Field Water Disinfection
Risk and Etiology
Infectious agents in contaminated drinking water most commonly associated with the potential for causing illness in a wilderness setting include bacteria, viruses, and protozoa. The main reason for treating drinking water is to prevent gastrointestinal illness from fecal pollution with enteric pathogens. Appearance, odor, and taste do not reliably estimate water safety. Risk for waterborne illness depends on the number of organisms consumed, which is determined by the volume of water, concentration of organisms, and treatment system efficiency (Boxes 45-1 and 45-2).
Specific Etiologic Agents
1. The infectious dose of enteric viruses is only a few infectious units in the most susceptible people.
2. Hepatitis A virus, norovirus, and rotavirus are the main viruses of concern for potable water supplies.
3. Many other viruses are capable and suspected of waterborne transmission, and more than 100 different virus types are known to be excreted in human feces.
Protozoa
1. Six protozoa that cause enteric disease and may be passed via waterborne transmission are Giardia lamblia, Cryptosporidium parvum, Entamoeba histolytica, Cyclospora cayetanensis, Isospora belli, and the microsporidia. The first two are the most important for wilderness travelers.
2. Giardia cysts have been found as frequently in pristine water and protected sources as in unprotected waters.
3. Many of the species seemingly capable of passing Giardia cysts to humans, including dogs, cattle, ungulates (deer), and beavers, are present in wilderness areas.
4. Cryptosporidium is an emerging enteric pathogen that has overtaken Giardia as the most common waterborne protozoa. Many aspects of the epidemiology and transmission appear similar to those related to Giardia.
Definitions
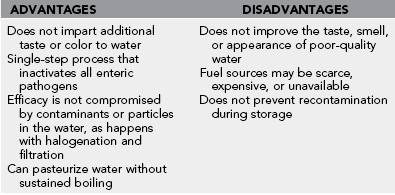
1. Disinfection, the desired result of field water treatment, means the removal or destruction of harmful microorganisms.
2. Pasteurization is similar to disinfection but specifically refers to the use of heat, usually at temperatures below 100° C (212° F), to kill most pathogenic organisms.
3. Disinfection and pasteurization should not be confused with sterilization, which is the destruction or removal of all life forms.
4. The goal of disinfection is to achieve potable water, indicating only that a water source, on average over a period of time, contains a “minimal microbial hazard” so that the statistical likelihood of illness is acceptable.
5. Water sterilization is not necessary because not all organisms are enteric human pathogens.
6. Purification is the removal of organic or inorganic chemicals and particulate matter to remove offensive color, taste, and odor. The term is frequently used interchangeably with “disinfection,” but purification may not remove or kill enough microorganisms to ensure microbiologic safety.
Heat
1. The boiling time required is important when fuel is limited.
2. Enteric pathogens, including cysts, bacteria, viruses, and parasites, can be killed at a temperature well below boiling (Table 45-1).
3. Thermal death is a function of both time and temperature; therefore lower temperatures are effective with longer contact times.
4. The boiling point decreases with the lower atmospheric pressure present at high elevations (Table 45-2).
Table 45-2
Boiling Temperatures at Various Altitudes
| ALTITUDE (ft) | ALTITUDE (m) | BOILING POINT |
| 5,000 | 1524 | 95° C (203° F) |
| 10,000 | 3048 | 90° C (194° F) |
| 14,000 | 4267 | 86° C (186.8° F) |
| 19,000 | 5791 | 81° C (177.8° F) |
5. The majority of the time required to raise the temperature of water to its boiling point works toward disinfection, so water is safe to drink by the time it has reached a full rolling boil. For an extra margin of safety (e.g., to kill hepatitis A virus), keep the water covered and hot for several minutes after boiling.
6. Pasteurization (at subboiling temperatures with extended contact times) has been successfully achieved using solar heating. A solar cooker constructed from a foil-lined cardboard box with a glass window in the lid can be used for disinfecting large amounts of water by pasteurization. This could be a low-cost method for improving water quality, especially in refugee camps and disaster areas.
7. When no other means are available, using hot tap water as drinking water may prevent traveler’s diarrhea in developing countries. As a rule of thumb, water too hot to touch is within the pasteurization range. However, lukewarm tap water can contain pathogenic microorganisms.
