Chapter 166 Fibromyalgia Syndrome
 Diagnostic Summary
Diagnostic Summary
 Diagnosis
Diagnosis
The 13 most common symptoms associated with the pain and tenderness of fibromyalgia syndrome (FMS) 2–4 are listed in Box 166-1.
Differential Diagnosis
The purpose of the 1990 American College of Rheumatology (ACR) criteria for FMS was to distinguish patients with a putative primary disorder designated FMS from those with similar symptoms due to other distinguishable medical disorders. The two major criteria for FMS are chronic (longer than 3 months) widespread pain and tenderness.1 The criteria were established mainly for use in research that would eventually identify the underlying pathologic mechanism of the symptoms, but they have come to serve as diagnostic criteria in clinical practice.
Some theorists have argued that widespread pain and tenderness at predictable anatomic sites are features of many medical disorders.5 Their argument is true only of hypothyroidism and cellular resistance to thyroid hormone, especially when the associated symptoms of FMS are also taken into account. When most patients with hypothyroidism or cellular resistance to thyroid hormone meet the ACR criteria for FMS and are effectively treated with thyroid hormone therapy, they no longer meet the FMS criteria.6–15 This finding indicates that these patients’ FMS symptoms and signs are a distinct clinical phenotype of inadequate thyroid hormone tissue regulation.16 It also justifies a trial of thyroid hormone therapy to distinguish whether a patient’s FMS symptoms and signs are features of hypothyroidism or cellular resistance to thyroid hormone.
The theorists’ argument does not apply to other medical disorders, however, and is refuted by two lines of evidence.17 First, studies have not established that chronic widespread pain and multiple TnPs are features of other medical disorders. Second, many patients with rheumatoid arthritis, osteoarthritis, Lyme disease, systemic lupus erythematosus, Chiari malformation, and spinal stenosis also meet the ACR criteria for FMS, but when these other disorders are effectively treated, the patients still meet the criteria for FMS.18–20
The argument is false, then, that chronic widespread pain and tenderness are features of many medical disorders. Nonetheless, the argument highlights the need to differentiate FMS from other disorders with similar symptoms that may lead the clinician to misdiagnose FMS. The main disorders the clinician should distinguish from FMS are arthritis, myopathy, polymyalgia rheumatica, diabetic polyneuropathy, ankylosing spondylitis, discopathy, cardiac or pleural pain,21 multiple muscle myofascial pain syndromes,22 and lupus erythematosus. A given patient may, of course, concurrently meet the criteria for FMS and one or more other disorders with overlapping symptoms. FMS can usually be distinguished from medical disorders other than hypothyroidism and cellular resistance to thyroid hormone by careful pathognomy. However, diagnostic scrutiny will show that the symptoms and signs of most FMS patients are indistinguishable from those of the subclass of hypothyroid and thyroid hormone–resistant patients for whom pain is a predominant symptom.
 General Considerations
General Considerations
Current Focus of the Conventional Fibromyalgia Research Community
The serotonin deficiency hypothesis is the oldest proposed mechanism of FMS.23–25 As a research-inspiring concept, it constituted the core theoretical underpinning of the rheumatology paradigm of FMS. This hypothesis proposed that a central nervous system (CNS) serotonin deficiency reduced the efficiency of the brainstem–spinal cord descending antinociceptive system, lowering the threshold for pain perception.26 By 2000, the serotonin deficiency hypothesis had been effectively refuted16 by three lines of evidence: First, the only site of low serotonin among FMS patients was their platelets.26–28 Second, serotonin-increasing drugs were no more effective than placebos29,30–34 and exacerbated some patients’ FMS status.35 And third, the low brain blood flow of FMS patients36,37 contradicts a serotonin deficiency; serotonin is a potent vasoconstrictor,38 and a low CNS level would produce cerebral vasodilation and increased blood flow.
This was further documented in the 2005 publication of the state of that paradigm.39 The central theoretical viewpoint of the former paradigm had shifted from a serotonin deficiency to a generalized problem of augmented pain processing resulting in a hyperalgesic state,39,40 which is plausibly explained by the extraordinarily high levels of substance P in patients with FMS.41 But researchers espousing the former paradigm have failed to address the most plausible mechanism of the patients’ high levels of substance P (see following section). Instead, their main efforts have been to develop pharmaceuticals to manage FMS pain. This has led to the approval of several drugs by the U.S. Food and Drug Administration for FMS and the recommendation that patients undergo education about FMS as well as psychotherapy and aerobic, strength, and flexibility training.40
Hypometabolism Hypothesis of Fibromyalgia Syndrome
The hypometabolism hypothesis posits that FMS is chronic hyperalgesia and other symptoms and signs of hypometabolism due to hypothyroidism, partial cellular resistance to thyroid hormone, or other metabolism-impeding factors. Among the other factors said to be responsible are pernicious diet, nutritional deficiencies, low physical fitness, and metabolism-impeding drugs. The term hypometabolism refers to the global impact on the patient of the underlying factors, most of which are catabolic and inhibitory, although some are anabolic or excitatory.16
• Tenderness (lowering of the pain threshold to mechanical stimuli)
• Hyperalgesia (increased responsiveness to noxious stimuli)43,44
ITHR can impair the antinociceptive system by two mechanisms. First, ITHR can severely increase production of substance P, which is extremely high in the cerebrospinal fluid (CSF) of tested FMS patients.41 Substance P is released from the terminals of nociceptive neurons42 and assists the summation of slow nociceptive signals.45 This facilitation amplifies the transmission of nociceptive signals in the spinal cord.46 Thyroid hormone normally inhibits the synthesis and secretion of substance P in many CNS cells. It does so by repressing the transcription of the preprotachykinin-A gene. Preprotachykinin-A is the precursor of substance P and its cognate substance P receptor.47,48 Lowering thyroid hormone levels by thyroidectomy increased the substance P level in astrocytes48; anterior pituitary49–51; many brain nuclei52; and, most relevant to pain, the dorsal horns of the lumbar spinal cord. The increase in dorsal horn substance P was highly elevated (100%),53,54 as in FMS patients.41 Thyroid hormone treatment lowered the substance P level in the anterior pituitary,50 brain nuclei,55 and dorsal horns.54 Excess thyroid hormone reduced substance P to subnormal levels.51
The second mechanism by which ITHR can reduce the effectiveness of the antinociceptive system is by reducing the synthesis and secretion of norepinephrine (NE) in cells of the brainstem locus ceruleus. Adequate NE is essential to normal function of the descending antinociceptive system.56,57
The antinociceptive pathways that descend from the brainstem to the dorsal horns contain two types of neurons: those that secrete serotonin and others that secrete NE.20,56,58,59 Serotonin secretion by the neurons is tonically augmented by NE secretion. Normal serotonin secretion is therefore dependent on NE secretion.54 The serotonin stimulates interneurons to secrete opiates.56,58,60 These then inhibit transmission partly by blocking release of neurotransmitter substances such as glutamate and substance P from the afferent neurons.61 They also block calcium influx and potassium efflux from the afferent terminals,62 mainly those of types C and A delta fibers.63 The decreased potassium efflux hyperpolarizes terminals, inhibiting the transmission of nociceptive signals to spinothalamic neurons that otherwise would transmit the signals to the brain.64 Low NE secretion by descending neurons, however, may reduce the secretion of serotonin selectively at dorsal horn interneurons and secondarily reduce opiate secretion. As a result, the transmission of nociceptive signals in the CNS will increase, thus heightening pain perception.
That decreased NE production is involved in the heightened pain perception of FMS patients is indicated by low metabolites of both dopamine and NE in patients’ CSF.27 That inadequate T3 regulation of locus ceruleus neurons accounts for the low NE production is suggested by the crucial role T3 plays in the synthesis of both dopamine and NE. The locus ceruleus is the brain site with the heaviest concentration of T3.65–67 Thyroid hormone regulates the activity levels of two rate-limiting enzymes in dopamine and NE synthesis.68 One enzyme, tyrosine hydroxylase, catalyzes the conversion of tyrosine to levodopa, which in turn is converted to dopamine.69 Tyrosine hydroxylase activity in the noradrenergic neurons of the locus ceruleus is low in hypothyroidism.70 Low activity of the enzyme and reduced conversion of levodopa to dopamine may be responsible for low dopamine levels in the striatum, hypothalamus, and superior cervical ganglia in hypothyroidism.68 Thyroid hormone therapy increases the activity of tyrosine hydroxylase.70 The second enzyme, dopamine-β-hydroxylase, catalyzes the conversion of dopamine to NE. Low activity of the enzyme in hypothyroidism can reduce NE levels.68 Unfortunately, NE levels in the antinociceptive system and other tissues in thyroid disorders have not been studied extensively71 enough to support this putative mechanism.
