37 Fetal Intervention and the EXIT Procedure
A Range of Anesthetic Options for Mother and Fetus
Physiologic Consequences of Pregnancy
Tocolysis and Tocolytic Agents
Congenital Cystic Adenomatoid Malformation: The Open Procedure
Other Diseases Eligible for Open Procedures
Hypoplastic Left Heart Syndrome: Percutaneous and Fetoscopic Procedures
Other Diseases Eligible for Fetoscopic Procedures
Movement toward Intervention for Non–Life-Threatening Diseases: Myelomeningocele
THE ADVENT OF FETAL intervention introduced the concept of surgically correcting a known congenital defect in utero to avoid certain fetal demise. With improvements in prenatal imaging and surgical techniques, fetal interventions have grown to include diagnoses associated with intrauterine demise, as well as diseases associated with significant postnatal morbidity. The goal of fetal intervention is to improve the chances of normal fetal development and minimize postnatal morbidity.1 Advances have changed some procedures from open in-utero interventions, which are associated with significant maternal risk, to percutaneous or fetoscopic techniques, thus improving the maternal risk-to-benefit ratio while diminishing postoperative uterine contractions associated with open procedures.
A Range of Anesthetic Options for Mother and Fetus
Mother
Fetal interventions have been successfully performed with various anesthetic techniques; both maternal and fetal anesthetic requirements must be considered and may, in fact, be quite different. With some endoscopic interventions, the site of surgical intervention is not innervated; thus the fetus may not sense a noxious stimulus, and its anesthetic requirements are presumably minimal. Nevertheless, fetal immobility remains essential to procedural safety and success. Other interventions may require that a needle be inserted into the fetus, which may elicit a noxious stimulus and possibly even cause pain. Open procedures can produce significant noxious stimuli. In addition to surgical demands, each mother and fetus exhibit a unique physiologic, pharmacologic, and pathophysiologic profile; the anesthesiologist must evaluate the advantages and disadvantages of each anesthetic technique and select the safest anesthetic.2
Regional Neuraxial Blockade with Sedation
The addition of IV sedation to regional anesthesia may provide the fetus with analgesia/anesthesia via placental drug transfer. Although IV fentanyl, propofol, and benzodiazepines can be administered to patients receiving regional anesthesia, they may place the mother at increased risk of bradyarrhythmias, respiratory depression, and pulmonary aspiration; the need for a T4 sensory block may produce alterations in respiratory mechanics additive to those caused by pregnancy. In addition, the level of sympathetic blockade is often two to six levels greater than the sensory level.3 Hence, a T4 sensory block may completely block cardiac accelerator fibers (T1 to T4); severe bradyarrhythmias and cardiac arrest have been reported.4–6 When IV agents with vagotonic properties are administered in this clinical setting, the risk of significant bradyarrhythmias may be increased.7
General Anesthesia
General anesthesia with inhalational anesthetics provides both maternal and fetal anesthesia and dose-dependent uterine relaxation even in patients who have received tocolytic therapy for preoperative premature uterine contractions.8–11
Combined Regional and General Anesthesia
A combined regional and general anesthesia technique is often used for open procedures, as well as for patients with anterior placentas, in whom externalization of the uterus for safe trocar insertion is anticipated. In addition to providing the advantages of both the regional and the general anesthetic techniques listed previously, this method allows for planned postoperative pain control.12 The physical window for trocar insertion is often smaller in this patient cohort, necessitating either externalization of the uterus or extreme lateral decubitus position. Externalization of the uterus requires a larger surgical incision than for standard cesarean sections.
Fetus
Transplacental Access
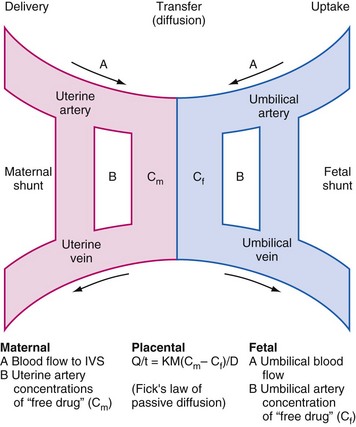
Many fetal interventions (open or endoscopic) employ transplacental drug administration to provide anesthesia and analgesia for both mother and fetus. Many, but not all, drugs cross the placenta via Fick’s law of passive diffusion (Fig. 37-1). Lipid solubility, the pH of both maternal and fetal blood, the degree of ionization, protein binding, perfusion, placental area and thickness, and drug concentration are factors that influence the extent of transplacental drug diffusion.13 The most obvious disadvantage with this approach is that the mother must be exposed to every drug that the fetus is intended to receive, often at large concentrations, to achieve adequate drug concentrations in the fetus. In addition, the uptake of drugs may be impaired if there is reduced placental blood flow. This has implications for successful anesthesia and analgesia both in terms of the delivered fetal dose and the time interval that must be allowed from maternal administration to the start of the fetal intervention. All inhaled anesthetics cross the placental barrier, but uptake in the fetus is slower than in the mother.13 However, this is offset by the reduced minimal alveolar concentration (MAC) for anesthesia in the fetus, resulting in a similar onset of anesthesia as in the mother.2 Fetal anesthesia is also important to reduce the fetal stress response, which, through catecholamine release, can reduce placental blood flow and exacerbate any asphyxia.14–17
Intravascular Access
Intravascular fetal drug administration ensures immediate drug levels, and no additional dosing calculations are necessary because placental perfusion does not significantly alter dosing. Intravascular access can be obtained via the umbilical cord (which is not innervated), larger fetal veins (e.g., hepatic vein), or intracardiac, as the specific intervention dictates.18 One advantage of administering drugs via the umbilical vein is the ability to provide analgesia before the surgical insult. Muscle relaxants, analgesics, and vagolytic agents, as well as resuscitation drugs, can be given with assurance of immediate access to the fetal circulation. This method is also useful when alterations in peripheral blood flow occur (i.e., a “central sparing response”), which significantly diminishes the blood distribution to sites of potential IM access.
Intraamniotic Access
Intraamniotic fentanyl, sufentanil, thyroxine, vasopressin, and digoxin have been safely administered in pregnant large-animal models, with only minimal drug detected in the mother.19,20 If the safety and efficacy of this method of drug delivery hold true in human trials, intraamniotic drug administration may become the preferred method for fetal drug delivery.
Fetal Development
Pathologic Lung Development
In the context of fetal interventions, there are two important causes of respiratory morbidity to consider: insufficient amniotic fluid and prematurity. With both, the timing of the insult in terms of the stage of lung development is critical to estimating the degree of likely morbidity. Deficiency of amniotic fluid may result from prelabor premature rupture of the amniotic membranes (PPROM), which may be spontaneous or iatrogenically induced either directly through trauma or by introducing infection into the uterus. Small amniotic fluid volume may also be secondary to reduced fetal urine output, from either poor renal function (e.g., with renal agenesis or urinary tract obstruction) or growth restriction secondary to placental insufficiency. Amniotic fluid deficiency contributes to pulmonary hypoplasia. In general, the likelihood of pulmonary insufficiency is inversely related to gestation at membrane rupture, a long latency to delivery, and the amount of residual amniotic fluid.21–23 The risk is relatively small if PPROM occurs after 24 weeks gestation,24 as demonstrated by one series of fetuses with PPROM before 26 weeks reporting pulmonary hypoplasia in 27% of fetuses.25 In contrast, with severe oligohydramnios of more than 2 weeks duration after PPROM arising before 25 weeks gestation, the predicted neonatal mortality exceeds 90%.26
Studies in sheep show that oligohydramnios causes spinal flexion, which compresses the abdominal contents, displacing the diaphragm upward and thus compressing the developing lungs.27 This increase in the pressure gradient between the lungs and the amniotic cavity causes a net loss of lung fluid through the trachea, preventing lung expansion.27 Lung fluid produced in the airways is thought to act as a stent for the developing lungs.28 Normally, it passes out through the trachea and is either swallowed or passes into the amniotic cavity. Ligation of the trachea causes lung hyperplasia29 or ipsilateral lung hyperplasia if a main bronchus is ligated.30 Experimental drainage of amniotic fluid in animals has been shown to result in pulmonary hypoplasia.31 Later restoration of amniotic fluid prevents the onset of pulmonary hypoplasia.32 There is evidence to support amnioinfusion in humans to maintain fluid volumes around the fetus after PPROM, in an effort to improve lung development.26,33
Surfactant is a complex of phospholipids secreted by type II alveolar cells, which reduces lung surface air tension, thereby preventing the lungs from collapsing at low volumes. Glucocorticoids, thyroid hormone, and β-adrenergic agonists stimulate surfactant synthesis. It is first detected in the lungs around 23 weeks gestation, but mature levels necessary for unassisted ventilation are not present until about 34 weeks. The degree of lung maturity can be evaluated by amniocentesis using the lecithin to sphingomyelin ratio or, more recently, by the lamellar body count.34 Acceleration of surfactant synthesis may be achieved with corticosteroids administered to the mother.35
FETAL Cardiovascular Development
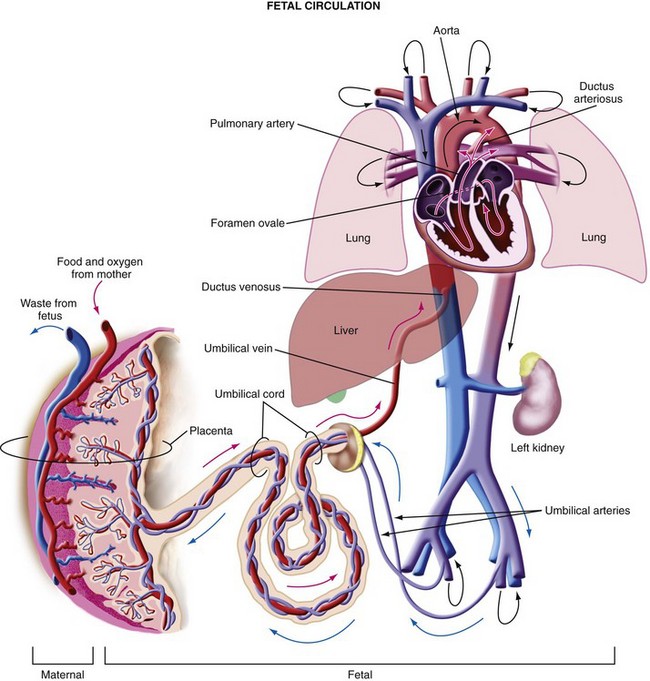
The differences between the fetal and postnatal circulations are complex (Fig. 37-2). In the fetal circulation, oxygenated blood returns from the placenta via the umbilical veins and ductus venosus (bypassing the liver) into the right atrium. At 20 weeks, 30% of the umbilical venous return (40 to 60 mL/kg/min) is shunted through the ductus venosus.36 This flow decreases over the second half of gestation as hepatic blood flow increases so that, by term only 20% of umbilical venous return (less than 20 mL/kg/min) is shunted through the ductus venosus.36 Hypoxia and hemorrhage increase the resistance in the liver, shunting a greater proportion of blood toward the brain and heart via the ductus venosus.37 The proportion of blood that perfuses the liver, which exits 15% less saturated in oxygen, rejoins the ductus venosus blood in the inferior vena cava. However, this deoxygenated blood has less kinetic energy and flows more slowly into the right atrium toward the right ventricle.37 The greater-velocity oxygenated blood from the ductus venosus is preferentially directed through the foramen ovale into the left side of the heart and out via the aortic arch to the developing head and upper body. The integrity of the foramen ovale is thus imperative. Blood returning from the placenta along the umbilical vein is 80% to 85% saturated. Despite this streaming within the right atrium, some mixing does occur, resulting in blood that is 65% saturated in the ascending aorta. The blood in the left ventricle, however, is 15% to 20% more saturated than the blood in the right ventricle. Most of the deoxygenated blood in the right ventricle bypasses the high-resistance pulmonary vasculature to enter the ductus arteriosus, and from there the descending aorta to supply the lower body, or pass via the umbilical arteries for reoxygenation in the placenta. In contrast to extrauterine life, when the two ventricles function in series and thus have equal outputs, before birth they function in parallel. Their outputs, therefore, do not have to be equal and, in fact, are not. In the third trimester, the right side of the heart has a greater output, as determined by Doppler ultrasonography studies, showing a 28% greater stroke volume than the left side.36
Fetal heart rate (FHR) is maintained above the intrinsic rate of the sinoatrial node by a combination of vagal and sympathetic inputs, as well as circulating catecholamines.38–40 FHR decreases throughout gestation,41,42 accompanied by an increase in stroke volume as the heart grows. Hypoxic stress in late gestation produces a reflex bradycardia, with a normal heart rate or tachycardia developing a few minutes later. The chemoreceptor reflex nature of the bradycardia is demonstrated by its abolition after section of the carotid sinus nerves.43 The later tachycardia is a result of an increase in plasma catecholamines causing β-adrenergic stimulation.44 Hemorrhage can also produce increases in FHR, probably via a baroreceptor reflex.