Filtration, Adsorption, and Clarification (Fig. 45-1)
1. Field filters that rely solely on the mechanical removal of microorganisms may be adequate for cysts and bacteria but may not reliably remove viruses, which are a major concern in water where high levels of fecal contamination are present (e.g., in developing countries).
2. They have the advantages of being simple and requiring no holding time.
3. Most viruses adhere to larger particles or clump together into larger aggregates that may be removed by a filter. However, filtration is not an adequate method to eliminate viruses because the infectious dose of an enteric virus may be quite small. Filters are often expensive and can add considerable weight and bulk to a backpack.
4. Some devices are designed as purely mechanical filters, whereas others combine filtration with granular activated carbon (GAC).
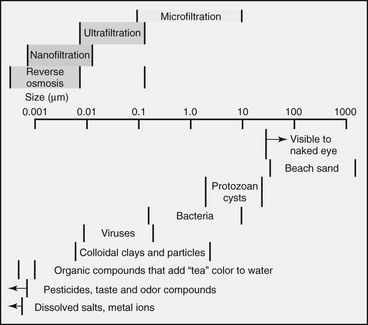
5. The size of a microorganism is the primary determinant of its susceptibility to filtration. Filters are rated by their ability to retain particles of a certain size.
6. All filters eventually clog from suspended particulate matter, present even in clear streams, requiring cleaning or replacement of the filter. The ability to easily service a unit in the field is an advantage. Flow can be partially restored to a clogged filter by back flushing or surface cleaning, which removes the larger particles trapped near the surface.
Microfiltration, Ultrafiltration, and Nanofiltration
1. In general, portable filters for water treatment can be divided into microfiltration with pores down to 0.1 µm, ultrafiltration that can remove particles as small as 0.01 µm, and nanofiltration with pore sizes as small as 0.001 µm or less.
2. Microfilters are effective for removing protozoa and bacteria, algae, most particles, and sediment but allow dissolved material, small colloids, and some viruses to pass through.
3. Ultrafiltration membranes are required for complete removal of viruses, colloids, and some dissolved solids.
4. Nanofilters can remove other dissolved substances, including sodium chloride, from water. All filters require pressure to drive the water through the filter element. The smaller the pore size, the more pressure required.
5. Filters are a reliable means to removing protozoan cysts.
Adsorption Using Granular Activated Carbon
1. Some, but not all, viral particles, bacteria, and protozoan cysts are removed by GAC filters.
2. GAC does not kill microorganisms.
3. No reliable means are available for determining precisely when GAC saturation is reached. Presence of unpleasant taste or color in the water can be the first sign that the charcoal is ineffective. To test the activity of the charcoal, one may filter iodinated water or water tinted with food coloring. With regular use, the lifetime of GAC is probably measured in months; it is substantially longer with infrequent use.
4. With increasing industrial and agricultural contamination of distant groundwater, final treatment of drinking water with GAC may be important for some wilderness users.
Reverse Osmosis
1. A reverse osmosis filter uses high pressure (100 to 800 psi) to force water through a semipermeable membrane that filters out dissolved ions, molecules, and solids.
2. Reverse osmosis is generally used for desalinating water.
3. It may also be used to remove biologic contaminants.
4. Small hand-pumped reverse osmosis units have been developed. High price and slow output currently limit their use by land-based wilderness travelers.
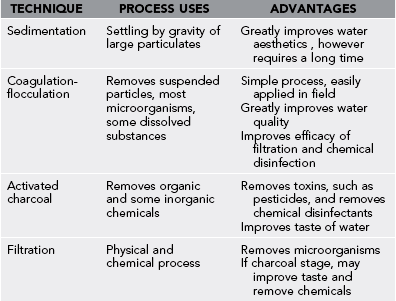
Clarification of cloudy water can be achieved by sedimentation, coagulation-flocculation (C-F), or adsorption (Table 45-3).
1. Large particles settle by gravity over 1 to 2 hours in sedimentation. Although filters remove particulate debris, thus improving the appearance and taste of “dirty” water, they clog quickly if the water contains large particles.