By raising substance P levels and possibly lowering NE levels in the spinal cord, ITHR can thus plausibly heighten FMS patients’ pain perception. Patients’ pain, however, is probably compounded by other factors. First, most FMS patients are physically inactive because of their pain,72,73 although low motor drive from low dopamine levels27 probably contributes to their inactivity. Their low physical activity level may further contribute to the inefficiency of the antinociceptive system.74–76
ITHR can also plausibly account for the other symptoms and objectively verified abnormalities of FMS: muscle and joint pain, paresthesias, cognitive dysfunction, depression, cold intolerance, exercise intolerance, weakness and fatigue, dry skin and mucous membranes, constipation, dysmenorrhea, and menorrhagia,77,78 increased platelet α2-adrenergic receptor density,79,80 reduced brain blood flow,81 reduced peripheral blood flow,82 sleep disturbance,83 deficient slow-wave sleep,84 hypotension,85 blunted sympathetic response to stress,86 stiffness and swelling,87 irritable bowel syndrome,88 excessive urination,83 high serum hyaluronic acid,89 low procollagen III,90 high ground substance proteoglycans,91 low pyridinoline92 and hydroxyproline,93 glycolysis abnormalities,94 low concentrations of high-energy phosphates in erythrocytes and muscle cells,95,96 and low growth hormone and somatomedin C levels.97 The cellular and genomic actions of thyroid hormone can explain the hormone’s relationship to all of these factors.16 Especially important is thyroid hormone’s effect on the adrenergic system. If the hypometabolism hypothesis is verified at some point in the future, the definition of fibromyalgia will include an explanation of FMS as a condition of α-adrenergic dominance.
Experimental Support for the Hypometabolism Hypothesis of Fibromyalgia Syndrome
The hypothesis that ITHR is an underlying mechanism of FMS has considerable experimental support.98 Many researchers have noted the virtually identical features of FMS, hypothyroidism, and the peripheral form of cellular resistance to thyroid hormone.16,99–114
Several research groups have used thyroid function testing to determine the incidence of thyroid disease among FMS patients. Each group has reported an incidence higher than in the general population.99–109 A thorough analysis of all the available evidence indicates that approximately 90% of FMS patients have some form of thyroid disease, either primary or central hypothyroidism or cellular resistance to thyroid hormone.115,116
In two studies, 15 female FMS patients had low resting metabolic rates (RMRs) and basal axillary temperatures compared with 15 matched healthy controls. In both studies, the controls’ mean RMR was within the reference range predicted by equations. However, in the first study, the mean RMR of FMS patients was 29.2% below normal based on their gender, age, height, and weight.117 In the second study, FMS patients’ mean RMR was 32.5% below normal.118 In both studies, FMS patients’ basal temperatures were significantly lower than those of controls. In the first study,117 FMS patients’ mean basal temperature was 96.95° F (36.08° C). In the second study,118 the mean temperature of FMS patients was 96.38° F (35.77° C).
The only clinical trials in which patients have been fully relieved of FMS have involved orally administered thyroid hormone. It should be noted, however, that in each study patients also used other metabolism-regulating therapies (see later discussion on metabolism-regulating therapies other than thyroid hormone). Euthyroid and hypothyroid FMS patients fully recovered in five open but highly systematic trials,6–10 three double-blind placebo-controlled crossover trials,11–13 and a randomized double-blind placebo-controlled trial.14 In another randomized double-blind trial, FMS patients had limited improvement with the use of transdermal T3.119 A 1- to 5-year follow-up study compared the status of control FMS patients with FMS patients who had either hypothyroidism or cellular resistance to thyroid hormone and underwent metabolic therapy, including the use of thyroid hormone. Although control patients’ FMS status deteriorated, treated patients recovered and maintained their recovery throughout the follow-up period. Results of this study were the first to demonstrate the long-term effectiveness of an FMS treatment.15
Metabolism-Regulating Therapies Other Than Thyroid Hormone
Thyroid hormone therapy is necessary in most cases of FMS to ensure recovery, but it is not sufficient. For example, in a study of 77 euthyroid FMS patients,6 those who declined to adopt a wholesome diet, take nutritional supplements, and exercise to tolerance were among the 25% who failed to benefit from T3 therapy. This result is consistent with years of clinical experience supporting the necessity for patients to use other metabolism-regulating therapies in addition to thyroid hormone. A common experience, for example, is a recovered patient stopping her use of B complex vitamins only to suffer a partial recurrence of FMS symptoms. The symptoms are again relieved when she resumes her use of the vitamins.
Wholesome Diet
Various diets and dietary supplements have been tested as FMS treatments. Mild-to-moderate improvements in FMS status have been reported for vegetarian diets120,121; elimination of the excitotoxins monosodium glutamate and aspartame122; Chlorella pyrenoidosa (a unicellular freshwater green alga rich in proteins, vitamins, and minerals)123,124; an uncooked vegan diet consisting of berries, fruits, vegetables and roots, nuts, and germinated seeds and sprouts125; and a strict, low-salt, uncooked vegan diet rich in lactobacteria.126
Nutritional Therapies
Studies indicate that vitamins B1, B6, B12,56,127–135 C,136–140 and E, and beta-carotene141 have antinociceptive properties. Other reports indicate that various nutritional supplements improve FMS status: the Myers’ cocktail (intravenous formula of B vitamins, vitamin C, calcium, and magnesium)142; 5-hydroxytryptophan143,144; S-adenosylmethionine145–148; magnesium and malic acid149,150; combined aloe vera extracts, plant saccharides, freeze-dried fruits and vegetables, Dioscorea, and a vitamin/mineral complex151; collagen hydrolysat152; and a blend of ascorbigen and broccoli powder.153
Exercise to Tolerance
The results of studies of exercise in the treatment of FMS have been mixed: some have shown no improvement in FMS status35,69,154,155–157 or only minimal improvement.158–163 Cardiovascular exercise has provided the most improvement,160–163 especially low-intensity endurance training.163,164 Endurance exercise is important in reducing the physical limitations associated with FMS.165 The importance of physical fitness and the high metabolic efficiency it provides166,167 was shown by deficient slow-wave sleep, inducing FMS-like symptoms in sedentary and not aerobically fit students.23 This finding suggests that low metabolic efficiency due to ITHR renders some individuals susceptible to FMS.16
Vigorous exercise tends to exacerbate patients’ FMS symptoms.72,73 As a result, some patients avoid exercise altogether and consequently are not physically fit.165,168–170 The most plausible explanation for the exacerbations on vigorous exercise is the high-density of α2-adrenergic receptors on FMS patients’ platelets.171 Platelet density of α2-adrenergic receptors is a reliable indicator of the receptor density in the CNS.172 Binding of catecholamines to a high density of the receptors on cells of most tissues inhibits energy metabolism. The inhibition of energy metabolism during vigorous exercise, mediated by the high density of the receptors, appears to worsen symptoms severely. During the early phase of treatment, then, exercise should be mild enough to avoid or minimize catecholamine secretion.16 With effective thyroid hormone therapy, the density of α2-adrenergic receptors decreases and the density of β-adrenergic receptors increases, enabling cells to respond appropriately to high levels of catecholamines. The shift away from α2-adrenergic receptor dominance probably explains in part the FMS patient’s ability to engage in vigorous physical activity after receiving thyroid hormone therapy.6,7,8,11–13,15
Physical Treatment
Extensive clinical experience and clinical trials7,8,11–13 show that despite the use of integrated metabolic therapies, many patients require physical treatment to fully relieve their FMS pain.173–176 Several studies have shown that spinal manipulation, soft tissue manipulation, and trigger point therapy provide palliative improvement in some FMS symptoms, especially pain.177–180
The most common lesions that exacerbate FMS symptoms are myofascial trigger points and spinal joint fixations.16,174,181 However, any nociception-generating neuromusculoskeletal lesion may exacerbate FMS patients’ pain, probably owing to their high levels of substance P.40 Neuromusculoskeletal lesions can also disturb sleep.182–184 This can, in turn, increase FMS symptoms.23
 Therapeutic Considerations
Therapeutic Considerations
Laboratory Testing for Thyroid Status
Thyroid Function Tests
In untreated primary hypothyroidism, the TSH is elevated. The recently revised upper limit of the serum TSH reference range is 2.5 µIU/mL.185
Thyroid Antibodies
The most common cause of primary hypothyroidism is autoimmune thyroiditis. The presence of this disorder can be determined by the patient’s titer of thyroglobulin and thyroid peroxidase (microsomal) antibodies. It is especially important to order tests of antibody levels in FMS patients. Many patients who have elevated thyroid antibodies have also had thyroid function test results within the reference ranges for many years.187,188 But compared with people without chronic, widespread musculoskeletal complaints, those with such complaints were found to have a significantly higher incidence of thyroid microsomal antibodies (16% vs. 7.3%, P <0.01). The prevalence of antibodies was also significantly higher in women than men (20.4% vs. 11.6%, P <0.02). However, thyroid function test results did not differ significantly between those with and without musculoskeletal complaints.119 This study indicates that in patients with autoimmune thyroiditis, thyroid hormone levels too low to properly regulate the CNS antinociceptive system and properly inhibit the production of substance P can escape detection by thyroid function tests, including the TSH.
Thyroid Hormone Therapy Based on Initial Thyroid Status
FMS patients whose test results indicate hypothyroidism should begin therapy with a thyroid hormone preparation containing both T4 and T3. Many hypothyroid FMS patients do not benefit from T4 alone no matter how high the dosage.189 Most do benefit, however, from the use of T4/T3 preparations in a 4:1 ratio. The dosage range at which most patients improve or recover with T4/T3 preparations is that which was used throughout the twentieth century without harmful effects190 before TSH assays came into widespread use in the early 1970s: 2 to 4 grains (76 mg T4 and 18 mg T3 to 152 mg T4 and 36 mg T3).16,191
The thyroid hormone therapy used in studies in which hypothyroid and euthyroid FMS patients recovered6–14 was not conventional T4-replacement therapy. Although T4 was used in some studies,9,10,14,15 it was T4/T3 preparations and T3 alone used in a way that violated the mandates of T4 replacement. Specifically, patients were permitted to use thyroid hormone despite laboratory test results indicating that they were euthyroid, and doses were not titrated according to TSH levels but by patients’ clinical responses to particular doses.