Cardiac output in the fetus is determined largely by heart rate.45 The combined ventricular output of the left and right ventricles in the human fetus is 450 mL/kg/min.46 During development, the ability of the fetus to increase stroke volume is limited by a reduced proportion of functioning contractile tissue and a limited ability to increase the heart rate because of a relatively reduced β-adrenergic receptor density and immature sympathetic drive. Thus if blood volume is reduced by hemorrhage, the heart cannot compensate by increasing stroke volume, or, conversely, if volume is increased, the walls are less able to distend and cardiac efficiency is reduced (although this second effect is reduced substantially by the huge, relatively compliant placental circulation). Thus the only way for the fetus to increase cardiac output is to increase heart rate. Despite this homeostatic limitation, the fetus is able to withstand significant hemorrhage. Sheep studies have shown that the fetal lamb can restore arterial blood pressure and heart rate very quickly, without any measurable disturbance in acid-base balance after acute loss of 20% of their blood volume.47 Even after a 40% reduction in blood volume, the ovine fetal blood pressure recovers to normal within 2 minutes and the heart rate within 35 minutes.48 Oxygen delivery to the brain and heart is maintained secondary to vascular redistribution (central sparing effect) and blood volume replacement from the placenta and extravascular space, with 40% of the hemorrhaged loss being corrected within 30 minutes.48 The development of acidemia indicates that the fetus is not able to compensate; acidosis shifts the oxygen dissociation curve to the right, thereby decreasing fetal hemoglobin oxygen saturation but improving release of oxygen from hemoglobin. Blood flow during periods of asphyxia increases more than 100% to the brainstem, but only 60% to the cerebral hemispheres.49
FETAL Oxygenation
The fetus exists in an environment of low oxygen tension, with arterial oxygen partial pressure (Po2) being approximately one-fourth that of the adult. The maximum Po2 of umbilical venous blood is approximately 30 mm Hg. The affinity of fetal hemoglobin for oxygen is modulated in utero by two principal factors: fetal hemoglobin and 2,3-diphosphoglycerate (2,3-DPG). The hemoglobin oxygen dissociation curve is shifted to the left because of fetal hemoglobin (hemoglobin F), thereby increasing the affinity for oxygen. 2,3-DPG is also present and might be expected to shift the oxyhemoglobin dissociation curve to the right, decreasing the affinity of the fetal hemoglobin for oxygen and favoring oxygen unloading. However, 2,3-DPG only appears to exert approximately 40% of the effect on fetal hemoglobin as it does on adult hemoglobin, thereby preserving a net leftward shift on the oxyhemoglobin dissociation curve. Thus for any given Po2, the fetus has a greater affinity for oxygen than does the mother. The P50 (the Po2 at which hemoglobin is 50% desaturated) is approximately 27 mm Hg for the adult and 20 mm Hg for the fetus. The concentration of 2,3-DPG increases with gestation, as does the concentration of hemoglobin A50; the greater hemoglobin concentration (18 g/dL) results in a greater total oxygen carrying capacity.
Oxygen supply to fetal tissues depends on a number of factors (Table 37-1). First, the mother must be adequately oxygenated. Second, there must be adequate flow of well-oxygenated blood to the uteroplacental circulation. This blood flow may be reduced from maternal hemorrhage (reduced maternal blood volume) or compression of the inferior vena cava (reduced venous return), which increases uterine venous pressure, thus reducing uterine perfusion. Additionally, aortic compression reduces uterine arterial blood flow.51 Care must be taken to position the mother in such a way as to prevent aortocaval compression. The surgical incision of hysterotomy itself reduces uteroplacental blood flow by as much as 73% in sheep, whereas fetoscopic procedures with uterine entry have no effect.52
TABLE 37-1 Causes of Impaired Blood Flow and Oxygenation to Fetal Tissues
| Causes of Impaired Uteroplacental Blood Flow/Oxygenation | Causes of Impaired Umbilical Blood Flow/Fetal Circulatory Redistribution |
|---|---|
| Reduced maternal oxygenation/hemoglobin concentration | Umbilical vessel spasm |
| Maternal hemorrhage | Reduced fetal cardiac output |
| Aortocaval compression | Fetal hemorrhage/reduced hemoglobin concentration |
| Drugs reducing uterine blood flow | Fetal hypothermia |
| Uterine trauma | Impaired uteroplacental blood flow/oxygenation |
| Uterine contractions | Umbilical cord kinking |
| Placental insufficiency (PET, IUGR) | |
| Polyhydramnios: pressure effect | |
| Maternal catecholamine production increasing uteroplacental vascular resistance | Fetal catecholamine production increasing fetoplacental vascular resistance |
IUGR, Intrauterine growth restriction; PET, preeclamptic toxemia.
Even if the uterine circulation is adequate, the fetus still depends on uteroplacental blood flow and umbilical venous blood flow for tissue oxygenation. Care must be taken not to interrupt umbilical vessel blood flow by manipulation or kinking the cord, which can cause vasospasm. Umbilical vasoconstriction can also occur as part of a fetal stress reaction resulting from a release of fetal stress hormones (Fig. 37-3). Increases in amniotic fluid volume increase amniotic pressure and impair uteroplacental perfusion.53,54 Placental vascular resistance can be increased, raising fetal cardiac afterload, by the surge in fetal catecholamine production stimulated by surgical stress.55 Fortunately, animal studies suggest that adverse effects on the arterial blood gas in the fetus do not occur until uteroplacental perfusion has been reduced by 50% or more.56
Inhalational anesthetics may cause maternal vasodilatation and, thus, in theory, could cause or exacerbate preexisting fetal hypoxia. Studies of anesthetics in hypoxic ovine fetuses have shown that isoflurane exacerbates preexisting acidosis.57 It also causes blunting of the usual vascular redistribution response to fetal hypoxia, but, owing to a reduction in cerebral oxygen demand, the balance of cerebral oxygen supply and demand is unaffected. β-Adrenergic blockade renders a fetus less able to cope with asphyxia. Compared with controls, these fetuses have a smaller increase in heart rate, cerebral blood flow, and cardiac output, and recover from acidosis more slowly.58
Central and Peripheral Nervous System Development
By the beginning of the second trimester, the spinal cord is largely formed; development of the brain and spinal cord begins as early as postconception week 3. Neural crest cells migrate laterally to form peripheral nerves from about 4 weeks, with the first synapses between them forming a week later.59 Synapses within the spinal cord develop from about 8 weeks gestation, suggesting the first spinal reflexes may be present at this time. Between 8 and 18 weeks gestation is the time of maximal neuronal development. The first neurons and glial cells develop in the ventricular zone (an epithelial layer) along which the newly formed neurons migrate out in waves to form the neocortex. Synaptogenesis occurs after neural proliferation, first in peripheral structures and then more centrally from around 20 weeks; this process is at least partly dependent on sensory stimulation.60
The development of the nociceptive apparatus proceeds in parallel with basic central nervous system development. The first essential requirement for nociception is the presence of sensory receptors, which develop first in the perioral area at around 7 weeks gestation. From here, they develop in the rest of the face and in the palmar surfaces of the hands and soles of the feet from about 11 weeks gestation. By 20 weeks, they are present throughout all of the skin and mucosal surfaces.61 The nociceptive apparatus is initially involved in local reflex movements at the spinal cord level without higher cortical integration. As these reflex responses become more complex, they, in turn, involve the brainstem, through which other responses, such as increases in heart rate and blood pressure, are mediated. However, such reflexes to noxious stimuli have not been shown to involve the cortex and, thus, are not thought to be available to conscious perception. The nature of fetal consciousness itself is complicated, both physiologically and philosophically, and a discussion of such is beyond the scope of this chapter. However, there is a working consensus that for consciousness to be present there must be electrical activity in the cerebral cortex.62 It appears that, far from being “switched on” at any one moment, consciousness evolves in a gradual process that has been likened to a “dimmer switch,” making attribution of fetal consciousness to any particular developmental moment a difficult undertaking.
Programming Effects
When considering the effects of noxious stimuli on the developing fetus and the rationale for fetal anesthesia and analgesia, we must consider not just the humanitarian need to alleviate the possible distress of pain sensation, but also whether being subjected to surgical stress during early development might cause permanent alterations in physiology. This concept is known as programming, defined as “the process whereby a stimulus or insult at a critical, sensitive period of development has permanent effects on structure, physiology, and metabolism.”63 Studies in rats and nonhuman primates have shown permanent reductions in the numbers of hippocampal and hypothalamic glucocorticoid receptors in the offspring of antenatally stressed animals. This attenuates the negative feedback response, resulting in increased basal and stress-induced cortisol levels in the offspring, which last into adulthood. Behavioral changes, such as poor coping behaviors, have also been observed.63
Fetal Monitoring
Use of FETAL Heart Rate Monitoring for FETAL Interventions
Currently, FHR monitoring with Doppler ultrasonography is the standard for the intrapartum assessment of fetal well-being. FHR monitoring is also used perioperatively during fetal interventions. The FHR is documented before maternal induction of anesthesia, to serve as a baseline for comparison and to reassure the perinatologist, surgeon, and anesthesiologist that the fetus is stable. The FHR may be continuously monitored intraoperatively by fetal echocardiography and with intermittent palpation of the umbilical cord in open cases. It is known that the most commonly used anesthetic induction agents at appropriate doses (propofol and thiopental) rapidly cross the placenta and thus also rapidly reach the fetus.64,65 The inhalation anesthetics also cross the placenta,66 but the uptake of the anesthetic occurs more slowly in the fetus than in the mother.67,68 These anesthetics decrease FHR and FHR variability. Although it is reassuring if the FHR is within the normal range for the gestational age, fetal bradycardia is a reliable indicator of fetal distress that needs to be immediately addressed.