2. Smaller suspended particles can be removed by C-F. This is accomplished in the field by adding alum (aluminum potassium sulfate). Alum is used in the food industry as a pickling powder and is nontoxic. C-F will remove contaminants that cause an unpleasant color and taste, some dissolved metals, and some microorganisms.
Chemical Disinfection (Tables 45-4 and 45-5)
Halogens (Chlorine and Iodine)
Table 45-4
Water Disinfection Techniques and Halogen Doses
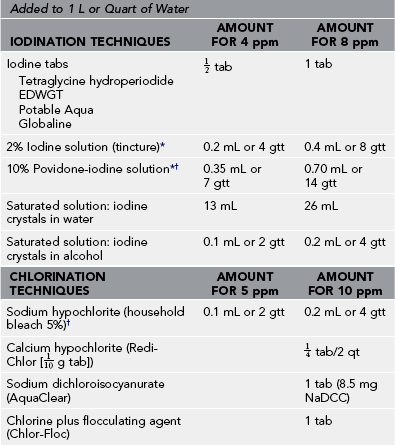
EDWGT, Emergency drinking water germicidal tablet; gtt, drops; ppm, parts per million.
*Measure with dropper (1 drop = 0.05 mL) or tuberculin syringe.
†Povidone-iodine solutions release free iodine in levels adequate for disinfection, but scant data are available.
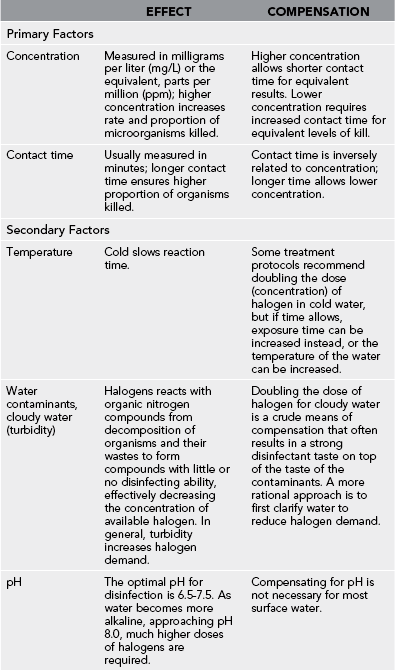
Factors Affecting Halogen Disinfection (Table 45-6)
Contaminants
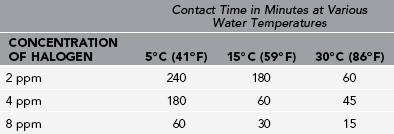
1. In cloudy water that will not settle out by sedimentation, the halogen dose should be at least 8 ppm. Ideally, use C-F to clarify the water before halogenation, and then use a smaller amount of halogen.
2. Several simple color strip tests are available for field use, similar to those used for swimming pools and spas to measure the amount of free (residual) halogen in water. Testing in the wilderness for halogen residual may be reasonable for large groups but is not practical for most. Smell of chlorine usually indicates some free residual. Color and taste of iodine can be used as indicators. Above 0.6 ppm, a yellow to brown tint is noted.
Pathogen Sensitivity
1. Bacteria are extremely sensitive to halogens.
2. Viruses and Giardia require higher concentrations or longer contact times.
3. Cryptosporidium cysts are extremely resistant to halogens.
4. The resistance of Cryptosporidium will require an alternative to halogens or a combination of methods to ensure removal and inactivation of all pathogens.
5. Relative resistance between organisms is similar for iodine and chlorine.
6. The physical state of the microbes also determines their susceptibility. Microbes that are aggregated in clumps or embedded in other matter or organisms may be shielded from disinfectants.
Iodine
Recommendations
1. Available data suggest the following:
a. High levels of iodine, such as those produced by recommended doses of iodine tablets, should be limited to periods of 1 month or less.
b. Iodine treatment that produces a low residual (1 mg/L or less) appears safe, even for long periods in people with normal thyroid function. This would require very low doses of iodine added to the water or an activated charcoal stage to remove residual iodine.
2. Persons planning to use iodine for a prolonged period should have the thyroid gland examined and thyroid function measured to ensure that a state of euthyroidism exists.
3. The following groups should not use iodine for water treatment because of their increased susceptibility to thyroid problems:
Improving the Taste of Water Disinfected With Halogens
1. Add flavoring to the water only after adequate contact time. Iodine will react with sugar additives, thereby reducing the free iodine available for disinfection.