FMS patients whose laboratory test results indicate euthyroidism should begin with T3. In studies of the thyroid status of FMS patients at intake, approximately 33% were euthyroid according to thyroid function testing.186,192 Of these patients, approximately 75% have partial peripheral cellular resistance to thyroid hormone according to four criteria115,116:
1. The patients are euthyroid (according to laboratory thyroid function test results) before beginning the use of T3.
2. The patients recover from their hypothyroid-like FMS symptoms and signs with supraphysiologic doses of T3.
3. The patients have high serum-free T3 levels after beginning T3 therapy.
4. The patients have no evidence of tissue thyrotoxicosis, according to the results of serial ECGs, serum and urine biochemical tests, and bone densitometry.
These patients usually benefit only from immediate-release T3 (not sustained-release T3) in a single daily dose.16 They are likely to respond only to supraphysiologic doses of T3. Effective T3 dosages for most patients are between 75 and 125 mg. For some patients, however, safe and effective doses are far higher.16
Some FMS patients have both hypothyroidism and cellular resistance to thyroid hormone.8 Most of these patients improve or recover only with T3 therapy, typically supraphysiologic doses.
The Basis of Dosage Adjustments
After a patient’s thyroid status has been determined and thyroid hormone therapy begun, dosage changes should be titrated according to symptom status and physiologic measures, not according to thyroid function test results. Thyroid function tests are of no value in finding the safest and most effective dosage for any particular patient.193 There is no scientific justification for inferring the metabolic status of cells (other than the thyrotrophs of the anterior pituitary) from thyroid function test results. The belief that tissue metabolic status can be accurately inferred from TSH or thyroid hormone levels is a postulate, not a research-derived conclusion. This postulate was found to be false, at least for rats, by studies showing that the levels of T3 and T4 in the cells of different tissues cannot be accurately predicted from plasma levels of TSH, T3, or T4.194,195 Moreover, some studies show that TSH levels correlate poorly with measures of the tissue effects of thyroid hormone, such as the speed of the relaxation phase of the Achilles reflex.196 Conversely, other studies show that the patient’s symptoms and signs are far more accurate and reliable indicators of tissue metabolic status than are the results of thyroid function tests.193,197
The RMR measured with indirect calorimetry is the most reliable measurement of oxidative metabolism, which is strongly regulated by thyroid hormone. In a study designed to find a correlation between patients’ RMRs and their TSH levels, a significant correlation was obtained only by log transforming the TSH values.198 The lack of a significant correlation without log transformation means that the TSH is not a useful tool for determining whether an individual patient’s thyroid hormone dosage is providing him or her with sufficient oxidative metabolism. Preferably, clinicians will include indirect calorimetry in their clinical practices. However, this instrument is not necessary for finding patients’ safe and effective dosages.
Objective Assessment of Patient Status
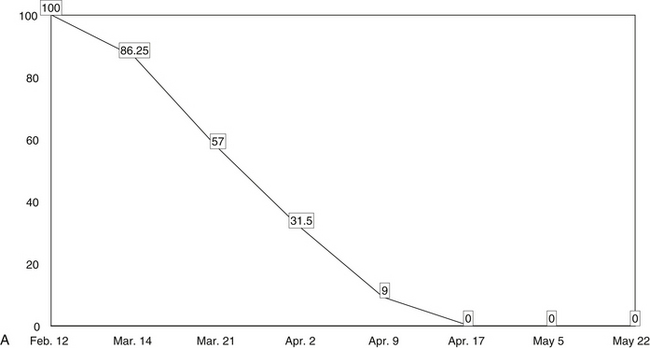
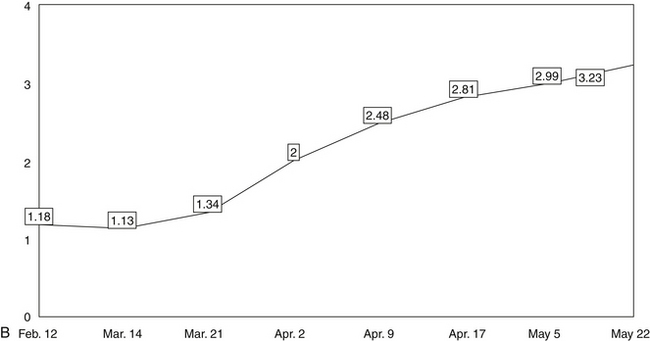
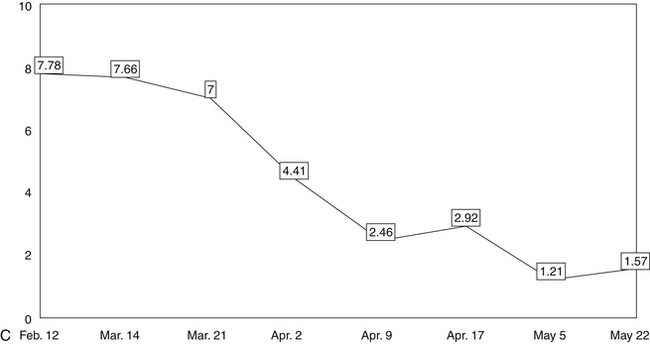
Next, a line should be drawn through the data points to create a trend line that makes obvious, on visual inspection, changes in the patient’s clinical status. The trend lines of the data points on the graphs thus help the clinician to assess the effectiveness of the patient’s regimen at any point during treatment. In this way, the lines enable the clinician to make informed, data-driven decisions about any treatment changes needed to optimize clinical results.
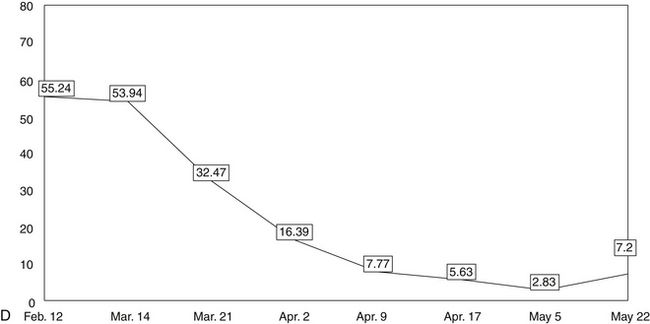
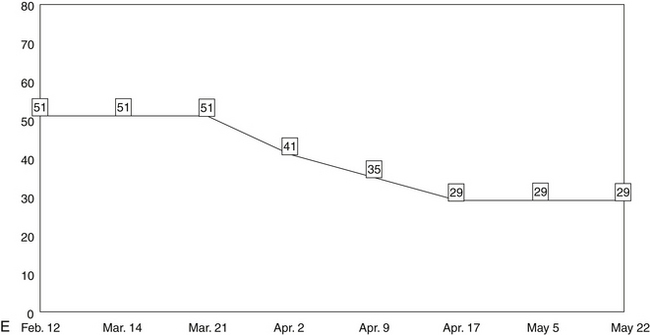
The five line graphs in Figure 166-2 show the changes in FMS measures during metabolic rehabilitation of a euthyroid FMS patient reported by Honeyman-Lowe.9 Typically, all measures change together in a direction of improvement, no improvement, or worse. The patient’s subjective status usually corresponds closely to what the trend lines indicate.
Pain Distribution Body Form
Four studies have shown that the most sensitive indicator for changes in FMS status is the pain distribution as a percentage of the body in pain.11–13,162,199 This is convenient in that chronic widespread pain is one of the two major criteria for the assessment of FMS status.1
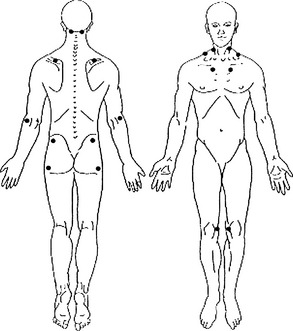
Small changes in pain distribution are assessed by using a form containing drawings of the body. The patient precisely shades in where he or she has had aching, pain, soreness, or tenderness since the last evaluation. Next, a template is placed over the patient’s shaded form, which shows the body form divided into 36 areas. Each of the 36 divisions has a percentage value.16,11–13 To obtain the patient’s pain distribution, the values of the shaded divisions are totaled. This percentage number is placed on a graph as a data point. The data points for each evaluation are connected by a line showing the trend of the pain distribution over time.
Mean Pressure/Pain Threshold of Tender Point Sites
The second major criterion for the assessment of FMS is the presence of tender point sites (TnPs).1 A modified version of the TnP examination measures the pressure/pain threshold of TnPs with algometry (numeric values in kilograms per square centimeter). This provides more precise quantification than the 1990 ACR method and enables the clinician to make more objective, evidence-based decisions.200 The mean of the threshold values is calculated and posted to a line graph.
