L | 0.5-3 L | 98-99 |
| Na+ | 26,000 mEq | 100-250 mEq | >99 |
| Cl− | 21,000 mEq | 100-250 mEq | >99 |
| HCO3− | 4800 mEq | 0 | ≈100 |
| K+ | 800 mEq | 40-120 mEq | 85-95† |
| Urea | 54 g | 27-32 g | 40-50 |
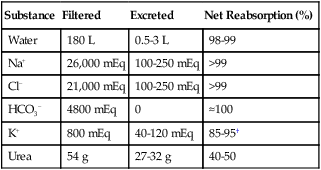
∗Values for normal men consuming a typical Western diet. The filtered load of water and solutes is about 25% less in women.
†Reflects the interplay of reabsorption and secretion of K+.
Modified from Rose BD: Clinical physiology of acid-base and electrolyte disorders, ed 3, New York, 1989, McGraw-Hill.
Determinants of Glomerular Filtration Rate
Renal Blood Flow
Increased renal blood flow increases the glomerular pressure and enhances filtration. It also enhances the GFR for another reason. Under normal conditions, glomerular blood protein concentration greatly increases as water passes through the filtration membrane into Bowman’s capsule. The subsequent increase in plasma osmotic pressure slows the filtration rate. Conversely, when blood flow is rapid, it spends less time in the glomerulus, and less water leaves the plasma. Consequently, the increase in plasma osmotic pressure and its inhibitory effect on the filtration rate are less severe.1
Efferent Arteriole Constriction
Efferent constriction increases glomerular outflow resistance and increases glomerular capillary pressure and the filtration rate. However, severe efferent arteriole constriction decreases blood flow through the glomerulus, allowing more water to be filtered into Bowman’s capsule. The subsequent increase in plasma osmotic pressure counteracts the filtration pressure, and the overall effect may be to slow the GFR.1
Autoregulation of Glomerular Filtration Rate
GFR remains fairly constant between arterial pressures of 75 mm Hg and 160 mm Hg.1 A constant GFR is important to eliminate waste products and reabsorb solutes appropriately. With an excessively slow GFR, filtrate passes so slowly through the tubules that most of it is reabsorbed; hence the kidneys fail to eliminate waste products adequately. An extremely rapid GFR does not give the tubules enough time to reabsorb needed substances, and the kidneys lose too much water and solute in the urine.
Two specialized feedback mechanisms automatically maintain a constant GFR between arterial blood pressures of 75 mm Hg and 160 mm Hg: (1) the afferent arteriolar vasodilator feedback mechanism and (2) the efferent arteriolar vasoconstrictor feedback mechanism.1 The juxtaglomerular apparatus controls both mechanisms.
Afferent Vasodilator Feedback Mechanism
A low GFR allows the tubules to reabsorb too much sodium and chloride ions from the filtrate. The specialized macula densa cells in the distal tubule sense the low sodium chloride concentration in the filtrate, which elicits a signal that causes afferent arteriole dilation.1 As a result, glomerular blood flow and GFR increase.
Efferent Vasoconstrictor Feedback Mechanism
In response to a decrease in the filtrate’s sodium chloride concentration, juxtaglomerular cells of both afferent and efferent arterioles release the enzyme renin; juxtaglomerular cells are major storage sites for renin.1 Renin is an enzyme that acts on circulating angiotensinogen and converts it to angiotensin I.1 A proteolytic enzyme called angiotensin-converting enzyme (ACE) converts angiotensin I to angiotensin II, a potent vasoconstrictor that preferentially constricts the kidney’s efferent arterioles.1 Consequently, glomerular capillary pressure increases, increasing the GFR. Together, the afferent vasodilator and efferent vasoconstrictor mechanisms keep GFR constant in the face of wide blood pressure swings.
Autoregulation of Renal Blood Flow
The same mechanisms just described also regulate renal blood flow. The most important autoregulatory mechanism in this regard is the afferent vasodilator mechanism. As described in the previous section, decreased blood flow tends to reduce GFR, which elicits afferent arteriolar dilation. Blood flow is restored toward normal. However, this mechanism is primarily designed to regulate GFR; autoregulation of renal blood flow is merely incidental.1
Myogenic Reflex Mechanism
Most arterioles in the body contract their smooth muscle to resist stretching in the face of increased blood pressure and vascular wall tension. This phenomenon, called the myogenic reflex mechanism, brings renal blood flow and GFR back to normal when blood pressure is too high.1 When vessel walls are stretched, calcium ions are allowed to move into vascular smooth muscle cells, facilitating contraction; the opposite effect occurs when blood pressure is low.2 The importance of the myogenic reflex mechanism in the autoregulation of renal blood flow and GFR is unclear.1,2
Processing the Glomerular Filtrate
Reabsorption and Secretion in the Tubules
In Figure 21-5, the glomerular filtrate entering juxtamedullary nephron tubules flows (1) into Bowman’s capsule, (2) through the proximal tubule, (3) through the loop of Henle, (4) through the distal tubule and past the juxtaglomerular apparatus, and (5) through the collecting duct. (Note the large osmotic pressure gradient in the interstitial fluid from the corticomedullary boundary to the papilla deep in the medulla.) In the juxtamedullary nephrons, Henle’s loop and the vasa recta extend deep into the medulla’s high osmotic pressure environment. As explained later, this design provides a mechanism for concentrating the filtrate, eliminating a maximum amount of unwanted solute with a minimum loss of water.