FETAL Electrocardiography
Several groups have used fetal ECG analysis to determine whether changes in time interval (PR and RR interval) and signal morphology (T to QRS ratio) correlate with fetal or neonatal outcome. Studies in animals and humans have shown that under normal conditions, there is a negative correlation between the PR interval and the FHR: as the FHR slows, the PR interval lengthens, and as the FHR increases, the PR interval shortens. The opposite relationship occurs in acidemic infants.69–75 During periods of fetal compromise, it is hypothesized that the sinoatrial node and the atrioventricular node respond differently.73 Periods of mild hypoxemia will induce increases in epinephrine levels, which will increase the FHR and shorten the PR interval. However, with periods of prolonged hypoxemia, the oxygen-dependent calcium channels of the sinoatrial node will demonstrate reduced sensitivity to epinephrine, resulting in a decrease in FHR. The fast sodium channels of the atrioventricular node are not affected by the reduction in the oxygen supply, and the increased levels of epinephrine will shorten the PR interval. As a result, the relationship between the PR interval and FHR changes from negative to positive.73 Measurements of this relationship have been divided into short-term and long-term measures.73,74 The short-term measure or the conduction index can be intermittently positive over short periods of time without an adverse outcome. However, a prolonged positive conduction index (greater than 20 minutes) has been associated with an increased risk of fetal acidemia.75
FETAL Pulse Oximetry
Standard pulse oximeters use the transmission and absorption of light through a vascular bed to a photodetector on the opposite side of the tissue. However, the development of reflectance oximetry allowed measurement of oxygen saturation from light-emitting diodes that are positioned next to each other on the same skin surface and absorption is determined from the light that scatters back to the tissue surface76,77; any fetal condition that decreases vascular pulsations (e.g., hypotension, vasoconstriction, shock, or strong uterine contractions) can produce inaccurate oximetry readings.78 Because direct contact of the oximeter must be made with the fetal skin surface, anything that interferes with light transmission or skin adhesion (e.g., fetal or maternal movement, vernix caseosa, caput succedaneum) can influence the quality and accuracy of the oximeter.79–83 Oximetry readings also vary in relation to the site of sensor application; several studies have found reduced baseline oxygen saturation values with the use of the oxygen sensor on the fetal buttock compared with the fetal head.84–87
The development of a 735/890-nm wavelength system (compared with the older 660/890-nm system) has improved the accuracy in monitoring arterial oxygen saturation (FSao2) in the fetus88; because the normal range of FSao2 of 30% to 70% lies in the middle of the oxygen-hemoglobin dissociation curve, small changes in pH or oxygen partial pressure cause large changes in FSao2.89 FPO can also identify an acidotic fetus. Increased concentrations of both the hydrogen ion and 2,3-DPG cause a rightward shift of the oxygen dissociation curve (Bohr effect) such that a chronically acidemic or hypoxemic fetus will have a low FSao2 even though the Po2 is within normal limits.89
FETAL Echocardiography
When technically feasible, fetal echocardiography should be available to assess fetal myocardial contractility and function, heart rate, intravascular volume status, and amniotic fluid volume. We have also used echocardiography to correctly identify proper endotracheal tube placement during an EXIT procedure90; a sterile sleeve is placed over the ultrasonographic probe that is then placed over the fetal chest.
Doppler Ultrasonography of FETAL Cerebral Blood Flow
Antepartum Doppler ultrasonography studies of the fetal circulation in cases of intrauterine growth restriction with presumed hypoxia have shown a compensatory redistribution, with an increase in peripheral vascular resistance in the fetal body and placenta and a compensatory reduction in peripheral vascular resistance in the fetal brain, producing a brain-sparing effect.91 Intrapartum Doppler ultrasonography and FPO have verified the brain-sparing response in the presence of intrapartum arterial hypoxemia (FSao2 less than 30% for 5 minutes or more), as reflected by increased mean flow velocity in the fetal middle cerebral artery.92 Preliminary studies of the middle cerebral artery pulsatility index in minimally invasive procedures, such as fetal blood sampling, transfusion, shunt insertion, tissue biopsy, and ovarian cyst aspiration, have demonstrated significant cerebral hemodynamic responses (decreases in the middle cerebral artery pulsatility index) in fetuses that underwent procedures involving transgression of the fetal body. This response was not noted in the fetuses undergoing procedures at the noninnervated placental cord insertion.93
Although not yet advocated for routine intrapartum management, it has been suggested that the combination of reduced arterial oxygen saturation and increased cerebral blood flow may indicate an ominous phase during labor. The redistribution of the fetal circulation is not an unlimited protective mechanism, and with persistent cerebral hypoxia, the active vasodilation of the cerebral vessels may fail, leading to disastrous consequences for the fetus.94
Physiologic Consequences of Pregnancy
Respiratory and Airway Considerations
There is an increase in metabolic demand of both the mother and the fetus, and this, along with anatomic and hormonal influences, accounts for the changes in maternal pulmonary physiology (Table 37-2). Pregnancy results in progressive increases in oxygen consumption and minute ventilation, along with a decreased residual volume and functional residual capacity.95 The increased metabolic demands and anatomic changes can make adequate oxygenation and perfusion of the parturient and the fetoplacental unit a constant challenge during maternal general anesthesia. During periods of apnea or hypoventilation, the parturient is prone to rapid development of hypoxia and hypercapnia. Even after adequate preoxygenation, the Pao2 in an apneic anesthetized parturient decreases by about 8 mm Hg more per minute than in nonpregnant women.96 Acidosis rapidly develops from hypoxia during difficult airway situations because of a decreased buffering capacity during pregnancy. The decreased pulmonary oxygen stores and increased oxygen consumption make parturients more susceptible than nonpregnant women to the consequences of airway mismanagement.
TABLE 37-2 Anesthetic Considerations of Respiratory Changes of Pregnancy
Petco2, End-tidal carbon dioxide pressure.
Not all physiologic changes of pregnancy are deleterious to the performance of anesthesia. For example, both the induction of and emergence from anesthesia with inhalational anesthetics occur faster in parturients than in nonpregnant women because the combination of increased alveolar ventilation and decreased functional residual capacity speeds the rate at which denitrogenation occurs and at which inspired and alveolar concentrations of inhalational agents reach equilibrium97; a faster induction, coupled with a decreased MAC, make parturients susceptible to relative anesthetic overdose and severe hypotension.98
Cardiovascular Considerations
Cardiovascular function is appropriately increased during pregnancy to meet the increased metabolic demands and oxygen requirements of the mother (Table 37-3). Cardiac output increases by 35% to 40% by the end of the first trimester and continues to increase throughout the second trimester until it reaches a level 50% greater than that in nonpregnant women.99 Heart rate increases 15% to 25% above prepregnancy rates and remains stable after the second trimester, and stroke volume progressively increases 25% to 30% by the end of the second trimester and remains stable until term.100,101 Aortocaval compression by the gravid uterus can cause a 30% to 50% decrease in cardiac output; lesser decreases occur in the sitting or semirecumbent positions. Maternal position is a major factor contributing to hypotension and fetal well-being.102
TABLE 37-3 Anesthetic Considerations of Cardiovascular Changes of Pregnancy
Maternal blood flow and pressure are directly linked to fetal perfusion via the placenta, and uterine blood flow represents about 10% of maternal cardiac output. The avoidance of aortocaval compression by left or right uterine displacement is imperative to prevent a decrease in the maternal blood pressure. Because large doses of inhalational agent are often necessary for uterine relaxation during fetal intervention, prompt treatment of hypotension is vital. Because uteroplacental blood flow is not autoregulated, a decrease in maternal blood pressure will eventually decrease placental blood flow and, therefore, blood flow to the fetus. IV ephedrine (5 to 10 mg) or phenylephrine (50 to 100 µg) per dose should be used to treat maternal hypotension unless contraindicated.103
Central and Peripheral Nervous Systems
Pregnancy-mediated analgesia is affected by changes in spinal opioid antinociceptive pathways and peripheral processes, including the effect of ovarian sex steroids (estrogen and progesterone) and uterine afferent neurotransmission. It is thought that pregnancy-mediated analgesia increases the woman’s threshold for pain during the latter stages of pregnancy before labor.104,105 Pregnant women are more sensitive to the action of many anesthetic agents and require less local anesthetic for spinal and epidural anesthesia and less inhalational anesthetics than their nonpregnant counterparts. The MAC of inhalational anesthetics in pregnancy is approximately 30% less than in nonpregnant females; and for this reason the concentration of inhalational anesthetic needs to be carefully titrated.106
Pharmacologic Consequences of Pregnancy
Physiologic changes of pregnancy alter the pharmacokinetics and pharmacodynamics of many anesthetic drugs. An increase in total body water and adipose tissue, and a decrease in plasma protein concentrations alter the volume of distribution. An increased renal blood flow and glomerular filtration rate can enhance the elimination of renally excreted drugs; hepatic metabolism of some drugs may be inhibited by competition with steroid hormones during pregnancy, whereas others may have a greater clearance associated with the increased basal metabolic rate. Therefore drug administration must consider the pharmacokinetics within the maternal-placental-fetal unit. Most drugs cross the placenta to some extent and the proportion transferred increases with the duration of gestation. The fetus has reduced plasma protein binding, producing relatively greater concentrations of free drug (i.e., unbound and available to cross biologic membranes).107 Despite detection of oxidation and reduction reactions in the fetal liver from as early as 16 weeks, enzyme concentrations and reaction rates are minimal, exposing the fetus to more prolonged drug effects than occur in the mother.108 Early in gestation, the primary mode of drug excretion is via blood flow to the placenta, but later, as the fetal kidneys mature, they become a route of drug excretion into the amniotic fluid for water-soluble drugs and metabolites. Amniotic fluid, however, can act as a reservoir for drugs, from which they can be reabsorbed.107
Induction
Pregnancy increases the parturient’s sensitivity to induction agents.109 Propofol has been safely used for induction of anesthesia for cesarean delivery, in doses of 2 mg/kg, with minimal effects on the neonate.110 Ketamine has also been used as the sole induction agent for parturients undergoing elective cesarean section; ketamine (1.5 mg/kg) has not been associated with maternal awareness or neonatal depression at delivery and parturients required fewer analgesics in the first 24 hours after delivery.111 It is speculated that ketamine’s analgesic properties may reduce the sensitization of pain pathways and subsequently confer extended benefit into the postoperative period. Induction agents decrease spontaneous uterine contractions of isolated pregnant rat myometrium, but only in concentrations greater than those seen in clinical obstetric practice.112
Neuromuscular Blocking Drugs
Although serum cholinesterase activity decreases 30% during pregnancy, recovery from a dose of 1 mg/kg of succinylcholine is not prolonged.113 Succinylcholine has a very low placental transfer, owing to its low lipid solubility and high degree of ionization.114 Similarly, cisatracurium has been safely used for cesarean section without routine neostigmine antagonism, despite decreased plasma cholinesterase activity.115 Pregnant women may be more sensitive to the action of nondepolarizing muscle relaxants, with the administration of vecuronium resulting in a more rapid onset and delayed recovery of neuromuscular block when compared with nonpregnant control patients. The prolonged action of vecuronium persists into the postpartum period for at least 4 days116; the clinical duration of vecuronium in term and postpartum women is twice that of nonpregnant women.117 However, in a study comparing cisatracurium 0.2 mg/kg for intubation in immediate postpartum and nonpregnant women, both the mean onset and recovery times in the postpartum period were significantly smaller.118 Nondepolarizing muscle relaxants have no effect on uterine relaxation and, as quaternary amines, do not cross the placenta.
Inhalational Anesthetics
Pregnant women are more sensitive to the anesthetic action of the inhalational anesthetics (MAC is reduced approximately 30% from nonpregnant females).119 This may lead to a deeper level of anesthesia than predicted during fetal surgery, and a relative overdose associated with maternal cardiac depression and hypotension. All inhalational anesthetics rapidly cross the placenta, but their uptake occurs more slowly in the fetus than in the mother.120,121 At light (1.0 MAC) isoflurane or halothane anesthesia, neither maternal pulse rate, cardiac output, and acid-base status, nor fetal pulse rate, acid-base status, and oxygen saturation changed significantly.122 During moderately deep (1.5 MAC) isoflurane or halothane anesthesia, maternal arterial pressure and cardiac output decreased. Uterine vasodilation occurred, but uteroplacental perfusion was maintained; fetal oxygenation and base excess were also maintained. However, at concentrations of inhalational anesthetics that exceeded 2.0 MAC, maternal hypotension decreased uteroplacental perfusion despite uterine vasodilation, leading to fetal hypoxia and acidosis. Inhalational anesthetics produced a dose-related uterine relaxation.123 At 0.5 MAC of enflurane, isoflurane, or halothane, uterine contractility decreases 20% whereas at 1.5 MAC, contractility decreases 60%.124 Sevoflurane produces a dose-dependent depression in uterine muscle contractility, with complete abolition of uterine activity at greater than 3.5 MAC.125 The large concentrations of inhalation anesthetic needed for profound uterine relaxation generally requires tracheal intubation and aggressive use of vasopressors.