FibroQuest Symptoms Survey
This assessment form consists of 100-mm visual analog scales (VASs), 1 for each of the 13 most common associated FMS symptoms.2 These symptoms are also characteristic symptoms of hypothyroidism and the peripheral form of cellular resistance to thyroid hormone (see Box 166-1).16 Readers can access and print this form free of charge from the author’s website, www.drlowe.com/forms.htm.
Fibromyalgia Impact Questionnaire
The Fibromyalgia Impact Questionnaire (FIQ)201 is a measure of the patient’s functional status. The total score from the FIQ is posted as a data point to a line graph. The assessment of functional ability is highly pertinent to the care of FMS patients. For example, in a recent study of the functional abilities of 1735 female FMS patients ages 31 to 78, researchers concluded that the average patient had less ability in activities of daily living than the average community-dwelling woman in her eighties.202
Zung’s Self-Rating Depression Scale
Depression is so common among FMS patients that an assessment specific for the presence and severity of depression is used in addition to the VAS scale for depression. Zung’s is one such assessment tool.203 The score from the depression scale is posted to a line graph.
Physical Examination
In the early twentieth century, indirect calorimeters came into use to measure patients’ RMRs.204 Based on the patient’s measured oxygen consumption, the instrument calculates the patient’s estimated 24-hour resting energy expenditure. Thyroid hormone potently and exquisitely regulates oxidative metabolism. Because of this, during the mid-twentieth century, indirect calorimeters were used as the most relevant instrument for the diagnosis of hypometabolism (subnormal oxidative metabolism) due to hypothyroidism and thyroid hormone resistance. In recent years, hand-held and other types of convenient-to-use, affordable, and accurate indirect calorimeters have become available for clinical use. Two studies have shown that FMS patients have abnormally low RMRs compared with matched controls.117,118
Integrated Metabolic Therapies Essential for All Fibromyalgia Syndrome Patients
The majority of FMS patients must control or eliminate at least four metabolism-impeding factors. First, for the approximately 90% of FMS patients who have some form of thyroid disease,115,116 the proper preparation and dosage of thyroid hormone are necessary for the patient’s recovery.
Second, most patients must also modify their diets so that they minimize the intake of arachidonic acid. A high intake of arachidonic acid can increase body levels of both the proinflammatory eicosanoids prostaglandin E2 and leukotriene B4,205 which can contribute to chronic pain.206 Patients must also reduce their intake of refined carbohydrate. ITHR of glycolysis, the citric acid cycle, and the electron transport chain results in low production of ATP and creatine phosphate. Dysglycemia and insulin resistance due to the intake of refined carbohydrates can worsen the low production of high-energy phosphates due to ITHR.16
Third, most patients must take a wide array of nutritional supplements. Various nutrients are synergistic to thyroid hormone in providing optimal intracellular metabolism, and patients must provide themselves with optimal amounts. For example, a deficiency of vitamin B1 can render patients intolerant of even low dosages of thyroid hormone—dosages too low to be therapeutic.207 In addition, supplemental thyroid hormone can increase the expenditure of vitamin B1 in cells. If a patient has a marginal deficiency of the vitamin, supplemental thyroid hormone could induce a frank deficiency. This can result in a beriberi-type of cardiomyopathy.207
Additional Metabolic Therapies Necessary for Some Patients
Adrenocortical Hypofunction
Some patients must be treated for adrenocortical hypofunction. Low cortisol levels can produce some symptoms that are the same as those associated with FMS. These include weakness, fatigue, exagerated responses to stress, exercise intolerance, hypotension, and low nociception threshold. Low cortisol levels can also render the patient intolerant of a dosage of thyroid hormone high enough to be fully therapeutic. The reason is that a minimally effective dosage of thyroid hormone is likely to accelerate the hepatic clearance of cortisol.208 This typically exacerbates the symptoms of cortisol deficiency symptoms.
Metabolism-Impairing Medications
Beta blockers impair metabolism directly by reducing the density of β-adrenergic receptors on cell membranes and rendering α2-adrenergic receptors predominant in the regulation of cell function, exactly as hypothyroidism does. In some patients, this shift in adrenoceptor isoform balance creates a general mental and physical inhibitory state that may be indistinguishable from hypothyroidism or the associated symptoms of FMS.209 The shift in adrenergic receptor isoform may also impair the function of the CNS’s descending antinociceptive system, resulting in the pain and tenderness characteristic of FMS.
 Therapeutic Approach
Therapeutic Approach
Assessment of Fibromyalgia Syndrome Status
The patient’s FMS status should be evaluated before treatment is begun and at intervals throughout the course of treatment. Reevaluations are best performed weekly to biweekly and before each increase in thyroid hormone dosage. If patients have taken measurements at home, such as the basal temperature, they should provide the clinicians with a record of these measurements. (An individualized self-monitoring form for patients is available at www.drlowe.com.forms.htm) A physical examination and five FMS measures should be used at each evaluation:
• Pain distribution as the percentage of 36 body divisions containing pain, according to a body drawing
• Mean TnP score in kg/cm2 measured by algometry
• Symptom intensity estimate according to visual analog scales (FibroQuest Symptoms Survey)
• Functional status according to the Fibromyalgia Impact Questionnaire
• Depression according to the Zung’s Self-Rating Depression Scale
Wholesome Diet
If they have not done so already, patients should adopt a diet that both stabilizes blood sugar regulation and insulin levels and reduces pain perception. Such a diet involves lean meats—especially poultry, fish, fruits, vegetables, and a minimal intake of whole grains. It also minimizes the intake of foods high in arachidonic acid (which increases the production of the proinflammatory eicosanoids prostaglandin E2 and leukotriene B4193) and additive excitotoxins such as monosodium glutamate and aspartame. The author recommends that patients use organic foods and purified drinking water to decrease the quantity of chemical contaminants ingested.
Nutritional Supplements
Patients should take at minimum the following nutrients in the approximate dosages given:
High potency multiple vitamin and mineral
Vitamin B12: 3000 to 5000 mcg a day of methylcobalamin
Vitamin C: 500 mg to 1 g three times a day
Vitamin E: 800 to 1600 IU a day
Carotenoids (mixed): 15 mg a day
Calcium: 2000 mg a day in divided dosages
Magnesium (preferably as aspartate, citrate, malate, or glycinate): 1000 mg a day in divided dosages
Exercise to Tolerance
Exercise is essential to help normalize metabolism. We recommend that patients engage in forms of exercise that they enjoy and gradually increase the intensity as their symptoms diminish. Weight-bearing exercise is encouraged as it supports bone health. Aerobic exercise is essential to strengthen the cardiovascular and pulmonary systems. Toning activities build lean muscle mass, which in turn improves metabolism. A combination of these forms of exercise is best. Many patients who have become severely deconditioned owing to their FMS symptoms find warm-water exercise an ideal way to begin.
Physical Treatment
Myofascial therapy to eliminate trigger points in muscles and spinal manipulation to regain flexibility of the spine and other joints are essential for completely eliminating some patients’ FMS pain. Both trigger points and spinal lesions can be perpetuated by self-sustaining skeletal muscle contractures. The contractures may result from deficient intramuscular high-energy phosphate production due to ITHR.20,22,195 Both forms of treatment may raise the nociceptive threshold and increase mobility.
If an examination indicates the need, orthotics should be prescribed to relieve pain-inducing chronic postural muscle tension. The therapeutic aim is to reduce nociceptive input of somatic origin to the CNS.
1. Wolfe F., Smythe H.A., Yunus M.B., et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis Rheum. 1990;33:160–172.
2. Wolfe F. Diagnosis of fibromyalgia. J Musculoskel Med. 1990;7:53–69.
3. Yunus M.B., Masi A.T. Fibromyalgia, restless legs syndrome, periodic limb movement disorder, and psychogenic pain. In: McCarty D.J., Koopman W.J. Arthritis and allied conditions: a textbook of rheumatology. Philadelphia: Lea & Febiger; 1993:1383–1405.
4. Nielson W.R., Grace G.M., Hopkins M., et al. Concentration and memory deficits in patients with fibromyalgia syndrome. J Musculoskel Pain. 1995;3:123.
5. Brady D.M., Schneider M.J. Commentary: fibromyalgia syndrome: a new paradigm for differential diagnosis and treatment. J Manipulative Physiol Ther. 2001;24:529–541.
6. Lowe J.C. Results of an open trial of T3 therapy with 77 euthyroid female fibromyalgia patients. Clin Bull Myofascial Ther. 1997;2:35–37.
7. Lowe J.C., Eichelberger J., Manso G., et al. Improvement in euthyroid fibromyalgia patients treated with T3. J Myofascial Ther. 1994;1:16–29.
8. Lowe J.C. T3-induced recovery from fibromyalgia by a hypothyroid patient resistant to T4 and desiccated thyroid. J Myofascial Ther. 1995;1:26–31.
9. Honeyman G.S. Metabolic therapy for hypothyroid and euthyroid fibromyalgia: two case reports. Clin Bull Myofascial Ther. 1997;2:19–49.
10. Teitelbaum J., Bird B. Effective treatment of severe chronic fatigue: a report of a series of 64 patients. J Musculoskel Pain. 1995;3:91–110.
11. Lowe J.C., Garrison R., Reichman A., et al. Effectiveness and safety of T3 therapy for euthyroid fibromyalgia: a double-blind, placebo-controlled response-driven crossover study. Clin Bull Myofascial Ther. 1997;2:31–57.
