Chapter 23 Fibromyalgia
AETIOLOGY, EPIDEMIOLOGY AND CLASSIFICATION
The American College of Rheumatology (ACR) definition of fibromyalgia (FM) has been based on generalised pain as the primary symptom; however, many of these patients present with a wide variety of complaints including, but not limited to, increased frequency of fatigue, non-restorative sleep, IBS, headache and impaired cognition and mood.1 It is more prevalent in women, ages 20–50 years, but has also been seen in paediatric and geriatric populations. It affects 0.5% to 5.8% of the population in North America and Europe.2 The diagnosis has been based primarily on subjective reporting of widespread pain, with the absence of objective findings or a known aetiology. Research has identified biochemical and metabolic abnormalities that are common to this particular group. Presently, there is a movement to redefine this disorder to include its multisystem effect and find objective, measurable biomarkers.3
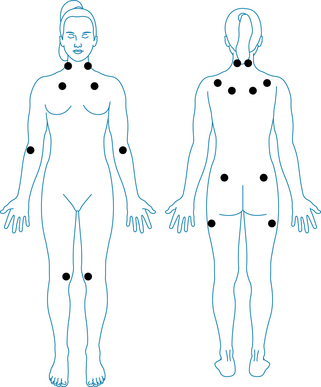
The 1990 American College of Rheumatology criteria for classification of fibromyalgia1 are widespread pain for at least 3 months, defined as the presence of all of the following:
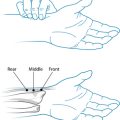
Pain, on digital palpation, must be present in at least 11 of the following 18 tender point sites (see Figure 23.1). All sites are bilateral:
The aetiology of fibromyalgia is not fully understood, but ongoing research has been elucidating some possible mechanisms for this syndrome. Biochemical, metabolic and cellular changes have been demonstrated in multiple systems including mitochondrial dysfunction with aberrations in ATP synthesis and use, central nervous system (CNS) changes affecting cerebral blood flow, neurotransmitter synthesis and function and increased pain perception.4 HPA axis function is affected, resulting in a hyporesponsiveness of the adrenals and circadian rhythm abnormalities.5 Somatic and visceral alterations have also been demonstrated in the literature.6 The result of these changes contributes to the high incidence of comorbidities associated with this syndrome.
RISK FACTORS
There are a variety of risk factors associated with FM patients, including genetics, and comorbidities with other rheumatological conditions and chronic fatigue syndrome (see Chapter 35 on chronic fatigue syndrome). One of the most consistent findings is that the prevalence among females compared to males is as high as 9:1,2 which is also common to these comorbid conditions. Previous history of domestic violence, abuse and emotional trauma appear to be factors as evidenced by the epidemiological data.7 The presence of poverty, poor support and lower educational status may be a predisposing factor associated with the high incidence of mood disorders and decreased pain threshold.8 These triggers, when combined with the prevalence of non-restorative sleep,
Table 23.1 Predictive risks factors for developing fibromyalgia7,8
| AGE 20–50 YEARS | FEMALE > MALE (9:1) |
|---|---|
| Lower education level and socioeconomic status | Non-restorative sleep |
| Increased incidence of anxiety, depression and somatisation | Comorbidity with other rheumatic diseases, such as rheumatoid arthritis and lupus |
| History of physical, sexual, emotional abuse or trauma | Comorbidity with chronic fatigue syndrome |
| Poor social support system | High prevalence of gastrointestinal dysfunction and dysbiosis |
| Abnormal stress response | Other family members with symptoms of fibromyalgia and common enzyme defect |
predispose the FM patient to irregularities in the HPA axis that are common in this cohort.9 This spectrum of systemic changes along with aberrations in the health of the gastrointestinal tract contribute to this cascade via alterations in serotonin synthesis and use, nutritional deficiencies and immune dysfunction and must be differentiated from other disease patterns presenting with myalgias.3
CONVENTIONAL TREATMENT
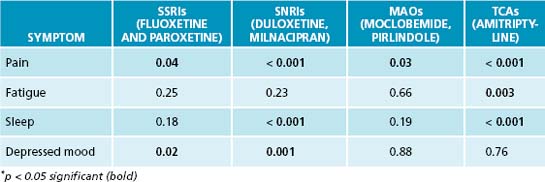
A variety of allopathic medications have been used in the treatment of FM, including muscle relaxants, antidepressants, anticonvulsants and other CNS agents. The antidepressants typically prescribed are tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), serotonin and noradrenaline reuptake inhibitors (SNRIs) and monoamine oxidase inhibitors (MAOIs).10 NSAIDs and steroids alone have not been shown to be effective.10 Review of a meta-analysis on patients using the muscle relaxant cyclobenzaprine revealed a reduction in pain initially and improvement in sleep, but no change in tender points or fatigue.11 A recent meta-analysis representing the efficacy of different antidepressant classes on the parameters associated with fibromyalgia revealed amitriptyline and duloxetine as having the most overall effectiveness. The author’s conclusion of the meta-analysis was that, based on the data, these two medications can justifiably be recommended, in the short term, for pain and sleep disturbances.12
Table 23.2 Medication overall effect using p value∗: meta-analysis of 18 randomised controlled trials (RCTs)12
TREATMENT OF GENERAL MUSCLE TENSION AND PAIN
Due to the increased side effects of the TCAs, low doses are typically prescribed; this may account for the lack of improvement in depression. The median duration for the RCTs was 8 weeks (4 to 28 weeks). The long-term effect of the medications and posttreatment improvement has not been elucidated in-depth in the literature. Median drop-out rates did not differ between the placebo and antidepressant groups due to adverse effects. Because of the possibility of suicidal thinking, in the United States of America most medications approved for fibromyalgia carry a black box warning from the Food and Drug Administration (FDA); these include duloxetine (SNRI), milnacipran (SNRI) and pregabalin, a GABA analogue typically used to treat neuropathic pain and seizures.13,14
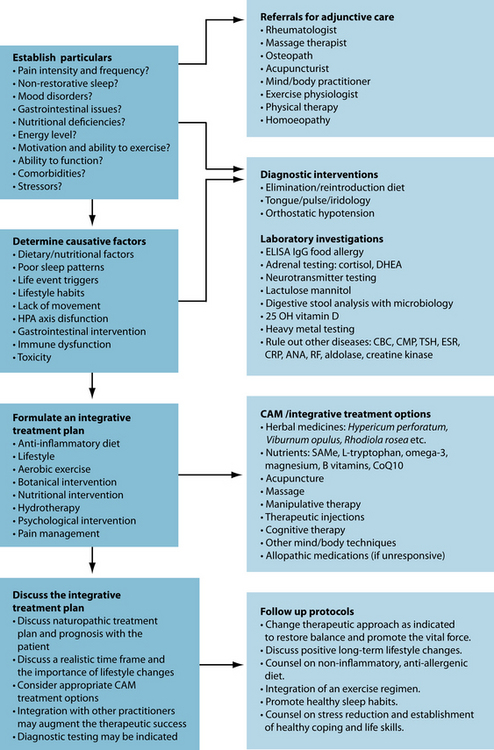
KEY TREATMENT PROTOCOLS
Mitochondrial and cellular changes
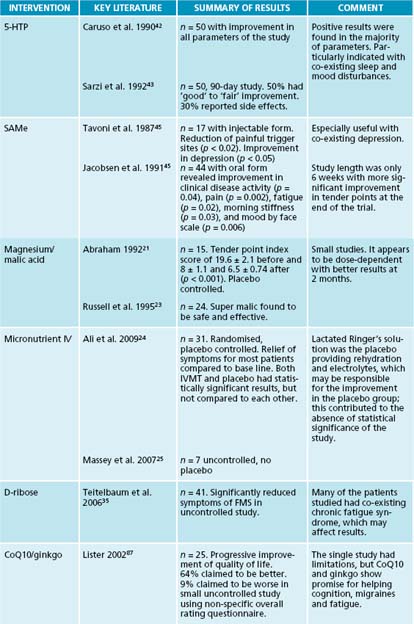
Metabolic changes occur at the cellular level, causing functional aberrations in FM patients. These include changes in glycolysis and isoenzyme production and reduction of energy reserves. Increased lactate production and decreased lactate dehydrogenase isoenzymes were present in the muscle tissue of FM patients.15 A small study using magnetic resonance spectroscopy revealed decreased phosphocreatine, an essential muscle energy storage form, and reduced ATP levels in the quadriceps of FM patients compared to controls during rest.16 Total oxidative capacity and phosphorylation potential was also reduced during rest and exercise.16 Another study found decreased platelet ATP, along with higher calcium and magnesium levels, implying irregularities in the calcium magnesium pump mechanisms at the cellular level.17 These biochemical aberrations contribute to the fatigue, weakness and exercise intolerance associated with FM.