Maternal Evaluation
The presence of fetal hydrops should alert the practitioner to the possibility of maternal mirror syndrome. Mirror syndrome refers to characteristic maternal pathophysiologic changes associated with a variety of fetal disorders, including nonimmune hydrops, molar pregnancies, congenital cystic adenomatoid malformation of the lung, and sacrococcygeal teratoma. Polyhydramnios and placentomegaly are usually present. Although the etiology of this condition is unclear, the end result is a maternal hyperdynamic state with associated hypertension and total body edema.126 Respiratory insufficiency or pulmonary edema may develop, requiring prompt and aggressive treatment. If preterm uterine contractions develop, treatment options may be limited because tocolytic agents can greatly exacerbate respiratory decompensation. Treatment is aimed at maternal supportive care; even correction of the underlying fetal pathology will not completely resolve the maternal abnormalities. Delivery of the fetus is the only sure method to completely reverse this maternal pathologic process.
The anesthesiologist should specifically investigate for the presence of placentomegaly; increased placental blood flow may alter pharmacologic treatment in both the mother and the fetus because increased drug metabolism may occur. The presence of placentomegaly may also increase risk for acute intraoperative bleeding, and preparation must be made to rapidly transfuse the mother. Several reports describe inadvertent inclusion of the placental edge during the hysterotomy incision, causing a sudden, massive loss of blood with a completely relaxed uterus127,128; immediate surgical control, resuscitation with blood products, and vasopressors that do not increase placental vascular resistance must be administered.
Tocolysis and Tocolytic Agents
Hormonal Receptors in Labor
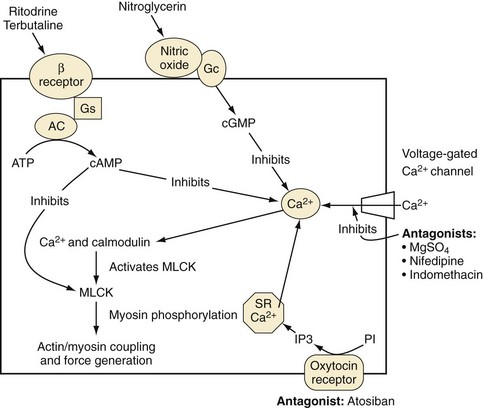
The adrenergic hormonal system plays a very influential part in the activity of the myometrium; several types of adrenergic receptors are found in the uterus (Fig. 37-4). Stimulation of the α-adrenergic receptor causes an increase in the rate and intensity of uterine contractions, whereas activation of the β2-adrenergic receptors produce myometrial relaxation.129 In addition, the term uterus is heavily populated with receptors for endogenously released oxytocin responsible for initiating uterine contractions. Prostaglandins also play a significant role in modulating myometrial tone. In general, prostaglandins are produced in or near the local environment where they exert their effect; both uterotonic and tocolytic prostaglandins have been identified. The balance of intrauterine and maternal uterotonic prostaglandins is thought to play an essential part in the preparation for both term and preterm labor. Prostaglandins, especially prostaglandin E2, are an essential component of every aspect of natural labor.130
Treatment of Acute Preterm Labor
β-Adrenergic–Mimetic Agents
Currently, only β2-adrenoceptor–selective medications are routinely used for acute preterm labor. Most adverse effects result from their lack of pure specificity, that is, simultaneous stimulation of β1– and β2-adrenergic receptors. Adverse effects include fetal tachycardia, maternal tremors, palpitations, tachycardia, a decreased or increased blood pressure, lethargy, sleepiness, ketoacidosis, and pulmonary edema. Pulmonary edema occurs in up to 5% of patients, especially when used with other tocolytics (e.g., magnesium).131 Because the β-adrenergic–mimetic agents are nonspecific receptor agonists, in large concentrations these agents can stimulate α-adrenergic receptors, which promotes uterine contractions, leading to treatment failure.
Magnesium
Magnesium competes with calcium for transmembrane channel entry into cells.132 Because the myometrium depends on stores of calcium for adequate contraction, a decrease in intracellular transport prevents the activation of the actin and myosin complex, resulting in uterine relaxation.
Nitric Oxide Donors
Nitroglycerin is an effective uterine relaxant used in select situations to produce rapid uterine relaxation (e.g., extraction of a retained placenta and uterine inversion). In pregnant sheep, nitroglycerin causes a decrease in mean maternal arterial pressure and increase in heart rate, without compromising uterine blood flow.133 During fetal surgery, nitroglycerin has been used to relax the myometrium and halt breakthrough contractions. Adverse effects include maternal hypotension, tachycardia, headache, development of tachyphylaxis, and a high incidence of maternal pulmonary edema.134
Calcium-Channel Blockers
Calcium-channel blockers are better tolerated than β-adrenergic–mimetic agents. Nifedipine may be more effective than β2-adrenergic agonists in postponing delivery, especially in those women with intact membranes.135 Neonates born to women treated with calcium-channel blockers have a reduced frequency of respiratory distress, necrotizing enterocolitis, and intraventricular hemorrhage.136 The most serious adverse effect is maternal hypotension; the combination of calcium-channel blockers and magnesium sulfate should generally be avoided.
FETAL Complications of Tocolytic Therapy
The adverse effects of tocolytics in the fetus present a number of problems, albeit usually less so than in the mother. Sympathomimetics that act through β-adrenoceptors cause fetal tachycardia.137 Whereas cyclooxygenase inhibitors have been shown to be more effective than other tocolytics in delaying labor,138 the adverse effects of fetal oliguria and ductus arteriosus constriction have limited their long-term use.139 However, after short-term use, these adverse effects are fully reversible within 72 hours from cessation of treatment.139 Longer-term use of indomethacin has been associated with renal dysfunction and increased rates of necrotizing enterocolitis, intracranial hemorrhage, and patent ductus arteriosus in infants delivered at less than 30 weeks gestation.140 Magnesium sulfate reduces FHR variability141 and depresses fetal right ventricular function.142 Because this drug rapidly crosses the placenta but is excreted more slowly by the fetal kidneys than by the maternal kidneys, there are concerns about fetal toxicity, resulting in respiratory and central nervous system depression.143 Nitric oxide donors, such as nitroglycerin, appear to have minimal fetal side effects.144
Postoperative Pulmonary Edema
Noncardiogenic pulmonary edema is a known complication of tocolysis. Most often, obstetric pulmonary edema is a result of increased hydrostatic pressures and resolves rapidly with diuretics, cessation of tocolytics, and fluid restriction. One study observed a prevalence of pulmonary edema of 0.5%, but that rate increased to 23% in fetal surgical patients; 93% of those with pulmonary edema required intensive care and 20% required tracheal intubation.134 It has been hypothesized that extensive uterine manipulation during surgery may result in release of mediators that increase the permeability of lung vasculature. The class of medications most strongly associated with pulmonary edema is β-adrenergic–mimetic agents. An additional important observation is that patients receiving nitroglycerin for tocolysis have demonstrated more pronounced pulmonary edema (more severe hypoxemia, greater time to resolution, worse chest radiograph, and a greater composite lung injury scores) than those who received other tocolytics.134
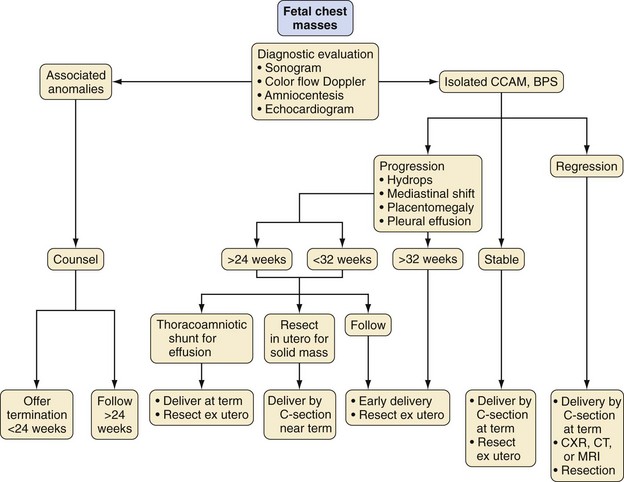
Congenital Cystic Adenomatoid Malformation: The Open Procedure
Congenital cystic adenomatoid malformation (CCAM) serves as a prime example of a fetal condition requiring open intervention. Fetuses with lung masses presenting before extrauterine viability represent a complex group of congenital disorders. Before the advent of preterm fetal intervention, management of fetal lung masses consisted of limited options, which included (1) delivery with hydrops once fetal viability was determined with regard to lung maturity while acknowledging the potential need for emergent postpartum resuscitation, (2) transplacental digoxin therapy in an effort to treat severe forms of cardiac dysfunction,145,146 and (3) termination of the pregnancy if the fetus was considered nonviable (Fig. 37-5). Fetuses demonstrating in-utero tumor regressions as documented by serial sonograms are allowed to progress to term gestation. Most infants with smaller lung masses or those with masses demonstrating in-utero regression do well with standard delivery and neonatal resection.147 However, a subset of fetuses experience significant fetal lung mass growth, ultimately compromising normal lung development. Treatment options for these fetuses have expanded to include cyst aspiration, thoracocentesis, double-J stents for permanent thoracic drainage, and in-utero resection of the lung mass.147–149 All treatment options aim to reduce the size of the lung mass to allow the remaining fetal lung to develop.
Congenital Cystic Adenomatoid Malformation of the Lung
CCAM of the lung consists of cystic masses of pulmonary tissue and bronchial structures, neither of which participate in gas exchange,150,151 that may represent a form of pulmonary hypoplasia.152 CCAMs can compress surrounding lung tissue and impede normal lung development, resulting in pulmonary hypoplasia.153 Of all the fetal lung masses, CCAM is the lesion most frequently associated with hydrops fetalis that often indicates a premorbid fetal state. Although the exact mechanisms for the development of hydrops are unclear, it has been suggested that it is secondary to either cardiac compression or vena caval obstruction from the intrathoracic mass.154,155 This condition is associated with a significant imbalance of fetal fluid, resulting in accumulation of fetal fluid causing increases in fetal interstitial and total body water, pericardial and pleural effusions, ascites, anasarca, polyhydramnios, or placental thickening.156,157
Fetal lung abnormalities themselves may lead to excessive fluid accumulation because the fetal lung is an important organ for amniotic fluid balance. The average fetal lung fluid production is estimated to be approximately 300 mL/day or about 4 mL/kg/hr.158 Fetal urine output is approximately 700 mL/day, and fetal swallowing is about 700 mL/day. The remaining 300 mL/day is postulated to exit the amnion through the chorioamnionic membrane. CCAMs may impair fetal swallowing via esophageal obstruction and therefore disrupt normal fluid balance; fetal swallowing is the major method by which amniotic fluid water is returned to the fetal vascular compartment. A second possibility is hypersecretion or transudation of fluid from the CCAM itself.
Management
Experts have formulated guidelines for the fetal surgical management of fetuses diagnosed with CCAM lesions; overall prognosis depends on the size of the lung mass and the presence of secondary physiologic derangements.147 Special consideration is given to fetuses exhibiting signs of hydrops fetalis, especially those who are less than 32 weeks gestation.159 Although these conclusions were based primarily on the experience with CCAM infants, it might be appropriate to extend this experience to the management of fetuses with other lung lesions. The primary goal of treatment is to reduce lesion size so that the fetal lung has an improved chance of normal development.
Operating Room Preparation
Like all other types of fetal intervention, ultrasonography should be performed before the induction of anesthesia to assess fetal well-being and to obtain an estimated fetal weight. In addition to the normal preanesthesia preparation checklist, additional maternal airway equipment, resuscitation drugs, and tocolytic agents should be prepared and immediately available. The availability of type-specific packed red blood cells for the mother and O-negative irradiated packed red blood cells, divided into 50 mL aliquots for the fetus must be confirmed. The operating room (OR) temperature should be warmed to at least 80° F (26.7° C) to prevent hypothermia of the partially exposed fetus during thoracotomy. Resuscitation drugs for the fetus (atropine 10 to 20 µg/kg, epinephrine 1 to 10 µg/kg), as well as a neuromuscular blocking drug (NMBD; e.g., vecuronium 0.2 mg/kg or pancuronium 0.1 mg/kg), and fentanyl (10 µg/kg) are prepared under sterile conditions, thus making them available during the procedure.160 A rapid infusion system with warmed isotonic saline is used to replace amniotic fluid loss during fetal lung resection, and is ready to administer onto the surgical field via a sterile tubing system. A pulse oximeter with a sterile extension cord should be available for application to the upper extremity of the fetus.