12. Lowe J.C., Garrison R., Reichman A., et al. Triiodothyronine (T3) treatment of euthyroid fibromyalgia: a small-n replication of a double-blind placebo-controlled crossover study. Clin Bull Myofascial Ther. 1997;2:71–88.
13. Lowe J.C., Reichman A., Yellin J. The process of change with T3 therapy for euthyroid fibromyalgia: a double-blind placebo-controlled crossover study. Clin Bull Myofascial Ther. 1997;2:91–124.
14. Teitelbaum J., Bird B., Greenfield R.M., et al. Effective treatment of CFS and FMS: a randomized, double-blind placebo controlled study. J Chronic Fatigue Syndr. 2001;8:3–28.
15. Lowe J.C., Reichman A.J., Yellin J. A case-control study of metabolic therapy for fibromyalgia: long-term follow-up comparison of treated and untreated patients. Clin Bull Myofascial Ther. 1998;3:65–79.
16. Lowe J.C. The metabolic treatment of fibromyalgia. Boulder, CO: McDowell Publishing; 2000.
17. Lowe J. The Brady/Schneider proposal: a misdirected reclassification of fibromyalgia. Dyn Chir. 2002;20(12, 14):25–27.
18. Rosner M.J. Decompression of craniovertebral stenosis leads to improvement in FMS and CFIDS symptoms. http://www.nfra.net/NewRosner1.htm. Accessed 8/5/2004
19. Alarcón G.S., Bradley L.A., Hadley M.N., et al. Does Chiari malformation contribute to fibromyalgia symptoms? http://www.nfra.net/Chiari5.htm. Accessed 8/5/2004
20. Mense S., Simons D.G., Russell I.J. Muscle pain: understanding its nature, diagnosis, and treatment. Philadelphia: Lippincott Williams & Wilkins; 2001.
21. Yunus M.B. Fibromyalgia syndrome and myofascial pain syndrome: clinical features, laboratory tests, diagnosis, and pathophysiologic mechanisms. In: Rachlin E.S., ed. Myofascial pain and fibromyal-gia: trigger point management. St. Louis: Mosby–Year Book; 1994:3–29.
22. Travell J.G., Simons D.G. Myofascial pain and dysfunction: the trigger point manual, vol 2. Baltimore: Williams & Wilkins. 1992.
23. Moldofsky H., Scarisbrick P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom Med. 1976;38:35–44.
24. Moldofsky H., Warsh J.J. Plasma tryptophan and musculoskeletal pain in non-articular rheumatism (“fibrositis syndrome”). Pain. 1978;5:65–71.
25. Moldofsky H. Rheumatic pain modulation syndrome: The interrelationship between sleep, central nervous system serotonin, and pain. Adv Neurol. 1982;33:51–57.
26. Russell I.J. Neurochemical pathogenesis of fibromyalgia syndrome. J Musculoskel Pain. 1999;7:183–191.
27. Russell I.J., Vaeroy H., Javors M., et al. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–556.
28. Russell I.J., Vipraio G.A. Serotonin (5HT) in serum and platelets (PLT) from fibromyalgia patients (FS) and normal controls (NC). Arthritis Rheum. 1994;37(suppl):S214.
29. O’Malley P.G., Balden E., Tomkins G., et al. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15:659–666.
30. Carette S., Bell M., Reynolds W.J., et al. A controlled trial of amitriptyline, cyclobenzaprine, and placebo in fibromyalgia. Arthritis Rheum. 1992;35(suppl 9):112.
31. Carette S., Bell M., Reynolds W.J., et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia: a randomized, double-blind clinical trial. Arthritis Rheum. 1994;37:32–40.
32. Carette S. What have clinical trials taught us about the treatment of fibromyalgia? J Musculoskel Pain. 1995;3:133–140.
33. Bennett R.M. Fibromyalgia review. J Musculoskel Pain. 1997;5:85–96.
34. Bennett R.M. Antidepressants do not have better results than placebo in the treatment of fibromyalgia in Brazil. Curr Rheumatol Rep. 2002;4:284–285.
35. Isomeri R., Mikkelsson M., Latikka P., et al. Effects of amitriptyline and cardiovascular fitness training on pain in patients with primary fibromyalgia. J Musculoskel Pain. 1993;1:253–260.
36. Kwiatek R., Barnden L., Tedman R., et al. Regional cerebral blood flow in fibromyalgia: single-photon-emission computed tomography evidence of reduction in the pontine tegmentum and thalami. Arthritis Rheum. 2000;43:2823–2833.
37. Mountz J.M., Bradley L.A., Modell J.G., et al. Fibromyalgia in women. Abnormalities of regional cerebral blood flow in the thalamus and the caudate nucleus are associated with low pain threshold levels. Arthritis Rheum. 1995;38:926–938.
38. Vanhoutte P.M. 5-hydroxytryptamine and vascular disease. Fed Proc. 1983;42(2):233–237.
39. Wallace D.J., Clauw D.J. Fibromyalgia and other central pain syndromes. Philadelphia: Lippincott Williams & Wilkins, 2005.
40. Arnold L.M., Clauw D.J. Fibromyalgia syndrome: practical strategies for improving diagnosis and patient outcomes. Am J Med. 2010;123(6):S2.
41. Vaeroy H., Helle R., Forre O., et al. Elevated CSF levels of substance P and high incidence of Raynaud phenomenon in patients with fibromyalgia: new features for diagnosis. Pain. 1988;32:21–26.
42. Messlinger K. [What is a nociceptor?] Anaesthesist. 1997;46:142–153.
43. Mense S. Descending antinociception and fibromyalgia. Z Rheumatol. 1998;57(suppl 2):23–26.
44. Henriksson K.G., Mense S. Pain and nociception in fibromyalgia: clinical and neurobiological considerations on aetiology and pathogenesis. Pain Rev. 1994;1:245–260.
45. Baranauskas G., Nistri A. Sensitization of pain pathways in the spinal cord: cellular mechanisms. Prog Neurobiol. 1998;54:349–365.
46. Satoh M. Transmission and modulation of nociceptive information in the spinal dorsal horn. Nippon Yakurigaku Zasshi. 1993;101:289–298.
47. Mendelson S.C., Quinn J.P. Characterization of potential regulatory elements within the rat preprotachykinin-A promoter. Neurosci Lett. 1995;184:125–128.
48. Too H.P., Marriott D.R., Wilkin G.P. Preprotachykinin-A and substance P receptor (NK1) gene expression in rat astrocytes in vitro. Neurosci Lett. 1994;182:185–187.
49. Jonassen J.A., Mullikin-Kirkpatrick D., McAdam A., et al. Thyroid hormone status regulates preprotachykinin-A gene expression in male rat anterior pituitary. Endocrinology. 1987;121:1555–1561.
50. Lam K.S., Lechan R.M., Minamitani N., et al. Vasoactive intestinal peptide in the anterior pituitary is increased in hypothyroidism. Endocrinology. 1989;124:1077–1084.
51. Jones P.M., Ghatei M.A., Wallis S.C., et al. Differential response to neuropeptide Y, substance P, and vasoactive intestinal polypeptide in the rat anterior pituitary gland to alterations in thyroid hormone status. J Endocrinol. 1994;143:393–397.
52. Savard P., Blanchard L.M., Merand Y., et al. Serotonin, 5-hydroxyindoleacetic acid and substance P content of discrete brain nuclei in rats made hypo- or hyperthyroid in the neonatal period: effect of growth hormone treatment. Brain Res. 1984;317:239–245.
53. Savard P., Merand Y., Bedard P., et al. Comparative effects of neonatal hypothyroidism and euthyroidism on TRH and substance P content of lumbar spinal cord in saline and PCPA-treated rats. Brain Res. 1983;277:263–268.
54. Savard P., Blanchard L.M., Merand Y., et al. Influences of both thyroid and bovine growth hormones on substance P, thyrotropin-releasing hormone, serotonin and 5-hydroxyindoleacetic acid contents in the lumbar spinal cord of developing rats. Brain Res. 1984;315:105–110.
55. Dupont A., Dussault J.H., Rouleau D., et al. Effect of neonatal thyroid deficiency on the catecholamine, substance P, and thyrotropin-releasing hormone contents of discrete rat brain nuclei. Endocrinology. 1981;108:2039–2045.
56. Hammond D.L. Pharmacology of central pain-modulating networks (biogenic amines and nonopioid analgesics). Fields H.L., Dubner R., Cervero F., eds., Advances in pain research and therapy. Proceedings of the Fourth World Congress on Pain: Seattle, vol 9. New York: Raven. 1985:499–511.
57. Hammond D.L. Control systems for nociceptive afferent processing: the descending inhibitory pathways. In: Yaksh T.L., ed. Spinal afferent processing. New York: Plenum Press; 1986:363–390.
58. Dubner R., Bennett G.J. Spinal and trigeminal mechanisms of nociception. Ann Rev Neurosci. 1983;6:381–418.
59. Cooper J.R., Bloom F.E., Roth R.H. The biochemical basis of neuropharmacology, 6th ed. New York: Oxford University Press; 1991.
60. Archer T., Arwestrem E., Jonsson G., et al. Noradrenergic-serotonergic interactions and nociception in the rat. Eur J Pharmac. 1986;120:295–307.
