Filamentary Keratitis
Introduction
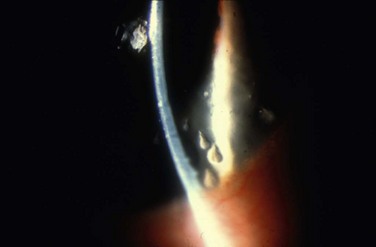
Filamentary keratitis is a condition in which mucoid filaments are attached to the anterior corneal surface and cause a foreign body sensation in the patient (Fig. 28.1). Filamentary keratitis is associated with dry eyes, superficial punctate keratopathy, ptosis, and tear film stasis. Patients affected with filamentary keratitis are often highly symptomatic; the filaments may cause a persistent foreign body sensation, photophobia, and redness, that can range from moderate to severe in intensity. Filamentary keratitis is associated with a wide range of conditions in which the ocular surface is abnormal. Treatment can be acute or chronic, and severe cases may require multiple therapeutic modalities.
Etiology
The filaments adherent to the corneal epithelium in filamentary keratitis are gelatinous and refractile in appearance and can vary from 0.5 mm sessile adhesions, to 10 mm strings;1 filaments are generally attached to the basement membrane of the corneal epithelium at one end, with the other end freely moveable. Movement of the lids causes the filament to elongate and coil, which is thought to produce patient discomfort. Filamentary keratitis is associated with underlying basement membrane abnormalities, most of which are related to hypertonic tear film states. A list of conditions that are associated with filamentary keratitis can be seen in Box 28.1.2–18
A relative deficiency in the aqueous component of the tear film is usually present in filamentary keratitis, which produces a relative increase in the mucinous component. The ratio of mucus to aqueous is usually increased because of a decrease in aqueous tear production, but can also occur from mucus production19 or abnormal mucus accumulation. Abnormalities of the ocular surface result in defects in the corneal epithelium that allow for anchoring of filaments. Ptosis may lead to a reduction in oxygenation of the corneal epithelium, as well as poor tear distribution underneath the lid, which can predispose to or exacerbate filament formation.2 Reactive ptosis from discomfort only serves to worsen the problem.
The disorder most frequently associated with filamentary keratitis is dry eye disease. The increase in the mucus-to-aqueous ratio in the tear film may be brought about by either reduced aqueous tear secretion or increased tear film stasis, both of which effectively increase tear film osmolarity. Mucus serves as a disposal system for exfoliated epithelial cells.20 For example, changes in the composition of the mucinous layer, in which mucus becomes more viscous, enhance the adherence of mucus to irregularities in the corneal epithelium. One example of this change occurs when the normally predominant sialomucin is replaced by more viscous sulfomucin in certain disease states.19 Adherence of mucus to the epithelium is more likely with the latter. The combination of epithelial damage, such as that seen in dry eyes or stasis conditions, combined with an increase in mucus viscosity, can increase the potential for filaments to form on the cornea. Aqueous tear insufficiency decreases lubrication and increases the viscosity of the mucin layer, thereby leading to increased sloughing of epithelial cells, epithelial defects, and viscous mucus – the basic constituents of the corneal filament.
Diagnosis
Objective
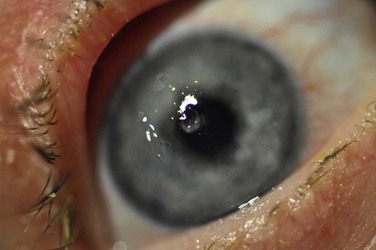
Filaments can vary in size from 0.5 mm to 10 mm in length. On biomicroscopy they appear as small, gray, mucoid threads that firmly adhere to the cornea (Fig. 28.2). Occasionally, blinking may dislodge large filaments. Small filaments can cause discomfort commenserate with larger filaments because of the underlying foreign body sensation, caused by the lids tugging on the filaments during blinking. Filaments stain with rose bengal, lissamine green and fluorescein; however, care should be taken during examination not to confuse frank epithelial defects, where a filament may have been dislodged, from sessile filaments that pool with fluorescein and may mimic small corneal abrasions.
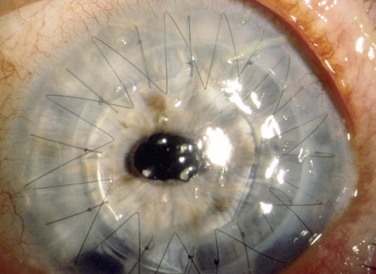
The location of the filaments may provide a clue as to the cause of the filamentary keratitis.1 Filaments associated with dry eye or exposure keratopathy are frequently found in the interpalpebral space. Filaments associated with ptosis, prolonged lid closure, or superior limbic keratoconjunctivitis are frequently found superiorly. Filaments related to ocular surgery are often found in the vicinity of the surgical trauma. For example, following corneal transplantation, filaments can be seen near sutures at the graft–host junction (Fig. 28.3). After cataract surgery, they may be overlying or alongside the corneal incision. Surgery-specific factors, such as the disruption of proper tear flow, pooling, toxicity from medications, and local trauma, may predispose to filament formation.