Induction
The preferred method of maternal anesthesia for these cases is general anesthesia with endotracheal intubation and neuromuscular blockade. Before entering the OR, an IV line is started and sedation is administered as needed. If the mother has not received indomethacin (50 mg rectal suppository) for tocolysis before arrival, it is administered after induction of general anesthesia. Indomethacin is used in conjunction with magnesium in the postoperative period for tocolysis but does not play a significant tocolytic role in the intraoperative period. After placement of standard monitors, a lumbar epidural catheter may be inserted for postoperative pain management. With the exception of a test dose, most practitioners avoid local anesthetic administration through the epidural catheter until the fetal intervention is completed. This is done to avoid possible decreases in maternal mean arterial pressure from an epidural-associated sympathectomy. The mother is then positioned in a uterine displacement position, preoxygenated, and a rapid-sequence induction is performed with an induction agent, succinylcholine (and subsequently followed with a short-acting nondepolarizing agent), and a rapid-acting opioid. Anesthesia is maintained with 1 MAC of the inhaled anesthetic of choice (usually sevoflurane or desflurane, should a rapid reinstatement of uterine tone be required) in 100% oxygen, while an ultrasonographic examination maps out surface anatomy with respect to the placenta and fetus, as well as reassuring fetal well-being after anesthetic induction. A second large-bore peripheral IV catheter, radial arterial catheter, urinary catheter, and nasogastric tube are then inserted. Because the maternal anesthesia induction is the same as a standard cesarean section, invasive blood pressure monitoring is not necessary until the inhalational anesthetic is increased to 2 to 3 MAC. Fetal hemodynamics (heart rate, right ventricular contractility) are monitored intraoperatively by continuous fetal echocardiography.160
Alternatively, in cases where a long period of time is expected to transpire between maternal induction and hysterotomy, a substitution or combination with an IV anesthetic (typically propofol and remifentanil) may reduce fetal cardiac acidosis seen with greater concentrations of inhalational anesthetics (most notably desflurane).161 In such cases, large concentrations of inhalational anesthetics should be reinstituted before uterine incision to assure adequate uterine relaxation. Alternatively, recent reports have described total IV anesthesia techniques employing remifentanil, nitrous oxide, midazolam as general anesthetic agents with IV nitroglycerin used for uterine relaxion.162 Remifentanil, which moves across the placenta freely, has also been described as an agent for fetal immobilization under combined spinal epidural anesthesia.163
Maintenance
Before hysterotomy the concentration of inhalational anesthetic is increased to 2 MAC to ensure myometrial relaxation and tocolysis.160,164 Satisfactory uterine relaxation can be achieved, but these concentrations may decrease maternal arterial pressure, uteroplacental perfusion, and fetal oxygenation, and may require pressor support.160,165 Although only small increases in fetal Pao2 occur with maternal inspired oxygen concentrations of 100%, this small increase may be advantageous. Additionally, the increased concentration of inhalational anesthetic needed for uterine relaxation dictates that only medications that augment uterine relaxation be administered.166 Given that nitrous oxide does not affect the uterine tone to any measurable degree and thus provides no direct surgical benefit, it is best omitted and 100% oxygen is used. Maternal eucapnia (Paco2 of 31-33 mm Hg) is the physiologic goal,156 because maternal hyperventilation may lead to decreases in fetal Pao2.167,168 Some have suggested that maternal hypercarbia can, in fact, increase fetal Po2.169 At this time, however, extrapolation of these conclusions to fetal intervention cases should be done with caution.
Meticulous attention to maternal blood pressure is essential to ensure adequate uterine blood flow and uterine perfusion; maternal systolic pressure is maintained at 110% of mean awake values with IV ephedrine or phenylephrine. Total IV fluids are limited unless blood loss is excessive, so as to minimize the risk of postoperative maternal pulmonary edema.170
Intervention
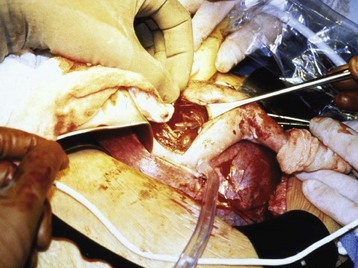
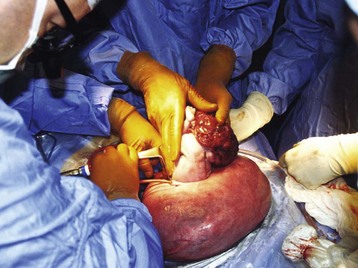
The fetal hemithorax and upper extremity are delivered through the hysterotomy. Warm fluids are continuously infused into the uterine cavity from a high-volume fluid warmer to replace amniotic fluid losses, provide a thermoneutral environment for the fetus, and prevent umbilical cord kinking or stretching. Limiting the size of the uterine incision helps prevent fetal evaporative fluid loss, uterine hemorrhage, and postoperative uterine contractions. Once the fetal hemithorax and upper extremity have been delivered into the operative field, fentanyl (5 to 20 µg/kg), atropine (20 µg/kg), and an NMBD (usually vecuronium 0.2 mg/kg or pancuronium 0.1 mg/kg) are given IM as a single injection into the exposed shoulder of the fetus.160 Fentanyl is administered for intraoperative and postoperative fetal analgesia and to suppress the fetal stress response, atropine ablates the expected bradycardic response with fetal surgical manipulation, and an NMBD will ensure an immobile fetus during surgery. Although the fetus receives anesthesia from transplacental transfer of maternal inhaled anesthetic, these additional IM medications augment fetal anesthesia and ensure fetal analgesia before thoracotomy.
A pulse oximetry probe can be applied to the exposed fetal extremity. Fetal echocardiography provides information about FHR and ventricular filling, which is particularly useful in those procedures in which fetal blood loss is anticipated (Fig. 37-6). Fetal lung lesions, especially if composed of multiple tissue types, may have a very irregular vascular supply, and significant fetal hemorrhage is possible. Direct vascular access in the exposed upper extremity allows immediate resuscitation and blood administration as needed. Even surgical manipulations alone can lead to hemodynamic instability, requiring urgent resuscitation. This may be secondary to mediastinal torsion, resulting in a sudden loss of cardiac preload.
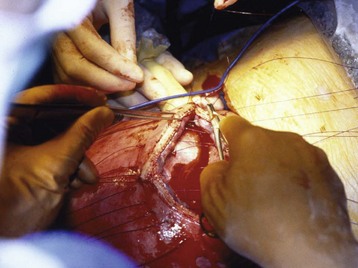
Closure
Once the lung lesion has been resected and fetal well-being is confirmed, the fetus is returned to the intrauterine environment and the hysterotomy incision closed. Closure consists of two separate layers, thus minimizing the risk for postoperative amniotic fluid leak and uterine wall dehiscence (Fig. 37-7). It is important to maintain complete uterine relaxation during closure, because uterine manipulation can alter blood flow and place the fetus at risk of hypoperfusion. Before the last uterine stitches, intraamniotic volume is assessed via ultrasonography and any deficit is replaced with warmed Ringer’s lactate solution.
Postoperative Management
Serious postoperative complications include premature labor, pulmonary edema, amniotic fluid leak, wound seroma, infection, and fetal demise.156,160,171–175 Virtually all patients experience premature uterine contractions in the immediate postoperative period, thereby necessitating a continuous magnesium sulfate infusion until premature labor is significantly diminished. In some instances, additional tocolytic agents may be necessary. Despite maximal tocolytic therapies, continued uterine irritability may result in premature delivery. Amniotic fluid leak can lead to oligohydramnios and significant reductions in amniotic fluid volume that may necessitate replacement. In refractory cases, the mother may return to the OR for reclosure of the hysterotomy incision.
The etiology of fetal demise after open fetal surgery is usually secondary to a primary complication (see earlier discussion). As such, every effort is made to minimize and promptly treat potential postoperative complications to ensure a positive fetal intervention and to provide an environment for a successful term gestation. Surgical stress and pain can lead to release of cortisol and inflammatory cytokines in both the mother and the fetus, which, in turn, may lead to premature uterine maturation and contractions.176 Maternal pain control can be provided by patient-controlled analgesia and epidural or spinal analgesia. One disadvantage of epidural analgesia is that the systemic opioid concentrations are reduced; therefore less is transferred to the fetus for postoperative analgesia. The benefit from a greater concentration of systemic opioids produced by IV analgesia is the possibility of improved fetal analgesia. However, IV analgesia does not reliably prevent a maternal stress response. To address this, the optimal choice for epidural analgesia may be a reduced concentration of the local anesthetic with a large concentration of a fat-soluble opioid, such as fentanyl (e.g., bupivacaine [0.05%] and fentanyl [10 µg/mL]).171
Other Diseases Eligible for Open Procedures
Pulmonary Sequestration
Pulmonary sequestration, also known as bronchopulmonary sequestration, accessory lung, or bronchopulmonary foregut malformation, represents 0.5% to 6% of congenital lung disease (0.15% and 1.7% of live births).30–32 Pulmonary sequestrations consist of nonfunctional lung tissue that does not communicate with the normal tracheobronchial tree and hence does not participate in gas exchange.31 Pulmonary sequestration may be differentiated from CCAM by investigation of its blood supply. Unlike pulmonary sequestrations, CCAMs derive their blood supply and venous drainage from the pulmonary circulation. A multitude of somatic anomalies have been associated with sequestration, most commonly diaphragmatic hernia. If not treated in utero, these lesions often present as respiratory distress in the neonatal period or as chronic respiratory infections in older children.
Bronchogenic Cysts and Mixed or Hybrid Pulmonary Lesions
Bronchogenic cysts are embryonic abnormalities considered to be a type of bronchopulmonary foregut malformation.177 These cysts are thought to result from an abnormal budding of the primitive bronchial tree between weeks 4 and 8 of gestation, thus representing abnormal lung development at an early stage of ontogeny.178 In most cases, bronchogenic cysts are asymptomatic in the first months of life. A notable exception is a mediastinal cyst that usually manifests as stridor.
Although in-utero complications are less likely than with the other fetal lung lesions previously described, the propensity of these lesions to cause life-threatening postnatal complications warrants close attention throughout the prenatal period. Fetal intervention with intermittent or continuous drainage of cysts can prevent the secondary morbidity; definitive fetal surgery with thoracotomy has also been successful.179
Sacrococcygeal Teratoma
Sacrococcygeal teratomas (SCTs, Fig. 37-8) are one of the most common congenital neonatal tumors (1 per 40,000 live births).180–182 A variety of tissues from the three primary germ layers are usually found, and the size of the tumor is quite variable.183,184 Most SCTs are external, usually protruding from the perineal region. The majority include both solid and cystic components, with only 15% being entirely cystic.185,186 Although usually benign, SCTs can cause significant secondary morbidity in selected cases because of the tumor’s mass effect and vast blood supply.187 With smaller tumors, complete surgical resection usually occurs after delivery under elective, controlled conditions. In extreme cases, the tumor can cause fetal congestive heart failure (usually high output failure), and even fetal demise if no treatment is performed.188 Death is usually secondary to an enlarged tumor mass and associated polyhydramnios, resulting in preterm labor and delivery, with ultimate survival dependent on fetal lung maturity. Massive hemorrhage into the tumor with fetal exsanguination may occur spontaneously in utero or be precipitated by labor and delivery. Prenatal intervention may be necessary, including intrauterine transfusion or fetal surgery for those fetuses that develop significant secondary morbidity (e.g., hydrops).
