Chapter 9 Fetal Surveillance during Labor
 Methods of Monitoring Fetal Heart Rate
Methods of Monitoring Fetal Heart Rate
CONTINUOUS ELECTRONIC FETAL MONITORING
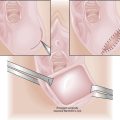
EFM allows continuous reporting of the FHR and uterine contractions (FHR-UC) by means of a monitor that prints results on a two-channel strip chart recorder. Uterine contractions result in a reduction in blood flow to the placenta, which can cause decreased fetal oxygenation and corresponding alterations in the FHR. The FHR-UC record can be obtained using external transducers that are placed on the maternal abdomen. This technique is used in early labor. Internal monitoring is carried out by placing a spiral electrode onto the fetal scalp to monitor heart rate and placing a plastic catheter transcervically into the amniotic cavity to monitor uterine contractions (Figure 9-1). To carry out this technique, the fetal membranes must be ruptured, and the cervix must be dilated to at least 2 cm.
 Etiology of Hypoxia, Acidosis, and Fetal Heart Rate Changes
Etiology of Hypoxia, Acidosis, and Fetal Heart Rate Changes
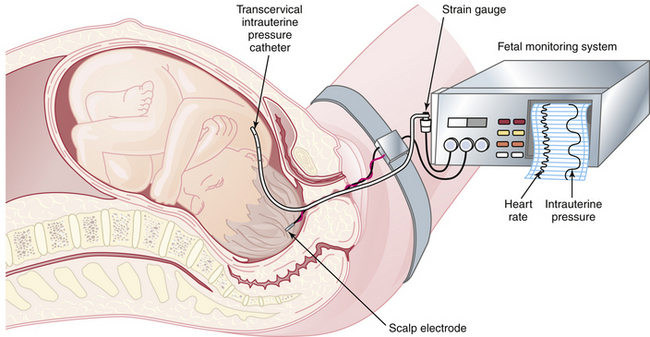
Fetal oxygenation can be impaired at different anatomic locations within the uteroplacental-fetal circulatory loop. For example, impairment of oxygen transportation to the intervillous space may occur as a result of maternal hypertension or anemia; oxygen diffusion may be impaired in the placenta because of infarction or abruption; or the oxygen content in the fetal blood may be impaired because of hemolytic anemia in Rh-isoimmunization. Figure 9-2 summarizes the clinical conditions that may be associated with fetal distress during labor.
FETAL HEART RATE PATTERNS
Baseline Assessment
This requires determination of the rate (in beats per minute) and the variability. Normal and abnormal rates are listed in Table 9-1. Baseline variability can be divided into short-term and long-term intervals. These are described as follows:
| Rate | Beats/Minute |
|---|---|
| Normal | 120-160 |
| Abnormal | |
| Tachycardia | >160 |
| Bradycardia | <120 |
Types of Patterns
EARLY DECELERATION (HEAD COMPRESSION)
This pattern usually has an onset, maximum fall, and recovery that are coincident with the onset, peak, and end of the uterine contraction (Figure 9-3). The nadir of the FHR coincides with the peak of the contraction. This pattern is seen when engagement of the fetal head has occurred. Early decelerations are not thought to be associated with fetal distress. The pressure on the fetal head leads to increased intracranial pressure that elicits a vagal response similar to the Valsalva maneuver in the adult. The vagal reflex can be abolished by the administration of atropine, but this approach is not used clinically.
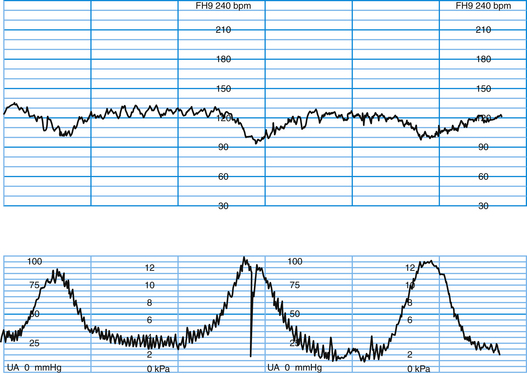
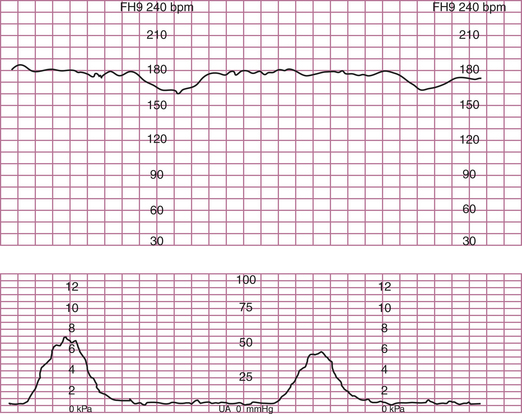
LATE DECELERATION (UTEROPLACENTAL INSUFFICIENCY)
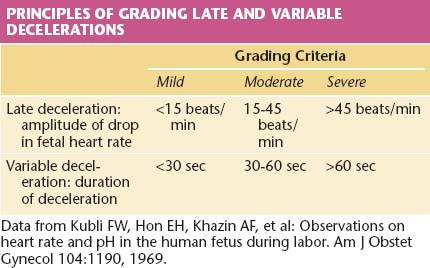
This pattern has an onset, maximal decrease, and recovery that are shifted to the right in relation to the contraction (Figure 9-4). The severity of late decelerations is graded by the magnitude of the decrease in FHR at the nadir of the deceleration (Table 9-2). Fetal hypoxia and acidosis are usually more pronounced with severe decelerations. Severe repetitive late decelerations usually indicate fetal metabolic acidosis, low arterial pH, and increased base deficit values. The partial pressure of carbon dioxide (PCO2) in the fetal blood is usually in the normal range, and the fetal blood oxygen partial pressure (PO2) is only slightly below normal because of the Bohr effect—the shift to the left of the oxygen dissociation curve caused by the acidosis.