Proximal Tubules
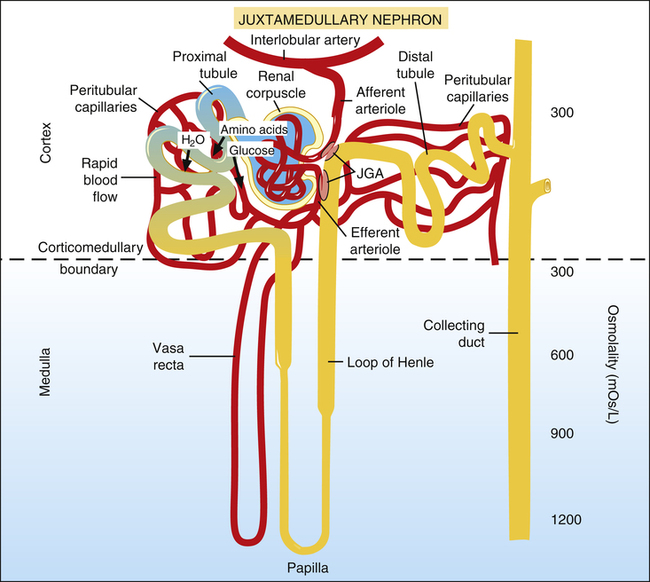
About 20% of the plasma of the glomerular capillary blood is filtered into Bowman’s capsule and then enters the proximal tubules (Figure 21-6). The low pressure and high blood flow rate of the peritubular capillaries, combined with the blood’s high osmotic pressure, provide an excellent reabsorption mechanism. (The process of filtration greatly increases the osmotic pressure of efferent arteriole blood, which is increased further with efferent vasoconstriction.)
About 65% of the filtrate volume is reabsorbed into the peritubular capillaries.1 The extensive brush border surface area on the luminal side of proximal tubule cells facilitates this reabsorption process. All amino acids and glucose are reabsorbed in conjunction with the active reabsorption of sodium.
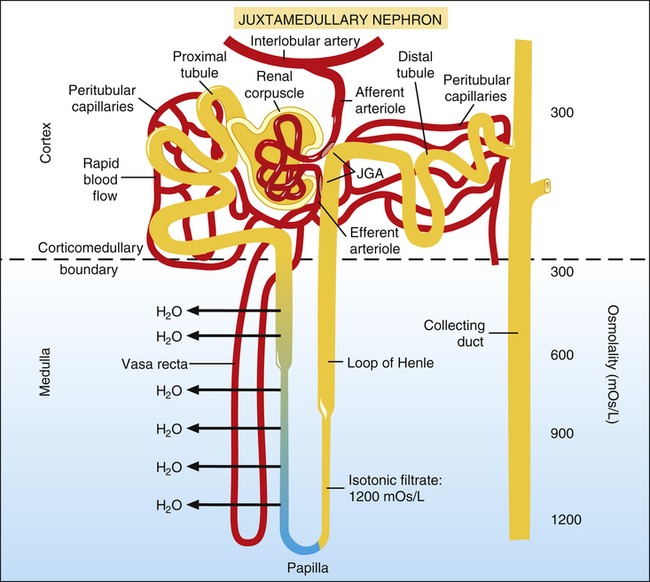
Descending Loop of Henle
The remaining 35% of the filtrate volume enters the descending limb of Henle’s loop (Figure 21-7), where it is isotonic with the peritubular interstitial fluid. The descending limb is freely permeable to water and moderately permeable to most ions.1 Thus, as the filtrate moves to the bottom of Henle’s loop, its osmotic pressure equalizes with that of the surrounding medullary interstitial fluid.