2. Use charcoal (GAC) to remove halogen after adequate contact time.
3. Reduce the concentration and increase the contact time in clean water. For a small group of people, use a collapsible plastic container to disinfect water with low doses of iodine during the day or overnight.
4. Iodine and chlorine taste and iodine color can be removed by chemical reduction. In addition, a much higher halogen dose (shorter contact time) can be used if followed by chemical reduction. To remove iodine and chlorine taste and iodine color by chemical reduction:
Superchlorination-Dechlorination
1. High doses of chlorine are added to the water in the form of calcium hypochlorite crystals to achieve concentrations of 30 to 200 ppm of free chlorine.
2. These extremely high levels are above the margin of safety for field conditions and rapidly kill all bacteria, viruses, and protozoa and could kill Cryptosporidium with overnight contact times.
3. After at least 10 to 15 minutes, several drops of 30% hydrogen peroxide solution are added. This reduces hypochlorite to chloride, forming calcium chloride and oxygen.
4. The minor disadvantage of a two-step process is offset by excellent taste.
5. This is a good technique for highly polluted or cloudy water and for disinfecting large quantities. It is the best technique for storing water on boats or for emergency use. Water is then dechlorinated in needed quantities when ready to use.
6. The ingredients can be easily obtained and packaged in small Nalgene bottles.
Miscellaneous Disinfectants
Table 45-7
Relative susceptibility of microorganisms to chlorine dioxide: bacteria > viruses > protozoa.
1. Chlorine dioxide is capable of inactivating most waterborne pathogens, including Cryptosporidium parvum oocysts, at practical doses and contact times.
2. It is at least as effective a bactericide as chlorine and in many cases is superior.
3. It is far superior as a virucide.
4. It does not form chlorinated compounds in the presence of organics and is efficacious over a wide pH range.
5. Cost-effective and portable chlorine dioxide treatment products include Micropur MP1, Aquamira, and Miox.
Mixed Species Disinfection (Miox Purifier)
1. Passing a current through a simple brine salt solution generates free available chlorine, as well as other “mixed species” disinfectants that have been demonstrated effective against bacteria, viruses, and bacterial spores.
2. The resulting solution has greater disinfectant ability than a simple solution of sodium hypochlorite.
3. It has even been demonstrated to inactivate Cryptosporidium.
Silver
1. The use of silver as a drinking water disinfectant has been much more popular in Europe, where silver tablets (Micropur) are sold widely for field water disinfection.
2. Silver ion has not been approved by the Environmental Protection Agency (EPA) for this purpose in the United States, but was approved as a water preservative to prevent bacterial growth in previously treated and stored water.
3. Micropur Forte tablets release free chlorine for disinfection and silver for prolonged persistence of antimicrobial activity.
4. Silver impregnation of filters may inactivate pathogens that pass through the filter pores or limit bacterial growth in the filter itself (bacteriostatic). Ceramic filters coated with silver have higher removal rates of bacteria than non-coated filters.
Potassium Permanganate
Potassium permanganate is a strong oxidizing agent with some disinfectant properties.
1. It is used in municipal disinfection to control taste and odor.
2. It has been used in a 1% to 5% solution as a drinking water disinfectant and is still used for this purpose in some countries, as well as for washing fruits and vegetables.
3. Although potassium permanganate clearly has disinfectant action and is frequently used in some parts of the world, it cannot be recommended for point-of-use water disinfection unless no other means are available, because quantitative data are not available for viruses and no data are available for protozoan cysts.
4. Packets of 1 g to be added to 1 L of water are sold in some countries.
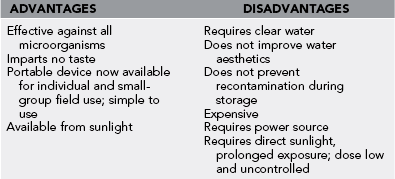
Ultraviolet Light and Sunlight
1. Using sufficient doses, all waterborne enteric pathogens are inactivated by ultraviolet (UV) radiation (Table 45-8).
2. Bacteria and protozoan parasites require lower doses than do enteric viruses and bacterial spores.
3. Giardia and Cryptosporidium are susceptible to practical doses of UV and may be more sensitive because of their relatively large size.