61. Riedel W., Neeck G. Nociception, pain, and antinociception: current concepts. Z Rheumatol. 2001;60:404–415.
62. Carr D.B., Lipkowski A.W. Neuropeptides and pain. Agressologie. 1990;31:173–177.
63. Chaouch A., Besson J.M. Peripheral and spinal mechanisms of nociception. Rev Neurol (Paris). 1986;142:173–200.
64. Jessell T.M., Kelly D.D. Pain and analgesia. In: Kandel E.R., Schwartz J.H., Jessell T.M. Principles of neural science. 3rd ed. Norwalk, CT: Appleton & Lange; 1991:385–389.
65. Rozanov C.B., Dratman M.B. Immunohistochemical mapping of brain triiodothyronine reveals prominent localization in central noradrenergic systems. Neuroscience. 1996;74:897–915.
66. Gordon J.T., Kaminski D.M., Rozanov C.B., et al. Evidence that 3,3¢,5-triiodothyronine is concentrated in and delivered from the locus coeruleus to its noradrenergic targets via anterograde axonal transport. Neuroscience. 1999;93:943–954.
67. Dratman M.B., Gordon J.T. Thyroid hormones as neurotransmitters. Thyroid. 1996;6:639–647.
68. de Lonlay A., Blouquit M.F., Valens M., et al. Tyrosine hydroxylase and dopamine b-hydroxylase inductions evoked by reserpine in the superior cervical ganglion of developing eu- and hypothyroid rats. J Auton Nerv Syst. 1991;36:33–38.
69. Schwartz J.H., Kandel E.R. Synaptic transmission mediated by second messengers. In: Kandel E.R., Schwartz J.H., Jessell T.M. Principles of neural science. 3rd ed. Norwalk, CT: Appleton & Lange; 1991:173–193.
70. Claustre J., Balende C., Pujol J.F. Influence of the thyroid hormone status on tyrosine hydroxylase in central and peripheral catecholaminergic structures. Neurochem Int. 1996;28:277–281.
71. Mano T., Sakamoto H., Fujita K., et al. Effects of thyroid hormone on catecholamine and its metabolite concentrations in rat cardiac muscle and cerebral cortex. Thyroid. 1998;8:353–358.
72. Nørregaard J., Lykkegaard J.J., Mehlsen J., et al. Exercise training in treatment of fibromyalgia. J Musculoskel Pain. 1996;5:71–79.
73. Verstappen F.T.J., van Santen-Hoeufft H.M.S., van Sloun S., et al. Fitness characteristics of female patients with fibromyalgia. J Musculoskel Pain. 1995;3:45–58.
74. Clark W.C., Yang J.C., Janal M.N. Altered pain and visual sensitivity in humans: the effects of acute and chronic stress. Ann N Y Acad Sci. 1986;467:116–129.
75. Guieu R., Blin O., Pouget J., et al. Nociceptive threshold and physical activity. Can J Neurol Sci. 1992;19:69–71.
76. Suaudeau C., Costentin J. Long lasting increase in nociceptive threshold induced in mice by forced swimming: involvement of an endorphinergic mechanism. Stress. 2000;3:221–227.
77. DeGroot L.J., Larsen P.R., Refetoff S., et al. The thyroid and its diseases, 5th ed. New York: Wiley; 1984.
78. DeGowin E.L., DeGowin R.L. Bedside diagnostic examination, 3rd ed. New York: Macmillan; 1976.
79. Kunos G., Ishac E.J.N. Mechanism of inverse regulation of alpha 1- and beta-adrenergic receptors. Biochem Pharmacol. 1987;36:1185–1191.
80. Kunos G., Vermes-Kunos I., Nickerson M. Effects of thyroid state on adrenoceptor properties. Nature. 1974;250:779–781.
81. Constant E.L., de Volder A.G., Ivanoiu A., et al. Cerebral blood flow and glucose metabolism in hypothyroidism: a positron emission tomography study. J Clin Endocrinol Metab. 2001;86:3864–3870.
82. Stewart J.H., Evans W.F. Peripheral blood flow in myxedema. Arch Intern Med. 1942;69:808.
83. Watanakunakorn C., Hodges R.E., Evans T.C. Myxedema: a study of 400 cases. Arch Intern Med. 1965;116:183–190.
84. Kales A., Heuser G., Jacobson A., et al. All night sleep studies in hypothyroid patients, before and after treatment. J Clin Endocrinol Metab. 1967;27:1593–1599.
85. Gay R.G., Raya T.E., Lancaster L.D., et al. Effects of thyroid state on venous compliance and left ventricular performance in rats. Amer J Physiol. 1988;254:H81–H88.
86. Guttler R.B., Shaw J.W., Otis C.L., et al. Epinephrine-induced alterations in urinary cyclic AMP in hyper- and hypothyroidism. J Clin Endocrinol Metab. 1975;41:707–711.
87. Kohn L.D. Connective tissue. Werner S.C., Ingbar S.H. The thyroid, 4th ed., New York: Harper & Row Publishers, 1978.
88. Thompson W.G., Heaton K.W. Functional bowel disorders in apparently healthy people. Gastroenterology. 1980;79:283–288.
89. Asboe-Hansen G. The variability in the hyaluronic acid content of the dermal connective tissue under the influence of thyroid hormone. Acta Dermato-Venereologica. 1950;30:221–229.
90. Faber J., Horslev-Petersen K., Perrild H., et al. Different effects of thyroid disease on serum levels of procollagen III N-peptide and hyaluronic acid. J Clin Endocrinol Metab. 1990;71:1016–1021.
91. Smith T.J., Murata Y., Horwitz A.L., et al. Regulation of glycosaminoglycan accumulation by thyroid hormone in vitro. J Clin Invest. 1982;70:1066–1073.
92. Nakamura H., Mori T., Genma R., et al. Urinary excretion of pyridinoline and deoxypyridinoline measured by immunoassay in hypothyroidism. Clin Endocrinol. 1996;44:447–451.
93. Foscolo G., Roiter I., De Menis E., et al. Bone metabolism in primary hypothyroidism in the adult. Minerva Endocrinol. 1991;7-10:16.
94. Eisinger J., Dupond J.L., Cozzone P.J. Anomalies de la glycolyse au cours des fibromyalgies: étude biologique et thérapeutique. Lyon Méd. 1996;27:2180–2181.
95. Taylor D.J., Rajagopalan B., Radda G.K. Cellular energetics in hypothyroid muscle. Eur J Clin Invest. 1992;22:358–365.
96. Sharma V.K., Banerjee S.P. Beta-adrenergic receptors in rat skeletal muscle: effects of thyroidectomy. Biochim Biophys Acta. 1978;539:538–542.
97. Chernausek S.D., Underwood L.E., Utiger R.D., et al. Growth hormone secretion and plasma somatomedin-C in primary hypothyroidism. Clin Endocrinol (Oxf). 1983;19:337–344.
98. Lowe J.C., Yellin J. Inadequate thyroid hormone regulation as the main mechanism of fibromyalgia: a review of the evidence. Thyroid Science. 2008;3(6):R1–R14.
99. Wilson J., Walton J.N. Some muscular manifestations of hypothyroidism. J Neurol Neurosurg Psychiatry. 1959;22:320–324.
100. Fessel W.J. Myopathy of hypothyroidism. Ann Rheum Dis. 1968;27:590–596.
101. Bland J.H., Frymoyer J.W. Rheumatic syndromes of myxedema. N Engl J Med. 1970;282:1171–1174.
102. Golding D.N. Hypothyroidism presenting with musculoskeletal symptoms. Ann Rheum Dis. 1970;29:10–14.
103. Hochberg M.C., Koppes G.M., Edwards C.Q., et al. Hypothyroidism presenting as a polymyositis-like syndrome: report of two cases. Arthritis Rheum. 1976;19:1363–1366.
104. Beetham W.P., Jr. Diagnosis and management of fibrositis syndrome and psychogenic rheumatism. Med Clin North Am. 1979;63:433–439.
105. Wilke S.W., Sheeler L.R., Makarowski W.S. Hypothyroidism with presenting symptoms of fibrositis. J Rheumatol. 1981;8:626–631.
106. Delamere J.P., Scott D.L., Felix-Davies D.D. Thyroid dysfunction and rheumatic diseases. J R Soc Med. 1982;75:102–106.
107. Sonkin L.S. Endocrine disorders and muscle dysfunction. In: Gelb H., ed. Clinical management of head, neck, and TMJ pain and dysfunction: a multidisciplinary approach to diagnosis and treatment. Philadelphia: Saunders; 1985:137–170.
108. Awad E.A. Histopathological changes in fibrositis. Fricton J.R., Awad E., eds., Advances in pain research and therapy: myofascial pain and fibromyalgia, vol 17. New York: Raven Press. 1990:249–258.
109. Awad E.A. Pathological changes in fibromyalgia. First International Symposium on Myofascial Pain and Fibromyalgia. Minneapolis; May 9, 1989.
110. Lowe J.C., Cullum M., Graf L., Jr., et al. Mutations in the c-erbA beta 1 gene: do they underlie euthyroid fibromyalgia? Med Hypotheses. 1997;48:125–135.