The role of magnesium includes glycolysis and mitochondrial function.18 ATP biosynthesis and metabolism in FM patients are affected by a disturbance in its use.19 Inconsistencies in the levels of magnesium are evident in the various blood components. Increased platelet magnesium and calcium levels have been found, and low serum and RBC magnesium levels are noted.20,21 Reduced serum magnesium levels appear to correlate with fatigue, but not to the number of tender points.22 In a small placebo controlled trial, malic acid, a precursor to malate (an intermediary in the citric acid cycle), was combined with magnesium. The effects of a 4-week course of magnesium/malic acid 300/1200 mg were compared to those of a 600/2400 mg dosage. Significant reductions in pain and tenderness measures were seen at higher dosages given for at least 2 months.21,23 A randomised, double blind, placebo controlled 8-week study using an IV micronutrient therapy (IVMT), Myers’ cocktail (which contains magnesium), thiamine, vitamin C, calcium and B vitamins demonstrated statistically significant improvement in tender points, pain, depression and quality of life in the IVMT group at 8 weeks, while the placebo group of IV lactated Ringer’s solution showed statistically significant improvement in tender points. The dramatic response of the placebo group negated a statistical significance between the groups. The response persisted for 4 weeks posttreatment for both groups.24 An 8-week uncontrolled case study of seven previously non-responsive patients by Massey revealed a 60% decrease in pain and 80% reduction in fatigue.25
The irregularities of the calcium–magnesium pump mechanism as suggested by Bazzichi may play a role in the muscle pain attributed to fibromyalgia.16 Other aberrations in calcium absorption and use may exist in the patient presenting with widespread pain. Vitamin D in the form of 1 alpha, 25-(OH)2-vitamin D3 promotes the calcium-dependent exocytotic activities of the cell when coupled with ATP, potentiating the bone anabolic effects of this nutrient.26,27 A deficiency of vitamin D may masquerade as a generalised non-specific pain, depression and poor fibromyalgia assessment scores.28 One author proposes that the widespread pain associated with low levels of vitamin D is secondary to reduced calcium absorption, which increases PTH levels.29 Elevated PTH causes increased urinary excretion of phosphorus, resulting in low levels of circulating calcium phosphate, ultimately leading to a poorly mineralised collagen matrix. Endosteal and periosteal swelling may occur secondary to the affected collagen on the surfaces, triggering bone and muscle pain.
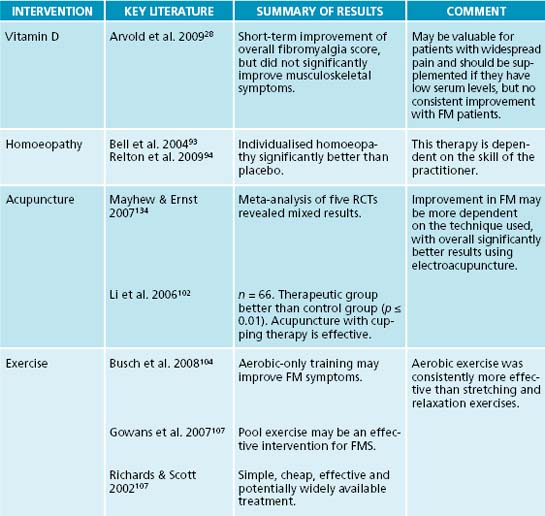
In multiple studies of patients presenting with persistent non-specific muscle pain as well as fibromyalgia, 25-OH vitamin D levels were frequently depressed.29,30 Supplementation of vitamin D2, 50,000 IU weekly for 8 weeks, yielded significant clinical improvement in mild to moderately (10–25 ng/mL) deficient patients, but not severely deficient patients.28 However, this regimen of supplementation has not consistently yielded pain reduction in moderately deficient patients, even after 3 months.31 Vitamin D deficiency has also been associated with decreased cognitive performance and mood disorders, especially anxiety and depression.32,33 Evaluation of 25-OH vitamin D levels and correction of low serum levels are warranted in all patients presenting with widespread pain. In addition to the possible benefit to FM, depressed levels of vitamin D have been associated with increased risk of breast cancer and osteoporosis in this primarily female population.34 The optimal level of 25-OH vitamin D remains a controversial topic and one study recommended a minimum of ≥ 40 ng/mL for breast cancer prevention.34 The optimal level for fibromyalgia patients still remains unclear.34
Phosphorylated D-ribose is a component of ATP and NADH as D-ribose-5-phosphate, and may be helpful to FM patients. A small uncontrolled trial of 41 patients with FM and/or chronic fatigue syndrome, given 5 g of ribose three times daily, revealed significant improvement in energy and wellbeing according to a visual analogue scale.35 Due to the limitations and positive results of the study, further investigation of D-ribose would be valuable.
Neurotransmitter effects
The concept of ‘somatisation’ has been associated with fibromyalgia secondary to the increased reporting of hypersensitivity to pain, cognitive dysfunction, depression, anxiety, social isolation and insomnia.36 There is an increased incidence of adverse life events and psychological distress common to this primarily female cohort.7 In addition to psychosocial stressors, the biochemical changes associated with neurotransmitter synthesis and use have been implicated and may contribute to the mental and emotional state of these patients (see Section 4 on the nervous system).