4. UV treatment does not require chemicals and does not affect the taste of the water.
5. UV works rapidly, and an overdose to the water presents no danger.
6. UV light has no residual disinfection power; water may become recontaminated, or regrowth of bacteria may occur.
7. Particulate matter can shield microorganisms from UV radiation.
8. Portable field units, such as SteriPEN and AquaStar UV Portable Water Purifier, require a power source (battery, human powered, and solar-charged units are available). Users must prefilter or clarify cloudy water.
9. A new technology is the SolarBag.
a. The SolarBag disinfects 3 L at a time and can be used several times per day, on sunny or cloudy days.
b. It uses sunlight to activate a mesh insert coated with titanium dioxide.
c. For disinfection the bag is placed flat or hanging in direct sunlight.
d. Disinfection requires 1 to 2 hours on a sunny day and 2 to 4 hours on a cloudy day.
e. For unknown water sources, a food-safe dye can be used as a tracer and timer. When the color has cleared, the water has been disinfected.
f. No chemicals or pumps are required, and it can be reused.
10. A unique, low-tech approach uses a simple solar disinfection (“SODIS”) technique (see http://www.sodis.ch/).
a. Transparent bottles (e.g., clear plastic beverage bottles), preferably lying on a dark surface, are exposed to sunlight for a minimum of 4 hours.
b. Oxygenation induces greater reductions of bacteria, so agitation is recommended before solar treatment in bottles.
c. Where strong sunshine is available, solar disinfection of drinking water is an effective, low-cost method for improving water quality and may be of particular use in refugee camps and disaster areas.
d. With a water temperature of 30° C (86° F), 6 hours of midlatitude midday summer sunshine are required to achieve a 3-log reduction of fecal coliforms.
Choosing the Preferred Technique (Table 45-9)
Table 45-9
Summary of Field Water Disinfection Techniques

*Most filters make no claims for viruses. Reverse osmosis is effective. The General Ecology filtration system claims virus removal.
†Eggs are not very susceptible to halogens but have very low risk of waterborne transmission.
1. The best technique for disinfection for either an individual or a group depends on the number of persons, space and weight available, quality of source water, personal taste preferences, and availability of fuel.
2. Optimal protection for all situations may require a two-step process of filtration or C-F and halogenation because halogens do not kill Cryptosporidium and filtration misses some viruses.
3. Heat works as a one-step process, but it will not improve the taste and appearance of water.
Alpine Camping
1. For alpine camping where a high-quality water source is available, heat, mechanical filtration, chlorine dioxide, or a low-dose halogen can be used.
2. The only limitation for halogens is Cryptosporidium cysts, but in high-quality pristine surface water, the cysts are generally found in insufficient numbers to pose significant risk.
3. Heat is limited by fuel supply.
4. Filtration has the advantage of imparting no taste and requiring no contact time.
Agricultural Runoff and Discharge From Upstream Towns
1. Treat water with agricultural runoff or sewage plant discharge from an upstream town or city with heat or a two-step process of filtration to remove Cryptosporidium, then with chlorine dioxide or a halogen to ensure destruction of all viruses.
2. A filter containing a charcoal element has the added advantage of removing many chemicals such as pesticides.
Surface Water in Undeveloped Countries
Systems Where Water Will Be Stored
1. Halogens have a distinct advantage in locations where the water will be stored for days or weeks, such as on a boat or in a home without running water.
2. Iodine works for short-term, but not prolonged, storage because it is a poor algicide.
3. Note that when only heat or filtration is used before storage, the water can become recontaminated and bacterial regrowth can occur. Superchlorination-dechlorination is particularly useful in this situation because a high level of chlorination can be maintained for a long period.
a. When ready to use the water, pour it into a smaller container and dechlorinate it.
b. If another means of chlorination is used, maintain a minimum residual of 3 to 5 mg/L in the water.
4. Silver has been approved by the EPA for preservation of stored water.
5. Chlorine dioxide is too unstable for long-term storage needs.
6. On oceangoing vessels where water must be desalinated during the voyage, only reverse-osmosis membrane filters are adequate. Halogens should then be added to the water in the storage tanks.