VARIABLE DECELERATION (CORD COMPRESSION)
The severity of variable decelerations is graded by their duration (see Table 9-2). When the FHR falls below 80 beats/minute during the nadir of the deceleration, there is usually a loss of the P wave in the fetal electrocardiogram, indicating a nodal rhythm or a second-degree heart block.
 Strategies for Intervention
Strategies for Intervention
LATE DECELERATIONS
 Fetal Blood Sampling
Fetal Blood Sampling
Fetal scalp blood sampling for pH determination has been used when clinical parameters, such as heavy meconium, are present or when FHR patterns are suggestive of acidosis, but is not the standard of practice in many centers. Fetal scalp pH correctly predicts neonatal outcome in 82% of cases as determined by the Apgar score. The false-positive rate is about 8% and the false-negative rate about 10%. Blood is obtained from the fetus by placing an amnioscope transvaginally against the fetal skull (Figure 9-5). Cervical mucus is removed with cotton swabs. Silicone grease is applied to the skull for blood bead formation. A 2 × 2-mm lancet is used for a stab incision, and a drop of blood is aspirated into a long heparinized capillary tube.
UMBILICAL CORD BLOOD SAMPLING
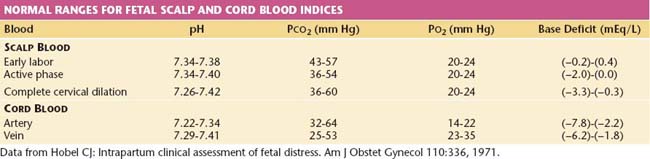
The Apgar scoring system has been classically used to assess the newborn condition. Over time, however, the Apgar score has come to be used inappropriately to define asphyxia, which is a misapplication, because many other conditions (e.g., prematurity, maternal drug administration) can result in low scores that are not reflective of asphyxia. Asphyxia implies hypoxia of sufficient degree to cause metabolic acidosis. Thus, the Apgar score alone cannot be used to define asphyxia. A more appropriate tool for defining this condition is assessment of the fetal and neonatal acid-base status. Normal ranges for these indices are given in Table 9-3. One reasonable protocol for umbilical cord blood pH and blood gas analysis is as follows:
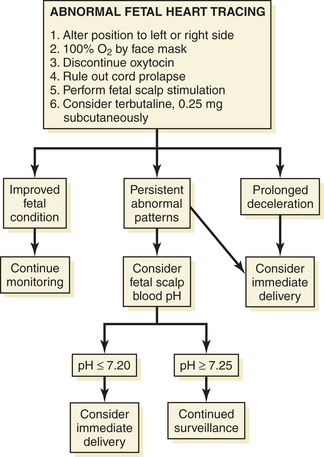
Newborn cerebral dysfunction, manifested as seizures and attributable to true birth asphyxia, does not seem to occur unless the Apgar score at 5 minutes is 3 or less, the umbilical artery blood pH is less than 7, and resuscitation is necessary at birth. The later onset of cerebral palsy can occur without these abnormalities and may be attributed to untoward events occurring earlier in the pregnancy or intrapartum infection in which various cytokines can affect the fetal brain. The impact of lesser degrees of asphyxia, as measured by the Apgar score and acid-base status at birth, requires further study. Figure 9-6 is an algorithm for the management of abnormal heart rate tracings during fetal monitoring.
American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Intrapartum Fetal Heart Rate Monitoring. Obstet Gynecol. 2005;106:1453-1461.
Liston R., Sawchuck D., Young D. Society of Obstetricians and Gynecologists of Canada: British Columbia Perinatal Health Program. Fetal health surveillance guideline: Antenatal and intrapartum consensus. [Erratum in: J Obstet Gynaecol Can 29:909, 2007.]. J Obstet Gynaecol Can. 2007;29:S3-S56.
Olagundoye V., Black R., Mackenzie I.Z. Impact of the severity of fetal distress on decision-to-delivery intervals for assisted vaginal delivery. J Obstet Gynaecol. 2008;28:51-55.
Parer J.T., Ideda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 197, 2007. 26e1–26e6
Wilson R.D. Fetal health surveillance guideline: Antenatal and intrapartum consensus. J Obstet Gynaecol Can. 2007;29:912.

 Meconium
Meconium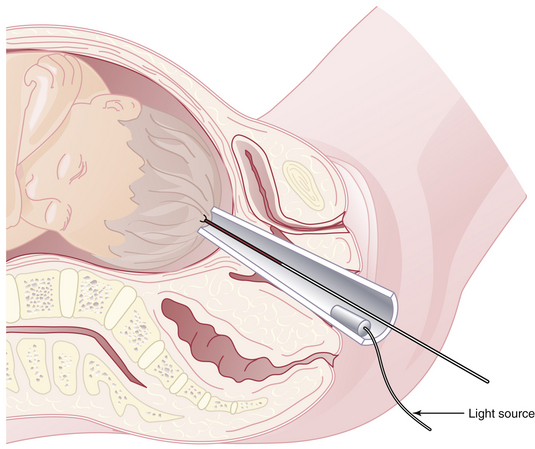
 Complications of Fetal Monitoring
Complications of Fetal Monitoring Controversies about Fetal Monitoring in the Diagnosis and Treatment of Fetal Distress
Controversies about Fetal Monitoring in the Diagnosis and Treatment of Fetal Distress