There is a genetic correlation of FM in families secondary to a defect in neurotransmitter metabolism. This polymorphic rate defect displays itself in catecholamine turnover secondary to catecholamine-O-methyl transferase enzyme and is linked to dopaminergic, adrenergic/non-adrenergic neurotransmission and the mu-opioid system.37 This aberration has been associated with increased nociceptive response to painful stimuli. In addition to dopamine and epinephrine metabolism, reduced levels of serotonin and its precursors, tryptophan and 5-hydroxytryptophan (5-HTP), are seen.38,39 Low levels of plasma and serum serotonin (5-HT) have been highlighted in the literature and pharmacological treatment has focused on the use of antidepressants, most recently SSRIs and SNRIs, for treatment of pain, sleep and mood disturbances.40 In FM patients, low levels of serotonin in combination with elevated levels of substance P, a neurotransmitter that has been associated with enhanced pain perception secondary to normal stimuli, has been considered to be a precipitating factor for the increased pain hypersensitivity found in fibromyalgia.41
The conversion of tryptophan to serotonin involves the intermediary, 5-hydroxytryptophan (5-HTP). This biochemical pathway may be inhibited by stress, insulin resistance, tetrahydrobiopterin, pyridoxal 5-phosphate and magnesium deficiency.42 Supplementation with 5-HTP alone has shown significant improvement in the number of tender points, anxiety, fatigue, pain intensity and quality of sleep during a 90-day trial of patients with primary fibromyalgia syndrome. Mild transient side effects were reported in 30% of the patients.42,43
Another metabolic pathway is the use of S-adenosyl methionine (SAMe) in the conversion of 5-HT to melatonin. It acts as a coenzyme and a methyl donor, and has been shown to be an effective antidepressant in psychiatric populations.44 In FM patients there is a correlation with depression and the number of trigger points. When patients were treated with 200 mg of injectable SAMe, both depression and the number of trigger points significantly improved.45 A 6-week trial of oral SAMe revealed improvement in fatigue, mood and morning stiffness.46 Reduction in pain, but not tender point count, occurred during week 6 of the trial and may warrant a longer investigation to realise the potential benefits.
The use of botanicals specifically for the treatment of FM has not been well documented in the scientific literature. Since combination formulas are the traditional mode of botanical dispensing, a balanced combination of botanicals would most likely best address the complex nature of a FM patient. However, single botanicals addressing the separate components of this syndrome will be presented, allowing the naturopathic practitioner to develop an individualised compound to best suit the needs of their patients (see Table 23.3).
Table 23.3 Fibromyalgia: herbal medicine actions and examples47,48
| 1. Adaptogens/tonics |
| Withania somnifera, Panax ginseng, Rhodiola rosea, Eleutherococcus senticosus |
| 2. Antispasmodics |
| Viburnum opulus, Piper methysticum, Piscidia erythrina, Scutellaria lateriflora |
| 3. Nervines |
| Scutellaria lateriflora, Passiflora incarnata, Matricaria recutita, Melissa officinalis |
| 4. Thymoleptics |
| Hypericum perforatum, Avena sativum, Lavandula angustifolia, Turnera diffusa |
| 5. Hypnotics |
| Humulus lupulus, Passiflora incarnata, Valeriana spp. |
| 6. Analgesics |
| Corydalis ambigua, Eschscholtzia californica |
| 7. Digestives (aromatics, bitters, mucilages) |
| Zingiber officinale, Taraxacum officinale, Ulmus fulva |
| 8. Circulatory stimulants |
| Zingiber officinale, Ginkgo biloba, Cinnamomum zeylanicum, Zanthoxylum spp. |
The botanical Rhodiola rosea was found to increase 5-HT levels in the hippocampus of depressive rats to normal levels using 1.5 g/kg, 3 g/kg and 6 g/kg dosages.49 A randomised placebo controlled double-blind study of Hypericum perforatum in the treatment of depressive patients with somatic complaints including depression, fatigue and disturbed sleep resulted in 70% of the patients being symptom-free after 4 weeks.50 H. perforatum administration in the unstressed rat population revealed a decrease in tryptophan, an increase in corticosterone and lower 5-HT in the hippocampus and amygdala under stressful physical conditions. Serum 5-HT levels increased more than 110%, 163% and 172% in the hypothalamus, amygdala and hippocampus respectively (p < 0.01), demonstrating an adaptive response to stress. Tryptophan and corticosterone levels did not change significantly.51 In addition, a meta-analysis, including the Cochrane database, comparing HP to key SSRIs revealed that there was a similar efficacy between the medications, but fewer side effects with the HP.52,53 The botanical Scutellaria baicalensis, which has traditionally been used as a ‘nervine’, contains a metabolite similar to hyperforin, which is found in H. perforatum and would demonstrate similar actions.54
The circadian rhythm
Dysregulated circadian rhythms, manifesting as poor sleep patterns, non-restorative sleep and chronic fatigue, have been reported in fibromyalgia patients.55 Elevation of late evening cortisol and alterations in melatonin levels may contribute to the poor sleep patterns seen in this cohort.56 Normal basal circadian rhythm is typically evidenced by a sharp rise in serotonin synthesis and a release of 5-HT in the early evening, preceding an elevation in melatonin production. There are conflicting data regarding melatonin secretion in FM patients. Decreased urinary melatonin secretion levels were found between the hours of 11 p.m. and 7 a.m.57–59 Meanwhile, in another study, plasma melatonin levels were elevated in FM patients between the hours of 11 p.m. and 6:50 a.m. compared to controls, but secretory patterns remained similar.5 This discrepancy between urinary and plasma melatonin levels was not demonstrated in normal non-depressed patients.60 Despite the discrepancy and different methods of evaluation, it was found that administration of 3 mg of melatonin at bedtime in a small uncontrolled study revealed significant improvement in tender point count, pain severity and sleep after 30 days.59
HPA-axis modulation
Another contributing factor to the non-restorative sleep, low energy and heightened pain perception may be associated with abnormalities in the hypothalamic–pituitary–adrenal (HPA) axis.61 Dexamethasone suppression tests in FM patients show statistically significant increased release of ACTH with no resulting change in cortisol, indicating a hyporesponsiveness of the adrenal function to stimuli.61 The increased ACTH/cortisol ratios, compared to controls, correlate to increased stress and anxiety measures. Also, the percentage of cortisol suppression is related to pain and fatigue as reported by patients.62 Corticoid response to acute exercise is also impaired in fibromyalgia patients.63 In addition to lower basal plasma and urinary cortisol levels, there is a decreased expression of corticosteroid receptors, all of these contributing to the abnormal glucocorticoid response.64 The combined dysfunction associated with the HPA axis and the adrenal response contributes to the aetiopathology and symptomology of fibromyalgia.