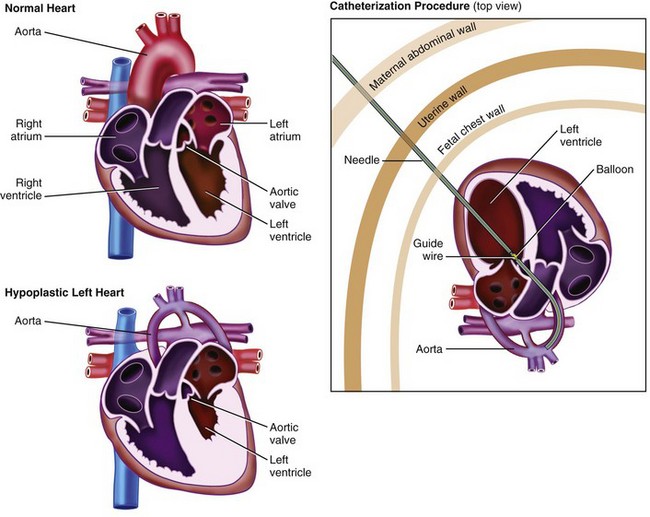
Hypoplastic Left Heart Syndrome: Percutaneous and Fetoscopic Procedures
A variety of congenital heart defects (CHDs) may be considered for fetal intervention. To date, the most studied defects include severe aortic stenosis with evolving hypoplastic left heart syndrome (HLHS) and pulmonary valve atresia with an intact ventricular septum with evolving hypoplastic right heart syndrome.189–193
Rationale for FETAL Cardiac Intervention
Most CHDs can be safely repaired in infancy, with excellent surgical survival and long-term prognosis. For these defects, there would be no need for in-utero intervention; and for many defects, in-utero intervention would not be technically possible (e.g., arterial switch procedure for transposition of the great arteries). For other defects, surgical correction itself may not be possible and the only option is staged surgical palliation, which is often associated with significant surgical morbidity and mortality.189,194,195 As such, the risk of performing any fetal intervention must be balanced against the potential benefits of improving the anticipated outcome of surgery performed in the neonatal period to correct the specific cardiac defect. It is the intention of prenatal intervention for certain types of CHD to reverse the pathologic process in an attempt to preserve cardiac structure and function and, thus, it is hoped, prevent serious postnatal disease. A secondary aim of prenatal intervention is to modify the severity of the disease and improve postnatal surgical outcomes.
Defects Amenable to in-Utero Repair
Certain congenital heart defects cause aberrations in blood flow, which are usually secondary to valvular stenosis or regurgitation. Regardless of the etiology, the end result is often an abnormally developed ventricle.196 Several case reports have characterized the progression of valvular stenosis to ventricular hypoplasia from reduced flow through the chamber during gestation.197–199 It has been hypothesized that relief of valvular stenosis in utero could reverse the progression toward ventricular hypoplasia. In these cases, there may be a window of opportunity in which ventricular growth can be salvaged. Because most routine prenatal ultrasonographic screening is performed between 16 and 24 weeks gestation, the window of opportunity for prenatal intervention is likely between 20 and 26 weeks gestation.
To date, the defect most amenable to correction is severe aortic stenosis with evolving HLHS.197–200 Without prenatal intervention, severe aortic stenosis can lead to marked left ventricular dysfunction, diminished flow through the left heart, arrest of left ventricular growth, ventricular fibroelastosis, and, consequently, HLHS. Aortic valve dilation may be performed percutaneously with ultrasound guidance. Optimal fetal positioning, placental location, or maternal habitus may require exposure of the uterus through an abdominal incision to obtain ideal access to the fetal thorax. These procedures have been performed under both maternal regional and general anesthesia, although general anesthesia is often preferred to obtain optimal uterine relaxation and an anesthetized fetus. Preliminary results are promising, but larger prospective investigations are warranted to determine long-term outcomes.201
Technical Aspects of FETAL Cardiac Interventions
Open cardiac surgery on the fetus is not presently technically possible.202–206 In humans, all of the reported procedures to date have been attempted using the transcutaneous or transuterine approach with ultrasound-guided access into the fetal heart.191–193 Although hysterotomy would provide means for more direct fetal access (e.g., femoral artery, transumbilical or carotid artery access), maternal morbidity would be significantly increased and postoperative premature labor certain. After valvuloplasty, the fetus requires time for the ventricle to recover. Therefore, any procedure that substantially increases the likelihood of early delivery would likely be counterproductive.
Although initial percutaneous techniques for fetal cardiac valvuloplasty were performed with only the mother receiving sedation,191,192 recent advances in surgical techniques have led to provision of maternal and fetal analgesia and anesthesia.207 The mother usually receives general anesthesia. After ultrasonographic confirmation of placental location, the maternal abdomen and uterus are punctured with a 22-gauge spinal needle. An IM injection of fentanyl, atropine, and a muscle relaxant is delivered to the fetus. A 19-gauge needle is subsequently directed into the fetal thorax, and access to the fetal heart is obtained. A small coronary balloon-tipped catheter is threaded over a guidewire through the needle, and passed through the stenotic valve or closed septum. The catheter balloon is then dilated, and blood flow is confirmed using Doppler ultrasonography (Fig. 37-9). The technique has been modified in certain cases, such that a laparotomy to expose the uterus is performed. Using this technique, better ultrasonography and ideal fetal positioning are possible to achieve optimal access to the fetal thorax.
Anesthetic Management for the Mother
Patients who received an epidural anesthetic technique required significantly more IV fluids but less IV opioid. The administration of large amounts of crystalloid and tocolytics during fetal surgery increases the risk of maternal pulmonary edema.208,209 Neuraxial techniques (e.g., spinal, epidural, and combined spinal-epidural anesthesia) have been used in other percutaneous and fetoscopic procedures; a T4 sensory level blockade is required. It should be noted that neuraxial anesthesia provides no uterine relaxation and no analgesia or anesthesia to the fetus unless supplemented with IV maternal analgesics and sedatives (e.g., fentanyl, benzodiazepines, propofol). Because of these issues, it is generally recommended, even in anticipated percutaneous procedures, to deliver a general anesthetic to the mother. If there is a high suspicion of a laparotomy being performed, a dose of spinal Duramorph (morphine sulfate) may be delivered to the mother before the anesthetic induction for postoperative pain relief and resultant suppression of myometrial contractility after laparotomy.197,198
Anesthetic Management for the Fetus
Anesthesia for percutaneous and fetoscopic interventions, of which fetal cardiac interventions are a significant subset, pose several unique challenges for the anesthesiologist. The combination of immature organ systems and the underlying cardiac anomaly places the fetus at considerable anesthetic risk. Unlike adults and older children, fetal cardiac output depends more on heart rate than on stroke volume. Because fetal myocardial contractility is likely maximally stimulated, the fetus has a limited ability to increase stroke volume. Therefore, it is plausible that fetal patients with congenital heart disease and evidence of failure (i.e., hydrops) will exhibit more pronounced physiologic limitations. Notably, anesthetic-induced decreases in contractility, combined with intracardiac catheter manipulation in a structurally compromised heart, can result in fetal hypotension, bradycardia, and eventual cardiac collapse and death. It is generally accepted that neonates manifest a greater degree of hypotension in response to isoflurane and halothane at equipotent anesthetic concentrations when compared with older children.210,211
Postoperative Considerations
The fetus is monitored postoperatively with intermittent ultrasonographic examinations. The incidence of premature contractions and labor is less after fetoscopic surgery than after open hysterotomy.212,213 Fetoscopic intervention also appears to have reduced requirements for tocolysis and a reduced rate of premature delivery.213 If early delivery should occur, many of these fetuses are considered nonviable owing to their young gestational age (usually less than 24 weeks gestation) and serious cardiac disease.
Other Diseases Eligible for Fetoscopic Procedures
TWIN–TWIN Transfusion Syndrome
Twin–twin transfusion syndrome (TTTS) is a serious complication occurring in 10% to 15% of monozygotic monochorionic twin pregnancies.214 Although all monochorionic twin pregnancies demonstrate one or more placental vascular anastomoses, TTTS represents a pathologic form of circulatory imbalance between the monochorionic twin fetuses.215 As a result of this imbalance, a net fetofetal transfusion occurs, from one twin (the donor) to the other (the recipient) (Fig. 37-10). Symptoms develop rapidly and, in the donor twin, include hypovolemia, oliguria, oligohydramnios, and growth retardation. In turn, the recipient twin develops hypervolemia, polyuria, polyhydramnios, and signs of circulatory volume overload, resulting in congestive heart failure.214–218 In severe cases, if untreated, TTTS may result in intrauterine fetal death and miscarriage. Even if twins with TTTS survive, there remains a high incidence of secondary neurologic and pulmonary morbidities.
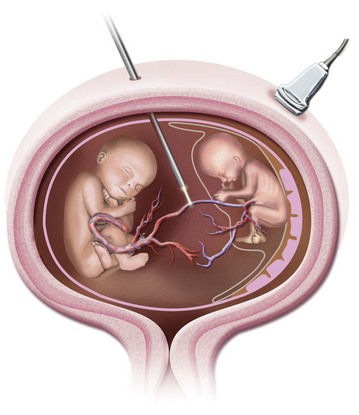
FIGURE 37-10 Schematic representation of umbilical cord ligation in twin reversed arterial perfusion sequence.
(Courtesy T.M. Crombleholme, MD.)
Fetoscopic laser photocoagulation of the communicating vessels associated with TTTS is based on three fundamental assumptions: (1) the syndrome occurs in the presence of vascular communications between fetuses in a monochorionic gestation, (2) obliteration of these vessels can halt the pathophysiologic process, and (3) both deep and superficial communications can be interrupted at the surface of the placenta.219 Fetoscopic laser surgical occlusion of superficial communicating vessels is associated with a reported survival rate of 55% to 83% and a reduced neurologic complication rate (5%) among survivors.214,216
There are few data on the reported anesthetic techniques used for fetoscopic laser ablation. The procedure has been performed with local, general, epidural, and combined general and epidural anesthesia.220–223 Factors that may influence the anesthetic technique include (1) the planned surgical approach and probability of converting to open fetal surgery; (2) the likelihood of surgical perturbation of innervated fetal tissues; (3) maternal preference; and (4) a history of prior uterine activity. The surgical approach for fetoscopic laser photocoagulation is determined by (1) the location of the placenta (anterior vs. posterior), (2) the position of the fetuses, and (3) the potential window(s) for trocar insertion.224
Twin Reversed Arterial Perfusion Sequence
Perinatal complications with TRAP sequence range in severity, with reported death rates for the pump twin ranging from 39% to 59% in untreated pregnancies.225 Treatment options include observation, medical therapy with digoxin and indomethacin, selective delivery, umbilical cord blockade with a coil, and fetoscopic cord ligation. Although all endoscopic procedures have the primary aim of interrupting umbilical cord blood flow to the nonviable twin, this invasive technique is generally employed after failed medical therapy or after signs of cardiac failure in the viable twin.226,227
Needle Aspiration and Placement of Shunts
A variety of fetal disorders may benefit from in-utero needle aspiration or shunt placement. These disorders include posterior urethral valves, aqueductal stenosis, fetal hydrothorax, ovarian cyst, and fetal ascites. Various shunts have been attempted to provide long-term decompression, with variable results.221
The EXIT Procedure
Ex utero intrapartum treatment, or the EXIT procedure, was initially described as a method for reversal of tracheal occlusion in fetuses with prenatally diagnosed severe congenital diaphragmatic hernia that had undergone in-utero tracheal clip application.228 Although these infants demonstrated no reduced morbidity compared with those who underwent conventional treatment, this novel technique provided a new therapeutic option for fetuses with a variety of potentially fatal diseases. Improvements in prenatal imaging and widespread use of prenatal ultrasonography have increased the identification of potentially lethal fetal structural malformations, which has had a direct impact on perinatal management and outcomes.