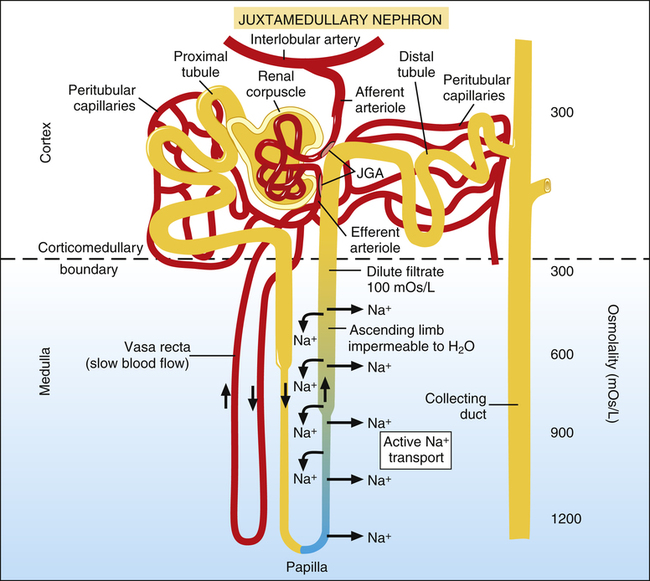
Ascending Loop of Henle
After it flows around the papillary tip of Henle’s loop, the filtrate moves up the ascending limb, flowing in a direction opposite to that of the filtrate in the descending limb (a process called countercurrent flow) (Figure 21-8). This portion of Henle’s loop is highly impermeable to water, especially in its thick portion.
Cells of the ascending limb actively pump sodium ions out of the filtrate into the interstitial fluid. Because this portion of Henle’s loop is impermeable to water, water cannot follow sodium ions, and the filtrate becomes mildly hypotonic with respect to the interstitial fluid outside of the tubule (see Figure 21-8).
1. The sodium ions pumped out of the ascending loop of Henle immediately diffuse into the descending limb of Henle’s loop and the vasa recta. The oppositely directed flow in these structures carries the sodium down to the papilla (see Figure 21-8).
2. Blood flow in the vasa recta is quite sluggish. As a result, it cannot effectively remove solutes from the deep medullary region.
3. New quantities of sodium continually flow from the proximal tubule into the loop of Henle. This influx of sodium, combined with the continual reabsorption of sodium from the ascending limb, is known as the countercurrent multiplier.1 The resulting high osmotic pressure in the medulla is necessary for excreting a concentrated urine, as explained in the next section.
Distal Tubules and Collecting Ducts
The filtrate leaving Henle’s loop enters the distal tubules slightly dilute, as just described (Figure 21-9). If the body’s vascular volume is too low, decreased renal blood flow causes the macula densa cells of the juxtaglomerular apparatus to secrete renin. Renin secretion ultimately results in angiotensin II formation, which causes the cortex of the adrenal glands to secrete the hormone aldosterone.1 Aldosterone causes sodium to be avidly reabsorbed from the distal tubular filtrate. When positively charged sodium ions leave the filtrate, negatively charged chloride ions passively follow. These events combine to create an osmotic pressure gradient that reabsorbs water from the filtrate back into the blood. In this way, the kidney restores intravascular volume toward normal.
Other hormones, atrial natriuretic hormone (ANH) and B-type natriuretic peptide (BNP), also influence water reabsorption (see Chapter 20, Clinical Focus 20-7). Specialized muscle fibers in the atria and ventricles of the heart secrete ANH and BNP in response to overstretch of their fibers. As their names imply, these hormones promote natriuresis, or loss of Na+ in the urine. In other words, ANH and BNP inhibit the effects of aldosterone and thus tend to prevent water reabsorption and increase urine volume.1,3
In addition to the renin-angiotensin-aldosterone mechanism just described, the antidiuretic hormone (ADH) feedback system also affects the distal tubule and collecting duct. For example, dehydration increases the blood’s osmolality, which stimulates osmoreceptors in the pituitary gland. As a result, the posterior pituitary gland secretes ADH into the general circulation.1 ADH increases distal tubule and collecting duct permeability, allowing more water to be reabsorbed into the blood from the filtrate. Figure 21-9 shows that as the filtrate travels deep into the medulla through the collecting duct (made permeable by ADH), water rapidly moves out of the collecting duct into the medullary interstitial fluid in response to the high osmotic pressure gradient created by the countercurrent mechanism (described earlier). Thus, under the influence of ADH, the kidney excretes a low-volume, highly concentrated urine; this conserves water and restores the body’s extracellular fluid volume. In the absence of ADH, water remains trapped in the collecting duct, and the kidney excretes a large volume of
dilute urine. It should now be apparent why the mechanisms that maintain the high corticomedullary osmotic gradient are essential for excreting concentrated urine.
Urine Output and Systemic Blood Pressure
Conversely, increased blood pressure slightly raises GFR (despite autoregulatory mechanisms) and presents the tubules with more solutes than can be reabsorbed. These solutes are excreted in the urine, “pulling” water with them because of an osmotic gradient. As a result, urine output increases. Doubling the systemic arterial blood pressure increases urine output seven to eight times greater than normal.1