A combination of Eleutherococcus senticocus, Schisandra chinensis and Rhodiola rosea revealed improved exercise endurance and tolerance to stress.65,66 In addition, a single administration of Panax ginseng up-regulated the release of ACTH and corticosteroid initially, but did not maintain this effect after 7 days of ginseng administration. However, ginseng was shown to modulate the effect of hydrocortisone administration on the pituitary adrenocortical system even after 7 days.67
Digestive dysbiosis
It is estimated that 30–70% of patients with FM have irritable bowel syndrome (IBS) concurrently.68,69 In addition to altered visceral and somatic perception, both patient subsets have decreased serotonin synthesis and use, which may contribute to increased sensitivity to abdominal pain.6,70 In addition, the low levels of serotonin may alter motor and secretory function that may result in diarrhoea or constipation, both manifestations of IBS.70 Increased intestinal and gastroduodenal permeability is associated with FM. In addition increased incidence of dysbiotic changes, as evidenced by the lactulose hydrogen breath test, implicates the prevalence of small intestinal bowel overgrowth (SIBO) in both these groups. The proximal small intestine typically has low levels of colonic type bacteria, but issues such as dysmotility and lack of gastric acid may exacerbate SIBO. The intensity of abdominal pain in FM patients with SIBO appears to correlate to the degree of bacterial overgrowth found in the small intestine.71 In a double-blind randomised placebo controlled trial, FM patients identified with SIBO were treated to achieve full eradication of the pathogenic flora compared to a non-eradicated group. The group that achieved complete eradication of dysbiotic flora had a significant improvement in pain and depression, in comparison to the non-eradicated group.72 Eradication with antibiotics is standard treatment for SIBO, but recurrence rate is high, especially in populations using proton pump inhibitors, which decrease gastric secretions.73 There have been a limited number of small studies and case reports showing improvement in SIBO via natural therapeutics.
Studies using glutamine and fibre in animal studies demonstrated a reduction of bacterial translocation into lymph nodes, along with improvement in gut barrier function secondary to glutamine.74–76 A nutrient solution of L-arginine alone at levels of 300 and 600 mg or in combination with nucleotides and omega-3 fatty acids showed a reduction in bacterial translocation.77 In addition the omega-3 fatty acids may improve the outcome of fibromyalgia patients over time by modulating inflammatory cytokines78 (see Chapter 28 on autoimmunity). Consideration should also be given to ensuring motility and normal gastric secretions, since their reduction may perpetuate SIBO. The modification of SIBO and bacterial translocation may decrease the inflammatory response that appears to develop with long-standing fibromyalgia.
Immune alterations
Immune activation was apparent in dermal biopsies of fibromyalgia patients that revealed significantly elevated levels of IgG deposits in the dermis. In addition there was higher mean number of mast cells and an association between degranulated mast cells and the individual IgG immunofluorescence scores.79 This IgG correlation may account, in part, for the prevalence of rhinitis in this cohort (70%) in the absence of positive skin allergy tests in 50–65% of this population.80 Both IBS and migraines, common manifestations associated with FM, also demonstrate elevated IgG levels.81,82 In a study of IBS patients, food antigen specific IgE antibodies were not significantly different between FM patients and controls. IgG4 antibodies were significantly elevated in one study, but did not significantly correlate with the severity of symptoms.83
IBS and migraines may both benefit by eliminating IgG positive foods, and reduction of circulating IgG may be beneficial in the treatment of fibromyalgia patients. In addition to the role of IgG in functional bowel disturbances, IBS and migraines, it is possible that dermal IgG may contribute to sensory alterations and widespread pain. It appears that IgG plays a more significant role than IgE in the pathogenesis of a variety of co-existing complaints and that standard scratch testing and IgE titres are not the most accurate method of testing for this cohort. Also, since the severity of symptoms does not always correlate with the antibody titres, it is important to eliminate all reactive foods, even low scoring ones.83 ELISA testing of food antigens and subsequent trial elimination would be justified in this population.
Circulatory disturbances
It has been hypothesised that circulatory changes may be partially responsible for the pain, presence of tender points, exercise intolerance, Raynaud’s phenomenon and cognitive changes present in FM.84 Alterations in microcirculation and temperature were at the level of tender points, resulting in lower skin temperature. A lack of blood flow was implicated by decreased erythrocyte velocity and increased concentration of erythrocytes at tender points.84 Muscle biopsy revealed increased dialysate lactate secondary to alterations in nitric oxide pathways; this may contribute to the reported exercise intolerance and exertion fatigue reported during aerobic exercise in these patients.85 Impaired cerebral perfusion flow is also evident by brain single photon emission computed tomography (SPECT) and clinically correlated with the severity and associated disability of the small group evaluated.86
The correlation between circulatory and oxygenation disturbances with FM may explain the beneficial effect of CoQ10 and Ginkgo biloba. A small, uncontrolled study of 200 mg daily of both coenzyme Q10 (CoQ10) and G. biloba showed promise for improving quality of life measures.87 Increased tissue perfusion was demonstrated by both substances. CoQ10 is present primarily in the mitochondria and assists in the generation of ATP via cellular respiration, but also has been shown to be protective against lipid peroxidation secondary to cerebral hypo/hyperperfusion in rats.88,89 CoQ10 is also effective in the treatment of fatigue and migraines.88,90 G. increased blood flow velocity cerebrally, as well as having an effect on promoting vasculogenesis capacity, via endothelial progenitor cells stimulation, in the peripheral blood, resulting in improved perfusion of the peripheral tissues.91,92
INTEGRATIVE MEDICAL CONSIDERATIONS
Homoeopathy
A double-blind, randomised, parallel-group, placebo controlled study using individualised homoeopathic medicine with daily LM potency over a 3-month period was been shown to be effective in treating fibromyalgia. The number of tender points, pain on palpation and quality of health all improved significantly.93 Another study using individualised homoeopathic prescriptions revealed more significant improvement on fatigue and function with less effect on pain.94
Acupuncture
Acupuncture diagnosis commonly assesses pain as ‘Blood and Qi [energy] Stagnation’ and focuses on movement of these vital substances.95 One study demonstrated an elevated number of erythrocytes in conjunction with a decrease in regional blood flow at the level of tender points of FM patients; this correlates to the Chinese theory of Blood Stagnation.84 Lower skin temperature was also found.84 A follow-up study measured these indices pre- and post-acupuncture treatment. The therapeutic effect of acupuncture revealed a significant increase in blood flow and temperature at the level of the tender points posttreatment.96 Acupuncture has been shown to increase serotonin levels in rats with the insertion of a bilateral acupoint, urinary bladder 23. Elevated levels began after 20 minutes and maintained for 60 minutes posttreatment.97 Substance P levels were reduced using electroacupuncture of local and distal points in a rat model.98
Acupuncture may have an adaptogenic response on cortisol levels. Animal studies on horses and rabbits using electroacupuncture showed an increase in cortisol levels post treatment.99–101 The inconsistencies of positive results with acupuncture, specifically on fibromyalgia patients, may be based in part on the techniques used. In a meta-analysis of
five randomised controlled studies, out of 11 evaluated, results were based on variables including reduction of pain, number of tender points and improvement in quality of life and sleep. The studies involving electroacupuncture overall achieved the best results. In addition, most of the positive studies used individualised treatments.100 The negative studies used manual therapy, which may not have had sufficient stimulation to ‘obtain Qi’ in a population that has sensory perception alterations.