Histopathology
Traditional teaching has centered on the premise that the filament is composed of mucus and degenerated epithelial cells, and is adherent to the corneal epithelium at the base. However, debate still exists over the exact structure of the filament because observations of the filament on a microscopic level have been heterogeneous in approach and conclusion. Wright used standard histochemical stains to suggest that filaments form when mucus in the tear film attaches to receptors on the corneal surface.21 Subsequent proliferation of a filament occurs as mucus accumulates cellular and other degenerative material. In contrast, Zaidman et al. used electron microscopy to report that scattered groups of inflammatory cells and fibroblasts are present below the basement membrane of the corneal epithelium where filaments are attached.22 The authors hypothesized that an underlying pathological process damages the basement epithelium, which leads to focal areas of basement membrane detachment from the underlying Bowman’s layer. This raised epithelium subsequently becomes a nidus for mucus and degenerated cells.22 Tabery confirmed these findings with in vivo photographs, showing aggregations of mucus and cell debris adherent to the corneal surface, adjacent to focal excrescences of the basement membrane.23 Recently, based on observations made with light microscope and immunohistochemical analysis of 13 cases of filamentary keratitis, Tanioka et al. proposed an alternate theory. They observed that corneal epithelial cells form the core of the filament and an admixture of multiple surface mucins, DNA material, and degenerated conjunctival epithelial cells become wound around the core to produce a braided shape.24 They theorize that blinking then transmits stress to the base of the filament, which in turn incites an inflammatory response below the corneal epithelial basement membrane.
Treatment
Initially, improving the ocular surface by treating any underlying dry eye and blepharitis is undertaken. The underlying causes of filament formation, such as keratoconjunctivitis sicca, contact lens over wear, medication toxicity, or ptosis, should initially be identified and treated. For example, resolution of filamentary keratitis in a patient with blepharoptosis has been reported following blepharoptosis surgery.8 Because surgical correction of ptosis may lead to increased corneal exposure and subsequent exacerbation of filamentary keratitis, the exact etiology of the condition should be clearly identified prior to electing for surgical intervention.
Medical
Hamilton and Wood reported a 95% success rate using 5% sodium chloride ophthalmic solution administered four times daily.25 This regimen reportedly works directly at the level of the corneal epithelium by reducing edema of the epithelium and obviating the focal detachments. While our experience with this modality is not quite as dramatic, it certainly can be useful in some patients.
N-acetylcysteine has similarly been used topically for treatment.26 N-acetylcysteine is a mucolytic agent that decreases the viscosity of mucus in the precorneal tear film. The ocular solution is not commercially available and must be compounded without preservatives from the commercially available respiratory preparation (20% solution) to create a 5% to 20% solution. However, the discomfort of the drops and the expense and difficulty of obtaining N-acetylcysteine in a preservative-free status, minimizes the usefulness of this treatment.
A bandage contact lens is frequently successful in treating cases of filamentary keratitis that do not respond to ocular lubrication.27 The contact lens protects the corneal epithelium from the shearing forces of the lids and may help to eliminate or reduce reactive ptosis that often occurs and exacerbates the condition in these patients. High Dk soft contact lenses should be used and are well tolerated with frequent topical artificial tears and a prophylactic topical antibiotic. Patients should be closely monitored because of the inherent risks of contact lens wear in dry eye patients with a suboptimal ocular surface.
Treatment may also target inflammation. Marsh and Pflugfelder demonstrated in a retrospective case series 100% efficacy of topical methylprednisolone for resolution of filamentary keratitis, in the setting of Sjögrens’s syndrome and symptomatic keratoconjunctivitis sicca.28 In their study, satisfactory control of symptoms and filaments was achieved with only short bursts of therapy in most cases.28 Administration of topical nonsteroidal anti-inflammatory drugs (NSAIDs) has also been shown to reduce patient discomfort and accelerate resolution of severe cases.29,30 The toxicity of NSAIDs on the damaged epithelium in a dry eye patient should be carefully weighed along with their beneficial effect in evaluating the pros and cons of this therapy.
References
1. Davidson, RS, Mannis, MJ, Filamentary keratitis. Cornea, 3rd ed. Krachmer, JH, Mannis, MJ, Holland, EJ, eds. Cornea, Vol 1. Elsevier/Mosby: Philadelphia, 2011:1093–1096.