111. Alajouanine T., Nick J. De l’existence d’une myopathie d’origine hypothyroidienne. Paris Med. 1945;35:346.
112. Bergouignan M., Vital C., Bataille J.M. Hypothyroid myopathies. Clinical and histopathological aspects. Presse Med. 1967;75:1551–1556.
113. Aarflot T., Bruusgaard D. Association between chronic widespread musculoskeletal complaints and thyroid autoimmunity. Results from a community survey. Scand J Prim Health Care. 1996;14:111–115.
114. Rodolico C., Toscano A., Benvenga S., et al. Myopathy as the persistently isolated symptomatology of primary autoimmune hypothyroidism. Thyroid. 1998;8:1033–1038.
115. Lowe JC, Honeyman-Lowe G. Fibromyalgia and thyroid disease. Paper presented and discussed in Grenoble, France May 6 (Conference of the French Fibromyalgia Association of Région Rhône-Alpes) and in Toulon, France, May 11 (Centre Hospitalier Intercommunal), 2000.Lowe J.C., Honeyman-Lowe G. Fibromyalgia and thyroid disease. Paper presented and discussed in Grenoble, France May 6 (Conference of the French Fibromyalgia Association of Région Rhône-Alpes) and in Toulon. (Centre Hospitalier Intercommunal). 2000. France, May 11
116. Lowe J.C., Honeyman-Lowe G. Thyroid disease and fibromyalgia syndrome. Lyon Méditerranée Médical: Médecine du Sud-Est. 2000;36:15–17.
117. Lowe J.C., Yellin J., et al. Female fibromyalgia patients: lower resting metabolic rates than matched healthy controls. Medical Science Monitor. 2006;12(8):CR1–CR8.
118. Lowe, JC, Honeyman, G, Yellin, J. Lower resting metabolic rate and basal body temperature of fibromyalgia patients compared to matched healthy controls. Thyroid Science 1006;1(9):CLS1-CLS18.
119. Starlanyl D.J., Jeffrey J.L., Roentsch G., et al. The effect of transdermal T3 (triiodothyronine) on geloid masses found in patients with both fibromyalgia and myofascial pain: double-blinded, crossover N of 1 clinical study. Myalgies Internat. 2001;2:8–18.
120. Donaldson M.S., Speight N., Loomis S. Fibromyalgia syndrome improved using a mostly raw vegetarian diet: an observational study. BMC Complement Altern Med. 2001;1:7.
121. Azad K.A., Alam M.N., Haq S.A., et al. Vegetarian diet in the treatment of fibromyalgia. Bangladesh Med Res Counc Bull. 2000;26:41–47.
122. Smith J.D., Terpening C.M., Schmidt S.O., et al. Relief of fibromyalgia symptoms following discontinuation of dietary excitotoxins. Ann Pharmacother. 2001;35:702–706.
123. Merchant R.E., Andre C.A. A review of recent clinical trials of the nutritional supplement Chlorella pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Altern Ther Health Med. 2001;7:79–91.
124. Merchant R.E., Carmack C.A., Wise C.M. Nutritional supplementation with Chlorella pyrenoidosa for patients with fibromyalgia syndrome: a pilot study. Phytother Res. 2000;14:167–173.
125. Hanninen O., Kaartinen K., Rauma A., et al. Antioxidants in vegan diet and rheumatic disorders. Toxicology. 2000;155:45–53.
126. Kaartinen K., Lammi K., Hypen M., et al. Vegan diet alleviates fibromyalgia symptoms. Scand J Rheumatol. 2000;29:308–313.
127. Mauro G.L., Martorana U., Cataldo P., et al. Vitamin B12 in low back pain: a randomised, double-blind, placebo-controlled study. Eur Rev Med Pharmacol Sci. 2000;4:53–58.
128. Franca D.S., Souza A.L., Almeida K.R., et al. B vitamins induce an antinociceptive effect in the acetic acid and formaldehyde models of nociception in mice. Eur J Pharmacol. 2001;421:157–164.
129. Waikakul W., Waikakul S. Methylcobalamin as an adjuvant medication in conservative treatment of lumbar spinal stenosis. J Med Assoc Thai. 2000;83:825–831.
130. Kuwabara S., Nakazawa R., Azuma N., et al. Intravenous methylcobalamin treatment for uremic and diabetic neuropathy in chronic hemodialysis patients. Intern Med. 1999;38:472–475.
131. Flynn M.A., Irvin W., Krause G. The effect of folate and cobalamin on osteoarthritic hands. J Am Coll Nutr. 1994;13:351–356.
132. Eckert M., Schejbal P. Therapy of neuropathies with a vitamin B combination: symptomatic treatment of painful diseases of the peripheral nervous system with a combination preparation of thiamine, pyridoxine and cyanocobalamin. Fortschr Med. 1992;20(110):544–548.
133. Leuschner J. Antinociceptive properties of thiamine, pyridoxine and cyanocobalamin following repeated oral administration to mice. Arzneimittelforschung. 1992;42:114–115.
134. Seror P. Sciatica cured by vitamin B12. Rev Rhum Mal Osteoartic. 1989;56:344.
135. Dordain G., Aumaitre O., Eschalier A., et al. Vitamin B12, an analgesic vitamin? Critical examination of the literature. Acta Neurol Belg. 1984;84:5–11.
136. Goldfarb A.H. Nutritional antioxidants as therapeutic and preventive modalities in exercise-induced muscle damage. Can J Appl Physiol. 1999;24:249–266.
137. Narchi H., Thomas M. A painful limp. J Paediatr Child Health. 2000;36:277–278.
138. Lytle R.L. Chronic dental pain: possible benefits of food restriction and sodium ascorbate. J Appl Nutr. 1988;40:95–98.
139. Hodges R.E., Hood J., Canham J.E., et al. Clinical manifestations of ascorbic acid deficiency in man. Am J Clin Nutr. 1971;24:432–443.
140. Kinsman R.A., Hood J. Some behavioral effects of ascorbic acid deficiency. Am J Clin Nutr. 1971;24:455–464.
141. Hirschmann J.V., Raugi G.J. Adult scurvy. J Am Acad Dermatol. 1999;41:895–906.
142. Gaby A.R. Intravenous nutrient therapy: the “Myers’ cocktail.”. Altern Med Rev. 2002;7:389–403.
143. Caruso I., Sarzi Puttini P., Cazzola M., et al. Double-blind study of 5-hydroxytryptophan versus placebo in the treatment of primary fibromyalgia syndrome. J Intern Med Res. 1990;18:201–209.
144. Puttini P.S., Caruso I. Primary fibromyalgia syndrome and 5-hydroxy-tryptophan: a 90-day open study. J Intern Med Res. 1992;20:182–189.
145. Jacobsen S., Danneskiold-Samsoe B., Andersen R.B. Oral S-adenosylmethionine in primary fibromyalgia: a double-blind clinical evaluation. Scand J Rheumatol. 1991;20:294–302.
146. Tavoni A., Vitali C., Bombardieri S., et al. Evaluation of S-adenosylmethionine in primary fibromyalgia: a double-blind crossover study. Am J Med. 1987;83(5A):107–110.
147. Volkmann H., Norregaard J., Jacobsen S., et al. Double-blind, placebo-controlled cross-over study of intravenous S-adenosyl-L-methionine in patients with fibromyalgia. Scand J Rheumatol. 1997;26:206–211.
148. Lieber C.S., Packer L. S-Adenosylmethionine: molecular, biological, and clinical aspects: an introduction. Am J Clin Nutr. 2002;76:S1148–S1150.
149. Abraham G.E., Flechas J.D. Management of fibromyalgia: rationale for the use of magnesium and malic acid. J Nutr Med. 1992;3:49–59.
150. Russell I.J., Michalek J.E., Flechas J.D., et al. Treatment of fibromyalgia syndrome with Super Malic: a randomized, double blind, placebo controlled, crossover pilot study. J Rheumatol. 1995;22:953–958.
151. Dykman K.D., Tone C., Ford C., et al. The effects of nutritional supplements on the symptoms of fibromyalgia and chronic fatigue syndrome. Integr Physiol Behav Sci. 1998;33:61–71.
152. Olson G.B., Savage S., Olson J. The effects of collagen hydrolysat on symptoms of chronic fibromyalgia and temporomandibular joint pain. Cranio. 2000;18:135–141.
153. Bramwell B., Ferguson S., Scarlett N., et al. The use of ascorbigen in the treatment of fibromyalgia patients: a preliminary trial. Altern Med Rev. 2000;5:455–462.
154. Mengshoel A.M. Effect of physical exercise in fibromyalgia. Tidsskr Nor Laegeforen. 1996;116:746–748.
155. McCain G.A. Role of physical fitness training in the fibrositis/fibromyalgia syndrome. Am J Med. 1986;81(3A):73–77.
156. Mengshoel A.M., Komnæ H.B., Forre O. The effects of 20 weeks of physical fitness training in female patients with fibromyalgia. Clin Exp Rheumatol. 1992;10:345–349.
157. Nichols D.S., Glen T.M. Effects of aerobic exercise on pain perception, affect, and level of disability in individuals with fibromyalgia. Phys Ther. 1994;74:327–332.
158. Bailey A., Starr L., Alderson M., et al. A comparative evaluation of a fibromyalgia rehabilitation program. Arthritis Care Res. 1999;12:336–340.