Cupping and moxibustion techniques, which were not addressed in the meta-analysis, may also have beneficial effects when included in the acupuncture treatment.102 The validity of sham acupuncture continues to be controversial, especially in a population with widespread pain. In acupuncture, insertion of needles into a painful point, termed an ‘ashi’ point, which is not otherwise considered a standard acupuncture point, is considered to be an effective treatment strategy for pain reduction. Meanwhile, the US National Institutes of Health consensus stance is that acupuncture is a sufficient adjuvant therapy to treat FM patients.103
Exercise and manual therapy
Exercise intolerance is a common manifestation in fibromyalgia patients. Multiple studies, including a meta-analysis of eight studies, showed that aerobic-only exercise training programs revealed significant improvement in outcome measures, including physical function and global wellbeing while reducing pain, tender points and fibromyalgia impact scores. Stretching and relaxation exercises did not yield the same effect. Improvement was seen as early as 6 weeks and maintained with exercise up to 12 months in some studies.104–105 A review of pool exercise studies yielded improvement in mood and sleep as well as physical function and pain. The improvements, as reviewed in follow-up studies, showed a positive response for up to 2 years.107
Muscle stimulation may also be achieved with manual therapies. A small study of FM patients receiving twice weekly 30-minute massage treatments showed a decrease of substance P, reduction in pain and anxiety, in addition to improvement in quality and number of sleep hours.108
Mind/body therapy
The psychological component of FM in this primarily female population has been explored. Violence against women is epidemic as demonstrated in a cross-sectional, self-administered, anonymous survey of 1952 female patients. Physical, sexual and alcohol abuse and emotional status were all investigated among a diverse community-based population. The results of the survey indicated that one in 20 women had experienced domestic violence in the past year, one in five in their adult life and one in three as either a child or an adult. Participants that had experienced abuse within the past year were more likely to have depression, anxiety and somatisation.109 Findings of another large case-controlled study of 574 women revealed an increased prevalence of abuse among FM patients over controls. There was a significant correlation between frequency of abuse and FM.7 A Spanish epidemiological and quality of life study revealed low educational level, low social class and self-reported depression are much more prevalent in this FM population.110 Depression co-existing with FM appears to decrease the pain threshold in these patients.111 Effective treatment of FM must address the psychological impact as well as the biochemical changes to effect a long-standing improvement.
The prognosis for FM patients is partially dependent on social support and self-efficacy, which has been shown to predict positive lifestyle changes in FM patients.112 A randomised controlled study of 91 patients with fibromyalgia using mindfulness training during eight weekly session of 2.5 hours revealed alleviation of depressive symptoms, as assessed by the Beck Depression Inventory.113 The effect of a 6-week guided imagery program was to improve functional status and self-efficacy in the management of pain. Actual pain level did not change significantly.114
Example treatment
The formula is designed to reduce pain, and improve sleep, cognition and mood. Chronic pain and lack of sleep create a vicious cycle, which contributes to the anxiety, cognitive changes and fatigue associated with this condition. This is due, in part, to a disruption in the hypothalamic–pituitary–adrenal axis.115 Reduction of pain and promotion of a restorative sleep are essential to daily functioning of this patient.
Herbal formula
| Rhodiola rosea 1:1 | 20 mL |
| Schisandra chinensis 1:1 | 25 mL |
| Viburnum opulus 1:2 | 15 mL |
| Hypericum perforatum 1:2 | 20 mL |
| Melissa officinalis 1:2 | 20 mL |
| 5 mL t.d.s. | 100 mL |
| Ginkgo biloba tablets 1 g t.d.s. | |
The presence of irritable bowel syndrome may indicate food intolerances and should be investigated. In addition, dysbiosis, which is common to this cohort, may exacerbate the condition and should be treated according to the presenting organisms and imbalances in the normal flora. To improve physical function, a graduated aerobic exercise program (water- or land-based) improves fibromyalgia scores, pain, cognition, anxiety and sleep. Manual therapies (massage, manual lymph drainage) have been helpful in pain, fatigue and anxiety.108,116
Nutritional intervention
Herbal intervention
Expected outcomes and follow-up protocols
The digestion must be addressed to improve barrier function and absorption. Dietary changes should be the focus, with botanical and nutraceuticals intervention as an adjunct. Dysbiotic changes are common to this cohort and re-establishment of normal gut flora should be promoted. Correction of nutritional deficiencies, especially vitamin D, may yield beneficial results and should be implemented. If insomnia, anxiety and fatigue continue, salivary cortisol may be valuable to assess aberrations in the HPA axis. Fibromyalgia affects patients on a profound level and modalities such as homoeopathy, acupuncture and mind/body techniques can provide a deep level of healing on the physical, mental, emotional and spiritual levels.
Busch A.J., et al. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35(6):1130-1144.
Dadabhoy D., et al. Biology and therapy of fibromyalgia. Evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res Ther. 2008;10(4):211.
Gran J.T. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17(4):547-561.
1. Wolfe F., et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160-172.
2. Gran J.T. The epidemiology of chronic generalized musculoskeletal pain. Best Pract Res Clin Rheumatol. 2003;17(4):547-561.
3. Dadabhoy D., et al. Biology and therapy of fibromyalgia. Evidence-based biomarkers for fibromyalgia syndrome. Arthritis Res Ther. 2008;10(4):211.
4. Sprott H., et al. Increased DNA fragmentation and ultrastructural changes in fibromyalgic muscle fibres. Ann Rheum Dis. 2004;63(3):245-251.
5. Korszun A., et al. Melatonin levels in women with fibromyalgia and chronic fatigue syndrome. J Rheumatol. 1999;26(12):2675-2680.
6. Mayer E.A., Raybould H.E. Role of visceral afferent mechanisms in functional bowel disorders. Gastroenterology. 1990;99(6):1688-1704.
7. Ruiz-Perez I., et al. Risk factors for fibromyalgia: the role of violence against women. Clin Rheumatol. 2009;28(7):777-786.
8. Holick M.F. The influence of vitamin D on bone health across the life cycle. J Nutr. 2005;135(11):2726S-2727S.
9. Gur A., Oktayoglu P. Central nervous system abnormalities in fibromyalgia and chronic fatigue syndrome: new concepts in treatment. Curr Pharm Des. 2008;14(13):1274-1294.
10. Lautenschlager J. Present state of medication therapy in fibromyalgia syndrome. Scand J Rheumatol Suppl. 2000;113:32-36.