2. Baum, JL. The Castroviejo Lecture. Prolonged eyelid closure is a risk to the cornea. Cornea. 1997;16:602–611.
3. Dada, VK. Contact lens induced filamentary keratitis. Am J Optom Physiol Opt. 1975;52:545–546.
4. Davis, WG, Drewry, RD, Wood, TO. Filamentary keratitis and stromal neovascularization associated with brain-stem injury. Am J Ophthalmol. 1980;9 0:489–491.
5. Demetriades, AM, Seitzman, GD. Isolated unilateral congenital lacrimal gland agenesis presenting as filamentary keratopathy in a child. Cornea. 2009;28:87–88.
6. Dodds, HT, Laibson, PR. Filamentary keratitis following cataract extraction. Arch Ophthalmol. 1972;88:609–612.
7. Good, WV, Whitcher, JP. Filamentary keratitis caused by corneal occlusion in large-angle strabismus. Ophthalmic Surg. 1992;23:66.
8. Kakizaki, H, Zako, M, Mito, H, et al. Filamentary keratitis improved by blepharoptosis surgery: two cases. Acta Ophthalmol Scand. 2003;81:669–671.
9. Mannis, MJ, Zadnik, K, Miller, MR, et al. Preoperative risk factors for surface disease after penetrating keratoplasty. Cornea. 1997;16:7–11.
10. Miri, A, Said, DG, Dua, HS. Donor site complications in autolimbal and living-related allolimbal transplantation. Ophthalmology. 2011;118:1265–1271.
11. Pons, ME, Rosenberg, SE. Filamentary keratitis occurring after strabismus surgery. Journal of AAPOS. 2004;8:190–191.
12. Rotkis, WM, Chandler, JW, Forstot, SL. Filamentary keratitis following penetrating keratoplasty. Ophthalmology. 1982;89:946–949.
13. Seedor, JA, Lamberts, D, Bergmann, RB, et al. Filamentary keratitis associated with diphenhydramine hydrochloride (Benadryl). Am J Ophthalmol. 1986;101:376–377.
14. Wolper, J, Laibson, PR. Hereditary hemorrhagic telangiectasis (Rendu–Osler–Weber disease) with filamentary keratitis. Arch Ophthalmol. 1969;81:272–277.
15. Diller, R, Sant, S. A case report and review of filamentary keratitis. Optometry. 2005;76:30–36.
16. Holly, FJ. Biophysical aspects of epithelial adhesion to stroma. Invest Ophthalmol Vis Sci. 1978;17:552–557.
17. Kawakami, H, Sugioka, K, Yonesaka, K, et al. Human epidermal growth factor eyedrops for cetuximab-related filamentary keratitis. J Clin Oncol. 2011;29:e678–e679.
18. Cher, I. Blink-related microtrauma: when the ocular surface harms itself. Clin Exp Ophthalmol. 2003;31:183–190.
19. Wright, P, Mackie, IA. Mucus in the healthy and diseased eye. Trans Ophthalmol Soc UK. 1977;97:1–7.
20. Adams, AD. The morphology of human conjunctival mucus. Arch Ophthalmol. 1979;97:730–734.
21. Wright, P. Filamentary keratitis. Trans Ophthalmol Soc UK. 1975;95:260–266.
22. Zaidman, GW, Geeraets, R, Paylor, RR, et al. The histopathology of filamentary keratitis. Arch Ophthalmol. 1985;103:1178–1181.
23. Tabery, HM. Filamentary keratopathy: a non-contact photomicrographic in vivo study in the human cornea. Eur J Ophthalmol. 2003;13:599–605.
24. Tanioka, H, Yokoi, N, Komuro, A, et al. Investigation of the corneal filament in filamentary keratitis. Invest Ophthalmol Vis Sci. 2009;50:3696–3702.
25. Hamilton, W, Wood, TO. Filamentary keratitis. Am J Ophthalmol. 1982;93:466–469.
26. Absolon, MJ, Brown, CA. Acetylcysteine in kerato-conjunctivitis sicca. Br J Ophthalmol. 1968;52:310–316.
27. Bloomfield, SE, Gasset, AR, Forstot, SL, et al. Treatment of filamentary keratitis with the soft contact lens. Am J Ophthalmol. 1973;76:978–980.
28. Marsh, P, Pflugfelder, SC. Topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjögren syndrome. Ophthalmology. 1999;106:811–816.
29. Avisar, R, Robinson, A, Appel, I, et al. Diclofenac sodium, 0.1% (Voltaren Ophtha), versus sodium chloride, 5%, in the treatment of filamentary keratitis. Cornea. 2000;19:145–147.
30. Grinbaum, A, Yassur, I, Avni, I. The beneficial effect of diclofenac sodium in the treatment of filamentary keratitis. Arch Ophthalmol. 2001;119:926–927.