Also referred to as the OOPS procedure (operation on placental support),229 the EXIT procedure allows for a controlled delivery and intrapartum assessment strategy to treat fetuses with certain life-threatening diseases. By maintaining uteroplacental circulation with only partial delivery of the infant, crucial time is provided to perform procedures critical to infant survival. These procedures include direct laryngoscopy, bronchoscopy, intubation, tracheostomy, tumor decompression and resection, and extracorporeal membrane oxygenation (ECMO) cannulation before clamping the umbilical cord (Fig. 37-11). In this way, continuous oxygenation is maintained at all times to the threatened infant, thereby improving the chances of overall survival. The EXIT procedure is now used for infants in whom prenatal imaging suggests a very low probability of survival with conventional treatment methods. This group includes fetuses with known tracheal obstruction and other life-threatening airway abnormalities, as well as those who will likely require ECMO support (i.e., congenital cardiac disease and diaphragmatic hernia).
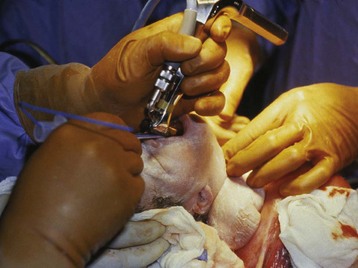
FIGURE 37-11 Fetal rigid bronchoscopy during an ex-utero intrapartum treatment procedure.
(Courtesy N. Scott Adzick, MD, Children’s Hospital of Philadelphia.)
Unlike many other fetal interventions, however, a planned delivery of the infant is the end result of these interventions. This unique difference creates significant increases in maternal morbidity because these procedures require complete uterine relaxation and serious maternal hemorrhage could result.230 An intimate understanding of the EXIT procedure, the fetal pathophysiology involved, and pregnancy-induced alterations directly affecting anesthesia care is required to minimize maternal and fetal morbidity and mortality.
FETAL Diseases Eligible for the EXIT Procedure
Cervical Teratoma
Cervical teratomas are rare (1 per 20,000 to 40,000 live births) and can extend from the mastoid process to the sternal notch inferiorly and to the trapezius muscle posteriorly. They can also invade the oral floor and extend into the anterior mediastinum. Many of the larger teratomas diagnosed prenatally cause maternal polyhydramnios, which is secondary to esophageal compression by the tumor and impaired fetal swallowing. Most of these tumors are benign but are associated with substantial mortality rates caused by airway compression and difficulty in establishing an adequate airway after delivery (Fig. 37-12).231 Of neonates with cervical teratomas, 30% die of airway obstruction shortly after delivery232; for infants not diagnosed prenatally, mortality rates are even greater.233,234 In addition, some larger tumors may interfere with normal delivery methods and necessitate emergent alterations in maternal care, placing the mother at increased risk.231,235
Until recently, treatment options for infants with cervical teratomas who survived the intrauterine period were limited. The standard of care incorporated scheduled cesarean section followed by various airway maneuvers, including the establishment of a surgical airway. Even with skilled help immediately available, dismal outcomes were common.231–233 Despite securing the airway, critical time is needed to perform this task, often at the expense of neonatal oxygenation. With the introduction of the EXIT procedure, precious time is provided to locate the trachea and provide a definitive airway before clamping the umbilical cord, thereby maintaining continuous fetal oxygenation and decreasing morbidity and mortality.
Cystic Hygroma
Cystic hygromas arise from the failure of the jugular lymph sacs to join the lymphatic system early in fetal development, resulting in the development of endothelium-lined cystic spaces that eventually compress normal surrounding structures. This compression may result in fetal hydrops, including skin edema, ascites, and pleural or pericardial effusions (Fig. 37-13).233,234 In infants with isolated cervical cystic hygroma and no evidence of hydrops, airway compromise at birth or shortly thereafter is the main therapeutic concern. These infants are considered candidates for EXIT procedures.
Congenital High Airway Obstruction Syndrome
Congenital high airway obstruction syndrome (CHAOS) is a clinical syndrome consisting of extremely large echogenic lungs, flattened or inverted diaphragms, a dilated tracheobronchial tree, ascites, and evidence of nonimmune hydrops, including fetal ascites, placentomegaly, and pleural or pericardial effusions.236–238 Airway obstruction may be because of laryngeal atresia, laryngeal cyst, or tracheal atresia. Diagnosis of prenatal CHAOS is confirmed by ultrasonographic evidence of complete or near-complete upper airway obstruction. Most diagnostic findings result from increased intratracheal pressure and distention of the tracheobronchial tree secondary to the accumulation of fluid in the lungs. Cardiac changes include the appearance of an elongated heart, septal shift, and small, compressed heart chambers.233
Management guidelines for fetuses with CHAOS are not definitive. In third trimester fetuses with a diagnosis of CHAOS and no evidence of hydrops, there is most probably incomplete airway obstruction, and management is aimed at establishing an airway before complete delivery. This subset of fetuses would likely benefit from an EXIT procedure.233,239 Those fetuses with a diagnosis of CHAOS made in the second trimester and those with evidence of complete airway obstruction and/or nonimmune hydrops present a dilemma, because insufficient data exist to determine their best treatment options.
Congenital Goiter
Congenital goiter is associated with fetal hypothyroidism, euthyroidism, or hyperthyroidism. Goiter associated with fetal hypothyroidism is almost always associated with the transplacental passage of a thyroid-stimulating immunoglobulin G antibody from the mother. Such antibodies are present in 90% of women with Graves disease. These antibody levels may not reflect maternal thyroid status, making the fetus of any woman with Graves disease at increased risk for fetal goiter. Less common causes include iodine deficiency, iodine intoxication, congenital metabolic disorders of thyroid hormone synthesis, or hypothalamic-pituitary hypothyroidism. Ultrasonographic findings of fetal hyperthyroidism include cardiac hypertrophy, tachycardia, or nonimmune hydrops fetalis. Fetal hypothyroidism may be associated with fetal cardiomegaly and heart block. Fetal blood sampling is required to determine the fetal thyroid status.233,240,241
EXIT to ECMO
In addition to airway management, the EXIT procedure may be considered for other instances in which separation from uteroplacental support is expected to cause critical cardiac or pulmonary compromise. Fetuses with congenital heart disease who are expected to need emergent ECMO at birth and fetuses with poor-prognosis congenital diaphragmatic hernias may benefit from the “EXIT to ECMO” strategy.230,242 Neonates undergoing this procedure are partially delivered via the EXIT procedure, and arterial and venous cannulas are inserted while uteroplacental perfusion is maintained. Although CHD remains the most common disease entity considered for potential EXIT to ECMO therapy, this technique has been used for neonates with other disease processes associated with almost certain chance of immediate cardiorespiratory collapse after conventional delivery.
Uterine Relaxation and Perfusion
To preserve maternal-fetal gas exchange at the placental interface, ensure fetal oxygenation, and avoid life-threatening hypoxemia, it is of primary importance to ensure complete uterine relaxation throughout the duration of fetal uteroplacental support. Factors affecting uterine blood flow include, but are not limited to, anesthetic induction agents, maternal hyperventilation, maternal hypotension, maternal catecholamine release, and other causes of increased noradrenergic activity and uterine tone. Any increase in uterine vascular resistance will decrease uterine perfusion, as is seen with uterine contractions. Of all factors ensuring the overall success of the EXIT procedure, minimal uterine vascular resistance is the most important, because decreases in uterine blood flow will cause fetal hypoxemia, acidosis, and, potentially, fetal demise.243
Surgical Procedure
After the hysterotomy site has been created and hemostasis achieved, the fetal head, neck, and shoulders are delivered. Because many of these procedures involve large neck masses, a generous hysterotomy incision is needed to partially deliver the fetus without injury to the mass or fetus. Furthermore, if a uterine contraction occurs at this time, inadvertent expulsion of the fetus could occur, interrupting the fetoplacental unit and thus critically jeopardizing the viability of the fetus. In some cases, a fetal extremity may be delivered to apply a pulse oximetry probe and to obtain IV access.244,245 Although the fetus is anesthetized via placental transfer of maternally administered inhaled anesthetics in most cases, additional analgesia and paralytics are administered (e.g., fentanyl, atropine, muscle relaxant). The additional medications may be given as a single IM dose in an upper extremity, or can alternatively be delivered under ultrasound guidance before hysterotomy. An advantage to earlier administration is increased time for fetal absorption via the IM route. If peripheral IV access is obtained, additional medications can be given through this route.
Access to the FETAL Airway
Most EXIT procedures are currently performed to access a compromised fetal airway before delivery; successful access depends on meticulous preoperative evaluation and careful preparation.246,247 Portions of the trachea can be completely compressed and distorted such that even successful intubation may result in an inability to achieve adequate ventilation. For this reason, most surgeons perform a direct laryngoscopy and rigid bronchoscopy to examine the status of the fetal airway. In one series, successful endotracheal intubation by conventional means was reported in 77% of cases.230 In those cases in which tracheal intubation is impossible, a surgical tracheostomy can be performed as soon as the trachea is identified. The trachea can be located with the aid of preoperative radiographic studies, often identifying the tracheal location relative to fixed external anatomic landmarks. Gentle surgical palpation may also aid in the identification of cartilaginous tracheal rings. In cases in which the former options have failed, ultrasonography with the sterile probe inserted directly into the surgical incision may help to locate the trachea.248 When tracheal rings are identified, the trachea may be accessed directly with an endotracheal tube by tunneling through the fetal soft tissue, or with the aid of a retrograde wire inserted by the Seldinger technique. The trachea, exposed through a neck incision, may be incised via a temporary tracheotomy to allow passage of a feeding tube or wire from the trachea to the mouth or nose. The guidewire is then attached to the endotracheal tube, which is then pulled down into the proper position. After suturing the endotracheal tube securely to the mouth, the tracheotomy can then be closed.
Delivery of the Infant and Maternal Management
Before umbilical cord clamping and delivery, coordination between the surgery and anesthesia teams is crucial to prevent uterine atony and excessive maternal hemorrhage. Because a decrease in the tocolytic agent, whether an inhalational or an IV agent, would result in increased uterine vascular resistance and decreased fetal oxygenation, reversal of the tocolysis must not occur before the umbilical cord is clamped. However, at clamping, a near-total reversal of tocolysis is required to limit uterine bleeding. This is best achieved with a low-solubility inhalational anesthetic (e.g., desflurane). As the cord is clamped, the anesthetic is immediately discontinued and oxytocin is administered as a bolus followed by a continuous infusion and titrated to uterine response (e.g., 40 units oxytocin in 500 mL of normal saline over 30 minutes, followed by 20 units over 8 hours). Additional uterotonic medications may be necessary and must be immediately available should uncontrolled maternal hemorrhage occur.249 These medications include methylergonovine, carboprost, and calcium carbonate. Anticipation of massive and rapid maternal hemorrhage is essential. Appropriate IV access (e.g., rapid infusion catheters, introducer sheaths) with a rapid infusion device in place for blood product administration may be life saving, should uncontrolled and persistent bleeding occur. In cases of uncontrolled hemorrhage despite maximal drug therapy, a hysterectomy may be necessary. When maternal hemostasis has been achieved, uterine tone restored, and the placenta delivered, then a low-dose inhalational anesthetic and nitrous oxide can be administered, provided that the mother is hemodynamically stable.
Postoperative Considerations
Mothers recovering from an EXIT procedure differ from those who undergo standard cesarean deliveries. Potential postoperative complications include wound dehiscence, infection, bleeding, and urinary retention.230 Although every attempt is made to place the hysterotomy incision in the lower uterine segment during EXIT procedures, those patients with anterior placentas may require incisions in different areas of the uterus. As a result, these patients are at increased risk of uterine rupture in any subsequent pregnancy. Practitioners should also consider the fact that, unlike with a standard cesarean section, the parents cannot immediately interact with or even view their neonates after delivery. Because many of these neonates undergo immediate surgical intervention, the parents’ first glimpse of their child will be of an intubated, sedated child with monitors, invasive catheters, and swollen, distorted facies. Continued emotional support, social services, and education will help ease this transition.