11. Tofferi J.K. Treatment of fibromyalgia with cyclobenzaprine: A meta-analysis. Arthritis Rheum. 2004;51(1):9-13.
12. Hauser W., et al. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. JAMA. 2009;301(2):198-209.
13. Russell I.J., et al. The effects of pregabalin on sleep disturbance symptoms among individuals with fibromyalgia syndrome. Sleep Med. 2009;10(6):604-610.
14. Food and Drug Administration News. FDA proposes new warnings about suicidal thinking, behavior in young adults who take antidepressant medications, in P07–77. May 2, 2007.
15. Is fibromyalgia caused by a glycolysis impairment? Nutr Rev. 1994;52(7):248-250.
16. Park J.H., et al. Use of P-31 magnetic resonance spectroscopy to detect metabolic abnormalities in muscles of patients with fibromyalgia. Arthritis Rheum. 1998;41(3):406-413.
17. Bazzichi L., et al. ATP, calcium and magnesium levels in platelets of patients with primary fibromyalgia. Clin Biochem. 2008;41(13):1084-1090.
18. Werbach M. Nutritional influences on illness, 2nd edn. Tarzana: Third Line Press; 1996.
19. Eisinger J. Metabolic abnormalities in fibromyalgia. Clinical Bulletin of Myofascial Therapy. 1998;3(1):3-21.
20. Eisinger J., et al. Selenium and magnesium status in fibromyalgia. Magnes Res. 1994;7(3–4):285-288.
21. Abraham G.E. Management of fibromyalgia: rationale for the use of magnesium and malic acid. Journal of Nutritional Medicine. 1992;3:49-59.
22. Sendur O.F., et al. The relationship between serum trace element levels and clinical parameters in patients with fibromyalgia. Rheumatol Int. 2008;28(11):1117-1121.
23. Russell I.J., et al. Treatment of fibromyalgia syndrome with Super Malic: a randomized, double blind, placebo controlled, crossover pilot study. J Rheumatol. 1995;22(5):953-958.
24. Ali A., et al. Intravenous micronutrient therapy (Myers’ Cocktail) for fibromyalgia: a placebo-controlled pilot study. J Altern Complement Med. 2009;15(3):247-257.
25. Massey P.B. Reduction of fibromyalgia symptoms through intravenous nutrient therapy: Results of a pilot clinical trial. Altern Ther Health Med. 2007;13(3):32-34.
26. Xiaoyu Z., et al. 1alpha,25(OH)2-vitamin D3 membrane-initiated calcium signaling modulates exocytosis and cell survival. J Steroid Biochem Mol Biol. 2007;103(3-5):457-461.
27. Biswas P., Zanello L.P. 1alpha,25(OH)(2) vitamin D(3) induction of ATP secretion in osteoblasts. J Bone Miner Res. 2009;24(8):1450-1460.
28. Arvold D.S., et al. Correlation of symptoms with vitamin D deficiency and symptom response to cholecalciferol treatment: a randomized controlled trial. Endocr Pract. 2009;15(3):203-212.
29. Plotnikoff G.A., Quigley J.M. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463-1470.
30. Badsha H. Myalgias or non-specific muscle pain in Arab or Indo-Pakistani patients may indicate vitamin D deficiency. Clin Rheumatol. 2009;28(8):971-973.
31. Warner A.E., Arnspiger S.A. Diffuse musculoskeletal pain is not associated with low vitamin D levels or improved by treatment with vitamin D. J Clin Rheumatol. 2008;14(1):12-16.
32. Wilkins C.H., et al. Vitamin D deficiency is associated with worse cognitive performance and lower bone density in older African Americans. J Natl Med Assoc. 2009;101(4):349-354.
33. Armstrong D.J., et al. Vitamin D deficiency is associated with anxiety and depression in fibromyalgia. Clin Rheumatol. 2007;26(4):551-554.
34. Crew K.D., et al. Association between plasma 25-hydroxyvitamin D and breast cancer risk. Cancer Prev Res (Phila Pa). 2009;2(6):598-604.
35. Teitelbaum J.E. The use of D-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. J Altern Complement Med. 2006;12(9):857-862.
36. Schochat T., Raspe H. Elements of fibromyalgia in an open population. Rheumatology (Oxford). 2003;42(7):829-835.
37. Zubieta J.K., et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240-1243.
38. Russell I.J., et al. Serum amino acids in fibrositis/fibromyalgia syndrome. J Rheumatol Suppl. 1989;19:158-163.
39. Yunus M.B., et al. Plasma tryptophan and other amino acids in primary fibromyalgia: a controlled study. J Rheumatol. 1992;19(1):90-94.
40. Wolfe F., et al. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol. 1997;24(3):555-559.
41. Russell I.J., et al. Elevated cerebrospinal fluid levels of substance P in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994;37(11):1593-1601.
42. Caruso I., et al. Double-blind study of 5-hydroxytryptophan versus placebo in the treatment of primary fibromyalgia syndrome. J Int Med Res. 1990;18(3):201-209.
43. Sarzi Puttini P., Caruso I. Primary fibromyalgia syndrome and 5-hydroxy-L-tryptophan: a 90-day open study. J Int Med Res. 1992;20(2):182-189.
44. Kagan B.L., et al. Oral S-adenosylmethionine in depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 1990;147(5):591-595.
45. Tavoni A., et al. Evaluation of S-adenosylmethionine in primary fibromyalgia. A double-blind crossover study. Am J Med. 1987;83(5A):107-110.
46. Jacobsen S., Danneskiold-Samsoe B., Andersen R.B. Oral S-adenosylmethionine in primary fibromyalgia. Double-blind clinical evaluation. Scand J Rheumatol. 1991;20(4):294-302.
47. Bone K. A clinical guide to blending liquid herbs: herbal formulations for the individual patient. St Louis: Churchill Livingstone; 2003.
48. Grieves B. A modern herbal. Chatham: Jonathan Cape Ltd, 1973. (1931)
49. Chen Q.G., et al. The effects of Rhodiola rosea extract on 5-HT level, cell proliferation and quantity of neurons at cerebral hippocampus of depressive rats. Phytomedicine. 2009;16(9):830-838.
50. Hubner W.D. Hypericum treatment of mild depressions with somatic symptoms. J Geriatr Psychiatry Neurol. 1994;7(Suppl 1):S12-S14.
51. Ara I., Bano S. St. John’s Wort modulates brain regional serotonin metabolism in swim stressed rats. Pak J Pharm Sci. 2009;22(1):94-101.
52. Rahimi R. Efficacy and tolerability of Hypericum perforatum in major depressive disorder in comparison with selective serotonin reuptake inhibitors: a meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):118-127.
53. Linde K. St John’s wort for major depression. Cochrane Database Syst Rev. (4):2008. CD000448
54. Dygai A.M., et al. [The modulating effects of preparations of Baikal skullcap (Scutellaria baicalensis) on erythron reactions under conditions of neurotic exposures]. Eksp Klin Farmakol. 1998;61(1):37-39.