159. Wolfe F. The clinical syndrome of fibrositis. Am J Med. 1986;81:7–14.
160. McCain G.A. Nonmedicinal treatments in primary fibromyalgia. Rheumat Dis Clin North Am. 1989;15:73–90.
161. McCain G.A., Bell D.A., Mai F.M., et al. A controlled study of the effects of a supervised cardiovascular fitness training program on the manifestations of primary fibromyalgia. Arthritis Rheum. 1988;31:1135–1141.
162. Wigers S.H., Stiles T.C., Vogel P.A. Effects of aerobic exercise versus stress management treatment in fibromyalgia. Scand J Rheumatol. 1996;25:77–86.
163. Burckhardt C.S., Mannerkorpi K., Hedenberg L., et al. A randomized, control clinical trial of education and physical training for women with fibromyalgia. J Rheumatol. 1994;21:714–720.
164. Mengshoel A.M., Førre O. Physical fitness training in patients with fibromyalgia. J Musculoskel Pain. 1993;1:267–272.
165. Clark S.R., Burckhardt C., Campbell S., et al. Fitness characteristics and perceived exertion in women with fibromyalgia. J Musculoskel Pain. 1993;1:191–197.
166. Poelhman E.T., Berke E.M., Joseph J.R., et al. Influence of aerobic capacity, body composition, and thyroid hormones on the age-related decline in resting metabolic rate. Metabolism. 1992;41:915–921.
167. Broeder C.E., Burrhus K.A., Svanevik L.S., et al. The effects of either high-intensity resistance or endurance training on resting metabolic rate. Am J Clin Nutri. 1992;55:802–810.
168. Mengshoel A.M., Haugen M. Health status in fibromyalgia: a follow-up study. J Rheumatol. 2001;28:2085–2089.
169. Bennett R.M., Clark S.R., Goldberg L., et al. Aerobic fitness in patients with fibrositis: a controlled study of respiratory gas exchange and 133xenon clearance from exercising muscle. Arthritis Rheum. 1989;32:454–460.
170. Klug G.A., McAuley E., Clark S. Factors influencing the development and maintenance of aerobic fitness: lessons applicable to the fibrositis syndrome. J Rheumatol Suppl. 1989;19:30–39.
171. Bennett R.M., Clark S.R., Campbell S.M., et al. Symptoms of Raynaud’s syndrome in patients with fibromyalgia: a study utilizing the Nielsen test, digital photoplethysmography and measurements of platelet alpha 2-adrenergic receptors. Arthritis Rheum. 1991;34:264–269.
172. Wirz-Justice A. Platelet research in psychiatry. Experientia. 1988;44:145–152.
173. Lowe J.C., Honeyman-Lowe G. The metabolic rehabilitation of fibromyalgia patients. In: Chaitow L., ed. Fibromyalgia syndrome: a practitioner’s guide to treatment. Edinburgh: Churchill Livingstone; 2000:131–143.
174. Lowe J.C., Honeyman-Lowe G. Facilitating the decrease in fibromyalgic pain during metabolic rehabilitation: an essential role for soft tissue therapies. J Bodywork Mov Ther. 1998;2:208–217.
175. Honeyman-Lowe G. Ultrasound treatment of the fibromyalgia patient. Grenoble, France: Paper presented at the French Fibromyalgia Association of Région Rhône-Alpes; May 6, 2000.
176. Honeyman-Lowe G. Ultrasound treatment of the fibromyalgia patient. Lyon Méditerranée Médical: Médecine du Sud-Est. 2000;36:30–31.
177. Pioro-Boisset M., Esdaile J.M., Fitzcharles M.A. Alternative medicine use in fibromyalgia syndrome. Arthritis Care Res. 1996;9:13–17.
178. Gamber R.G., Shores J.H., Russo D.P., et al. Osteopathic manipulative treatment in conjunction with medication relieves pain associated with fibromyalgia syndrome: results of a randomized clinical pilot project. J Am Osteopath Assoc. 2002;102:321–325.
179. Hains G., Hains F. A combined ischemic compression and spinal manipulation in the treatment of fibromyalgia: a preliminary estimate of dose and efficacy. J Manipulative Physiol Ther. 2000;23:225–230.
180. Blunt K.L., Rajwani M.H., Guerriero R.C. The effectiveness of chiropractic management of fibromyalgia patients: a pilot study. J Manipulative Physiol Ther. 1997;20:389–399.
181. Offenbacher M., Stucki G. Physical therapy in the treatment of fibromyalgia. Scand J Rheumatol Suppl. 2000;113:78–85.
182. Lowe J.C. The myofascial genesis of unpleasant thoughts and emotions: its neural basis. Dig Chiro Econ. 1989;32(78):80–81.
183. Harding S.M. Sleep in fibromyalgia patients: subjective and objective findings. Am J Med Sci. 1998;315:367–376.
184. Moldofsky H. Sleep and fibrositis syndrome. Rheum Dis Clin North Am. 1989;15:91–103.
185. National Academy of Clinical Chemists. Available online at http://www.nacb.org/lmpg/thyroid_LMPG_PDF.stm Accessed July 5, 2004
186. Lowe J.C. Thyroid status of 38 fibromyalgia patients: implications for the etiology of fibromyalgia. Clin Bull Myofascial Ther. 1997;2:47–64.
187. Volpé R. Autoimmune thyroiditis. In: Braverman L.E., Utiger R.D. Werner and Ingbar’s the thyroid: a fundamental and clinical text. 6th ed. Philadelphia: Lippincott; 1991:921–933.
188. Wikland B. Redefining hypothyroidism: a paradigm shift. Thyroid Science. 2008;3(1):1.
189. Lowe J.C. Thyroid hormone replacement therapies: ineffective and harmful for many hypothyroid patients. Thyroid Science. 2006;1(1):C1–C21.
190. Pearce C.J., Himsworth R.L. Total and free thyroid hormone concentrations in patients receiving maintenance replacement treatment with thyroxine. Br Med J. 1984;288:693.
191. Hamburger J.I. Strategies for cost-effective thyroid function testing with modern methods. In: Hamburger J.I., ed. Diagnostic methods in clinical thyroidology. New York: Springer-Verlag; 1989:63–109.
192. Lowe J.C., Reichman A., Honeyman G.S., et al. Thyroid status of fibromyalgia patients (abstract). Clin Bull Myofascial Ther. 1998;3:69–70.
193. Fraser W.D., Biggart E.M., O’Reilly D.S., et al. Are biochemical tests of thyroid function of any value in monitoring patients receiving thyroxine replacement? Br Med J (Clin Res Ed). 1986;293:808–810.
194. Escobar-Morreale H.F., del Rey F.E., Obregón M.J., et al. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology. 1996;137:2490–2502.
195. Escobar-Morreale H.F., Obregón M.J., Escobar del Rey F., et al. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838.
196. Costin G., Kaplan S.A., Ling S.M. The Achilles reflex time in thyroid disorders. J Pediatr. 1970;76:277–282.
197. Johansen K., Hansen J.M., Skovsted L. Myxedema and thyrotoxicosis: relations between clinical state and concentrations of thyroxine and triiodothyronine in blood. Acta Med Scand. 1978;204:361–364.
198. Al-Adsani H., Hoffer L.J., Silva J.E. Resting energy expenditure is sensitive to small dose changes in patients on chronic thyroid hormone replacement. J Clin Endocrinol Metab. 1997;82:1118–1125.
199. Wigers S.H., Skrondal A., Finset A., et al. Measuring change in fibromyalgic pain: the relevance of pain distribution. J Musculoskel Pain. 1997;5:29–41.
200. Chaves T.C., Nagamine H.M., de Sousa L.M., et al. Comparison between the reliability levels of manual palpation and pressure pain threshold in children who reported orofacial pain. Man Ther. 2010;15(5):508–512.
201. Burckhardt C.S., Clark S.R., Bennett R.M. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–733.
202. Jones J., Rutledge D.N., Jones K.D., et al. Self-assessed physical function levels of women with fibromyalgia: a national survey. Women’s Health Issues. 2008;18(5):406–412.
203. Zung W.W. A self-rating depression scale. Arch Gen Psychiatry. 1965;12:63–70.
204. Harris J., Benedict F. A biometric study of human basal metabolism. Proc Sci USA. 1918;4(12):370–373.
205. James M.J., Gibson R.A., Cleland L.G. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(suppl 1):S343–S348.
206. Seaman D.R. The diet-induced proinflammatory state: a cause of chronic pain and other degenerative diseases? J Manipulative Physiol Ther. 2002;25:168–179.
207. Travell J.G., Simons D.G. Myofascial pain and dysfunction: the trigger point manual, vol 1. Baltimore: Williams & Wilkins. 1983.
208. Christy N.P. The adrenal cortex in hypothyroidism. In: Braverman L.E., Utiger. R.D. Werner’s The thyroid: a fundamental and clinical text. 6th ed. Philadelphia: JB Lippincott Co; 1991:1045–1049.
209. Hoffman B.B., Lefkowitz R.J. Adrenergic receptor antagonists. In: Gilman A.G., Rall T.W., Nies A.S., Tayleor P. Goodman and Gilman’s the pharmacological basis of therapeutics. 8th ed. New York: Pergamon Press; 1990:221–243.