55. Korszun A. Sleep and circadian rhythm disorders in fibromyalgia. Curr Rheumatol Rep. 2000;2(2):124-130.
56. Crofford L.J., et al. Basal circadian and pulsatile ACTH and cortisol secretion in patients with fibromyalgia and/or chronic fatigue syndrome. Brain Behav Immun. 2004;18(4):314-325.
56. Klein D.C., et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307-357. discussion 357–358
58. Wikner J., et al. Fibromyalgia—a syndrome associated with decreased nocturnal melatonin secretion. Clin Endocrinol (Oxf). 1998;49(2):179-183.
59. Citera G., et al. The effect of melatonin in patients with fibromyalgia: a pilot study. Clin Rheumatol. 2000;19(1):9-13.
60. Matthews C.D. Human plasma melatonin and urinary 6-sulphatoxy melatonin: studies in natural annual photoperiod and in extended darkness. Clin Endocrinol (Oxf). 1991;35(1):21-27.
61. Griep E.N. Altered reactivity of the hypothalamic-pituitary-adrenal axis in the primary fibromyalgia syndrome. J Rheumatol. 1993;20(3):469-474.
62. Wingenfeld K., et al. The low-dose dexamethasone suppression test in fibromyalgia. J Psychosom Res. 2007;62(1):85-91.
63. Paiva E.S., et al. Impaired growth hormone secretion in fibromyalgia patients: evidence for augmented hypothalamic somatostatin tone. Arthritis Rheum. 2002;46(5):1344-1350.
64. Macedo J.A., et al. Glucocorticoid sensitivity in fibromyalgia patients: decreased expression of corticosteroid receptors and glucocorticoid-induced leucine zipper. Psychoneuroendocrinology. 2008;33(6):799-809.
65. Sun L.J., et al. [Effects of schisandra on the function of the pituitary-adrenal cortex, gonadal axes and carbohydrate metabolism in rats undergoing experimental chronic psychological stress, navigation and strenuous exercise]. Zhonghua Nan Ke Xue. 2009;15(2):126-129.
66. Panossian A., et al. Adaptogens exert a stress-protective effect by modulation of expression of molecular chaperones. Phytomedicine. 2009;16(6–7):617-622.
67. Filaretov A.A., et al. Role of pituitary-adrenocortical system in body adaptation abilities. Exp Clin Endocrinol. 1988;92(2):129-136.
68. Almansa C., et al. Prevalence of functional gastrointestinal disorders in patients with fibromyalgia and the role of psychologic distress. Clin Gastroenterol Hepatol. 2009;7(4):438-445.
69. Wallace D.J., Hallegua D.S. Fibromyalgia: the gastrointestinal link. Curr Pain Headache Rep. 2004;8(5):364-368.
70. Crowell M.D. Role of serotonin in the pathophysiology of the irritable bowel syndrome. Br J Pharmacol. 2004;141(8):1285-1293.
71. Goebel A., et al. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology (Oxford). 2008;47(8):1223-1227.
72. Pimentel M. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95(12):3503-3506.
73. Lauritano E.C., et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am J Gastroenterol. 2008;103(8):2031-2205.
74. Spaeth G. Fibre is an essential ingredient of enteral diets to limit bacterial translocation in rats. Eur J Surg. 1995;161(7):513-518.
75. Evans M.A., Shronts E.P. Intestinal fuels: glutamine, short-chain fatty acids, and dietary fiber. J Am Diet Assoc. 1992;92(10):1239-1246. 1249
76. Xu D. Elemental diet-induced bacterial translocation associated with systemic and intestinal immune suppression. J Parenter Enteral Nutr. 1998;22(1):37-41.
77. Aydogan A., et al. Effects of various enteral nutrition solutions on bacterial translocation and intestinal morphology during the postoperative period. Adv Ther. 2007;24(1):41-49.
78. Kang J.X., Weylandt K.H. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell Biochem. 2008;49:133-143.
79. Enestrom S. Dermal IgG deposits and increase of mast cells in patients with fibromyalgia—relevant findings or epiphenomena? Scand J Rheumatol. 1997;26(4):308-313.
80. Baraniuk J.N., et al. Nasal secretion analysis in allergic rhinitis, cystic fibrosis, and nonallergic fibromyalgia/chronic fatigue syndrome subjects. Am J Rhinol. 1998;12(6):435-440.
81. Zuo X.L., et al. Alterations of food antigen-specific serum immunoglobulins G and E antibodies in patients with irritable bowel syndrome and functional dyspepsia. Clin Exp Allergy. 2007;37(6):823-830.
82. Arroyave Hernandez C.M. Food allergy mediated by IgG antibodies associated with migraine in adults. Rev Alerg Mex. 2007;54(5):162-168.
83. Zar S., et al. Food-specific IgG4 antibody-guided exclusion diet improves symptoms and rectal compliance in irritable bowel syndrome. Scand J Gastroenterol. 2005;40(7):800-807.
84. Jeschonneck M., et al. Abnormal microcirculation and temperature in skin above tender points in patients with fibromyalgia. Rheumatology (Oxford). 2000;39(8):917-921.
85. McIver K.L., et al. NO-mediated alterations in skeletal muscle nutritive blood flow and lactate metabolism in fibromyalgia. Pain. 2006;120(1–2):161-169.
86. Guedj E., et al. Clinical correlate of brain SPECT perfusion abnormalities in fibromyalgia. J Nucl Med. 2008;49(11):1798-1803.
87. Lister R.E. An open, pilot study to evaluate the potential benefits of coenzyme Q10 combined with Ginkgo biloba extract in fibromyalgia syndrome. J Int Med Res. 2002;30(2):195-199.
88. Mizuno K., et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24(4):293-299.
89. Tsukahara Y. Antioxidant role of endogenous coenzyme Q against the ischemia and reperfusion-induced lipid peroxidation in fetal rat brain. Acta Obstet Gynecol Scand. 1999;78(8):669-674.
90. Sandor P.S., et al. Efficacy of coenzyme Q10 in migraine prophylaxis: a randomized controlled trial. Neurology. 2005;64(4):713-715.
91. Zhang J., et al. The therapeutic effect of Ginkgo biloba extract in SHR rats and its possible mechanisms based on cerebral microvascular flow and vasomotion. Clin Hemorheol Microcirc. 2000;23(2–4):133-138.
92. Chen J., et al. Effects of Ginkgo biloba extract on number and activity of endothelial progenitor cells from peripheral blood. J Cardiovasc Pharmacol. 2004;43(3):347-352.
93. Bell I.R., et al. Improved clinical status in fibromyalgia patients treated with individualized homeopathic remedies versus placebo. Rheumatology (Oxford). 2004;43(5):577-582.
94. Relton C., et al. Healthcare provided by a homeopath as an adjunct to usual care for fibromyalgia (FMS): results of a pilot randomised controlled trial. Homeopathy. 2009;98(2):77-82.
95. Maciocia G. The practice of Chinese medicine: the treatment of diseases with acupuncture and Chinese herbs. London: Churchill Livingstone; 1994.
96. Sprott H., et al. [Microcirculatory changes over the tender points in fibromyalgia patients after acupuncture therapy (measured with laser-Doppler flowmetry)]. Wien Klin Wochenschr. 2000;112(13):580-586.
97. Yoshimoto K., et al. Acupuncture stimulates the release of serotonin, but not dopamine, in the rat nucleus accumbens. Tohoku J Exp Med. 2006;208(4):321-326.
98. Tu W.Z., et al. [Effect of electroacupuncture of local plus distal acupoints in the same segments of spinal cord on spinal substance P expression in rats with chronic radicular pain]. Zhen Ci Yan Jiu. 2008;33(1):7-12.
99. Schneider A., et al. Neuroendocrinological effects of acupuncture treatment in patients with irritable bowel syndrome. Complement Ther Med. 2007;15(4):255-263.
100. Cheng R., et al. Electroacupuncture elevates blood cortisol levels in naive horses; sham treatment has no effect. Int J Neurosci. 1980;10(2–3):95-97.
101. Liao Y.Y., et al. Effect of acupuncture on adrenocortical hormone production: I. Variation in the ability for adrenocortical hormone production in relation to the duration of acupuncture stimulation. Am J Chin Med. 1979;7(4):362-371.
102. Li C.D., et al. [Clinical study on combination of acupuncture, cupping and medicine for treatment of fibromyalgia syndrome]. Zhongguo Zhen Jiu. 2006;26(1):8-10.
103. Acupuncture. NIH consensus statement online 1997 Nov 3-5;15(5):1–34.
104. Busch A.J., et al. Exercise for fibromyalgia: a systematic review. J Rheumatol. 2008;35(6):1130-1144.
105. Richards S.C., Scott D.L. Prescribed exercise in people with fibromyalgia: parallel group randomised controlled trial. BMJ. 2002;325(7357):185.
106. Valim V., et al. Aerobic fitness effects in fibromyalgia. J Rheumatol. 2003;30(5):1060-1109.
107. Gowans S.E., deHueck A. Pool exercise for individuals with fibromyalgia. Curr Opin Rheumatol. 2007;19(2):168-173.
108. Field T., et al. Fibromyalgia pain and substance P decrease and sleep improves after massage therapy. J Clin Rheumatol. 2002;8(2):72-76.
109. McCauley J., et al. The ‘battering syndrome’: prevalence and clinical characteristics of domestic violence in primary care internal medicine practices. Ann Intern Med. 1995;123(10):737-746.
110. Mas A.J., et al. Prevalence and impact of fibromyalgia on function and quality of life in individuals from the general population: results from a nationwide study in Spain. Clin Exp Rheumatol. 2008;26(4):519-526.
111. de Souza J.B., et al. The deficit of pain inhibition in fibromyalgia is more pronounced in patients with comorbid depressive symptoms. Clin J Pain. 2009;25(2):123-127.
112. Beal C.C., Stuifbergen A.K., Brown A. Predictors of a health promoting lifestyle in women with fibromyalgia syndrome. Psychol Health Med. 2009;14(3):343-353.
113. Sephton S.E., et al. Mindfulness meditation alleviates depressive symptoms in women with fibromyalgia: results of a randomized clinical trial. Arthritis Rheum. 2007;57(1):77-85.
114. Menzies V. Effects of guided imagery on outcomes of pain, functional status, and self-efficacy in persons diagnosed with fibromyalgia. J Altern Complement Med. 2006;12(1):23-30.
115. Novati A., et al. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. Sleep. 2008;31(11):1579-1585.
116. Ekici G., et al. Comparison of manual lymph drainage therapy and connective tissue massage in women with fibromyalgia: a randomized controlled trial. J Manipulative Physiol Ther. 2009;32(2):127-133.
117. Kalueff A.V., et al. Increased anxiety in mice lacking vitamin D receptor gene. Neuroreport. 2004;15(8):1271-1274.
118. Kelly G.S. Rhodiola rosea: a possible plant adaptogen. Altern Med Rev. 2001;6(3):293-302.
119. Fintelmann V., Gruenwald J. Efficacy and tolerability of a Rhodiola rosea extract in adults with physical and cognitive deficiencies. Adv Ther. 2007;24(4):929-939.
120. Spasov A.A., et al. A double-blind, placebo-controlled pilot study of the stimulating and adaptogenic effect of Rhodiola rosea SHR-5 extract on the fatigue of students caused by stress during an examination period with a repeated low-dose regimen. Phytomedicine. 2000;7(2):85-89.
121. Birks J., Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. Cochrane Database Syst Rev. (1):2009;. CD003120
122. Shah Z.A. Ginkgo biloba normalises stress-elevated alterations in brain catecholamines, serotonin and plasma corticosterone levels. Eur Neuropsychopharmacol. 2003;13(5):321-325.
123. Biddlestone L. Oral administration of Ginkgo biloba extract, EGb-761 inhibits thermal hyperalgesia in rodent models of inflammatory and post-surgical pain. Br J Pharmacol. 2007;151(2):285-291.
124. Huang F., et al. Sedative and hypnotic activities of the ethanol fraction from Fructus schisandrae in mice and rats. J Ethnopharmacol. 2007;110(3):471-475.
125. Panossian A., Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. J Ethnopharmacol. 2008;118(2):183-212.
126. Lee S.B., et al. Induction of the phase II detoxification enzyme NQO1 in hepatocarcinoma cells by lignans from the fruit of Schisandra chinensis through nuclear accumulation of Nrf2. Planta Med. 2009. In press
127. Calle J., et al. Antinociceptive and uterine relaxant activities of Viburnum toronis alive (Caprifoliaceae). J Ethnopharmacol. 1999;66(1):71-73.
128. Zayachkivska O.S., et al. Influence of Viburnum opulus proanthocyanidins on stress-induced gastrointestinal mucosal damage. J Physiol Pharmacol. 2006;57(Suppl 5):155-167.
129. Uchida S., et al. Antinociceptive effects of St. John’s wort, Harpagophytum procumbens extract and grape seed proanthocyanidins extract in mice. Biol Pharm Bull. 2008;31(2):240-245.
130. Guginski G., et al. Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol Biochem Behav. 2009;93(1):10-16.
131. Awad R., et al. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother Res. 2009;23(8):1075-1081.
132. Soulimani R., et al. Neurotropic action of the hydroalcoholic extract of Melissa officinalis in the mouse. Planta Med. 1991;57(2):105-109.
133. Sadraei H. Relaxant effect of essential oil of Melissa officinalis and citral on rat ileum contractions. Fitoterapia. 2003;74(5):445-452.
134. Mayhew E., Ernst E. Acupuncture for fibromyalgia—a systematic review of randomized clinical trials. Rheumatology (Oxford). 2007;46(5):801-804.