Movement toward Intervention for Non–Life-Threatening Diseases: Myelomeningocele
At present, nearly all human fetal interventions are performed to prevent almost certain fetal demise secondary to a known congenital defect or pathophysiologic process. Myelomeningocele (MMC) is the first nonfatal birth defect to be treated in utero. MMC affects 0.5 to 1 per 1000 live births annually, with variations in both population and geography.250–252 At least 75% of affected individuals reach early adulthood; most deaths occur during infancy and the preschool years secondary to respiratory and neurologic complications.253 There is significant risk associated with this early fetal intervention. Many infants with MMC may be delivered prematurely as a direct result of intrauterine intervention, further adding to the risks of an already compromised infant.252 Some have argued that because MMC is a nonlethal defect, intrauterine intervention for potential reduced secondary morbidity may not justify the significant maternal morbidity or fetal mortality associated with this procedure. However, the severe morbidity associated with MMC, combined with the promising results of animal research, have led to consideration of prenatal intervention for this disorder. Initial human outcomes have demonstrated some improvement in secondary morbidity.252,254,255 Recent publication of the results of the Management of Myelomeningocele Study (or the MOMS trial) demonstrated a reduced need for placement of cerebrospinal fluid shunts and improved motor outcomes (e.g., earlier ambulation) at 30 months in the fetal intervention group, compared to infants whose repairs were deferred until after delivery. Nevertheless, significant maternal and fetal morbidities were reported.256
Future Considerations
With the advances in surgical and anesthetic techniques and technologies, significant progress may be made in mid-gestation fetal intervention, and in moving from treatment of only life-threatening fetal pathologic processes toward preemptive management of fetal disorders that are not necessarily life-threatening but have significant, disabling postpartum morbidities. However, these benefits may have to be balanced against the possibility of long-term neurocognitive disorders in anesthetized fetuses, particularly in those fetuses undergoing prenatal repair of anomalies that are not life threatening.257–259
Boat A, Mahmoud M, Michelfelder EC, et al. Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Paediatr Anaesth. 2010;20:748–756.
Courtier J, Poder L, Wang ZJ, et al. Fetal tracheolaryngeal airway obstruction: prenatal evaluation by sonography and MRI. Pediatr Radiol. 2010;40:1800–1805.
Fink RJ, Allen TK, Habib AS. Remifentanil for fetal immobilization and analgesia during the ex utero intrapartum treatment procedure under combined spinal-epidural anaesthesia. Br J Anaesth. 2011;106:851–855.
Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
Tran KM, Maxwell LG, Cohen DE, et al. Quantification of serum fentanyl concentrations from umbilical cord blood during ex utero intrapartum therapy. Anesth Analg. 2012;114:1265–1267.
1 Olutoye OO. Fetal surgery: coming to a center near you? J Intensive Care Med. 2008;23:67–69.
2 Garcia PJ, Olutoye OO, Ivey RT, Olutoye OA. Case scenario: anesthesia for maternal-fetal surgery: the ex utero intrapartum therapy (EXIT) procedure. Anesthesiology. 2011;114:1446–1452.
3 Pollard JB. Cardiac arrest during spinal anesthesia: common mechanisms and strategies for prevention. Anesth Analg. 2001;92:252–256.
4 Geffin B, Shapiro L. Sinus bradycardia and asystole during spinal and epidural anesthesia: a report of 13 cases. J Clin Anesth. 1998;10:278–285.
5 Auroy Y, Narchi P, Messiah A. Serious complications related to epidural anesthesia. Anesthesiology. 1997;87:479–486.
6 Baron JJ, Decaux-Jacolot A, Edourd A. Influence of venous return on baroreflex control of heart rate during lumbar epidural anesthesia in humans. Anesthesiology. 1986;64:188–193.
7 Ekholm EM, Erkkola RU, Piha SJ. Changes in autonomic cardiovascular control in mid-pregnancy. Clin Physiol. 1992;12:527–536.
8 Galinkin JL, Gaiser RR, Cohen DE, et al. Anesthesia for fetoscopic surgery: twin-reverse arterial perfusion sequence and twin-twin transfusion syndrome. Anesth Analg. 2000;91:1394–1397.
9 Sabik JF, Assad RS, Hanley FL. Halothane as an anesthetic for fetal surgery. J Pediatr Surg. 1993;28:542–546.
10 Biehl DR, Yarnell R, Wade JG. The uptake of isoflurane by the fetal lamb in utero: effect on epidural blood flow. J Can Anaesth Soc. 1983;30:581–586.
11 Palahnuik RJ, Shnider SM. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462–472.
12 Gaiser RR, Kurth CD. Anesthetic considerations for fetal surgery. Semin Perinatol. 1999;23:507.
13 Sibley C, D’Souza S, Glazier J, Greenwood S. Mechanisms of solute transfer across the human placenta: effects of intrauterine growth restriction. Fetal Maternal Med Rev. 1998;10:197–206.
14 Giannakoulopoulos X, Sepulveda W, Kourtis P, et al. Fetal plasma cortisol and beta-endorphin response to intrauterine needling. Lancet. 1994;344:77–81.
15 Gitau R, Fisk NM, Teixeira JM, et al. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–109.
16 Fisk NM, Gitau R, Teixeira JM, et al. Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling. Anesthesiology. 2001;95:828–835.
17 Giannakoulopoulos X, Teixeira J, Fisk N, Glover V. Human fetal and maternal noradrenaline responses to invasive procedures. Pediatr Res. 1999;45:494–499.
18 Tran KA, Maxwell LG, Cohen DE, et al. Quantification of serum fentanyl concentrations from umbilical cord blood during ex utero intrapartum therapy. Anesth Analg. 2012;114:1265–1267.
19 Hamamoto K, Iwamoto HS, Roman CM, et al. Fetal uptake of intraamniotic digoxin in sheep. Pediatr Res. 1990;27:282–285.
20 Strumper D, Durieux ME, Gogarten W, et al. Plasma concentrations after intraamniotic sufentanil in chronically instrumented pregnant sheep. Anesthesiology. 2003;98:1400–1406.
21 Locatelli A, Vergani P, Di Pirro G, et al. Role of amnioinfusion in the management of premature rupture of the membranes at <26 weeks’ gestation. Am J Obstet Gynecol. 2000;183:878–882.
22 Rotschild A, Ling EW, Puterman ML, Farquharson D. Neonatal outcome after prolonged preterm rupture of the membranes. Am J Obstet Gynecol. 1990;162:46–52.
23 Laudy JA, Tibboel D, Robben SG, et al. Prenatal prediction of pulmonary hypoplasia: clinical, biometric, and Doppler velocity correlates. Pediatrics. 2002;109:250–258.
24 Spong CY. Preterm premature rupture of the fetal membranes complicated by oligohydramnios. Clin Perinatol. 2001;28:753–759.
25 Nimrod C, Varela-Gittings F, Machin G, et al. The effect of very prolonged membrane rupture on fetal development. Am J Obstet Gynecol. 1984;148:540–543.
26 Kilbride HW, Yeast J, Thibeault DW. Defining limits of survival: lethal pulmonary hypoplasia after midtrimester premature rupture of membranes. Am J Obstet Gynecol. 1996;175:675–681.
27 Harding R, Hooper SB, Dickson KA. A mechanism leading to reduced lung expansion and lung hypoplasia in fetal sheep during oligohydramnios. Am J Obstet Gynecol. 1990;163:1904–1913.
28 Mescher EJ, Platzker AC, Ballard PL, et al. Ontogeny of tracheal fluid, pulmonary surfactant, and plasma corticoids in the fetal lamb. J Appl Physiol. 1975;39:1017–1021.
29 Carmel JFF, Adams F. Fetal tracheal ligation and lung development. Am J Dis Child. 1965;109:452–456.
30 Moessinger AC, Harding R, Adamson TM, et al. Role of lung fluid volume in growth and maturation of the fetal sheep lung. J Clin Invest. 1990;86:1270–1277.
31 Moessinger AC, Bassi GA, Ballantyne G, et al. Experimental production of pulmonary hypoplasia following amniocentesis and oligohydramnios. Early Hum Dev. 1983;8:343–350.
32 Harrison MR, Nakayama DK, Noall R, de Lorimier AA. Correction of congenital hydronephrosis in utero: II. Decompression reverses the effects of obstruction on the fetal lung and urinary tract. J Pediatr Surg. 1982;17:965–974.
33 Hadi HA, Hodson CA, Strickland D. Premature rupture of the membranes between 20 and 25 weeks’ gestation: role of amniotic fluid volume in perinatal outcome. Am J Obstet Gynecol. 1994;170:1139–1144.
34 Neerhof MG, Haney EI, Silver RK, et al. Lamellar body counts compared with traditional phospholipid analysis as an assay for evaluating fetal lung maturity. Obstet Gynecol. 2001;97:305–309.
35 Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525.
36 Kiserud T, Rasmussen S, Skulstad S. Blood flow and the degree of shunting through the ductus venosus in the human fetus. Am J Obstet Gynecol. 2000;182:147–153.
37 Kiserud T. The ductus venosus. Semin Perinatol. 2001;25:11–20.
38 Hanson MA, Spencer JAD, Rodeck CH. The circulation. Cambridge, UK: Cambridge University Press; 1993.
39 Jones CT, Robinson RO. Plasma catecholamines in foetal and adult sheep. J Physiol. 1975;248:15–33.
40 Martin AA, Kapoor R, Scroop GC. Hormonal factors in the control of heart rate in normoxaemic and hypoxaemic fetal, neonatal and adult sheep. J Dev Physiol. 1987;9:465–480.
41 Eden RD, Seifert LS, Frese-Gallo J, et al. Effect of gestational age on baseline fetal heart rate during the third trimester of pregnancy. J Reprod Med. 1987;32:285–286.
42 Snijders RJ, Sherrod C, Gosden CM, Nicolaides KH. Fetal growth retardation: associated malformations and chromosomal abnormalities. Am J Obstet Gynecol. 1993;168:547–555.
43 Giussani DA, Spencer JA, Moore PJ, Hanson MA. The effect of carotid sinus nerve section on the initial cardiovascular response to acute isocapnic hypoxia in fetal sheep in utero. J Physiol. 1990;432:33P.
44 Cohn HE, Piasecki GJ, Jackson BT. The effect of beta-adrenergic stimulation on fetal cardiovascular function during hypoxemia. Am J Obstet Gynecol. 1982;144:810–816.
45 Gilbert RD. Control of fetal cardiac output during changes in blood volume. Am J Physiol. 1980;238:H80–H86.
46 Cohen LS, Friedman JM, Jefferson JW, et al. A reevaluation of risk of in utero exposure to lithium. JAMA. 1994;271:146–150.
47 Itskovitz J, Goetzman BW, Rudolph AM. Effects of hemorrhage on umbilical venous return and oxygen delivery in fetal lambs. Am J Physiol. 1982;242:H543–H548.
48 Meyers RL, Paulick RP, Rudolph CD, Rudolph AM. Cardiovascular responses to acute, severe haemorrhage in fetal sheep. J Dev Physiol. 1991;15:189–197.
49 Myers LB, Cohen D, Galinkin J, et al. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569–578.
50 Delivoria-Papadopoulos M, Oski FA, Gottlieb AJ. Oxygen-hemoglobin dissociation curves: effect of inherited enzyme defects of the red cell. Science. 1969;165:601–602.
51 Gaiser RR, Kurth CD. Anesthetic considerations for fetal surgery. Semin Perinatol. 1999;23:507–514.
52 Luks FI, Johnson BD, Papadakis K, et al. Predictive value of monitoring parameters in fetal surgery. J Pediatr Surg. 1998;33:1297–1301.
53 Bower SJ, Flack NJ, Sepulveda W, et al. Uterine artery blood flow response to correction of amniotic fluid volume. Am J Obstet Gynecol. 1995;173:502–507.
54 Fisk NM, Tannirandorn Y, Nicolini U, et al. Amniotic pressure in disorders of amniotic fluid volume. Obstet Gynecol. 1990;76:210–221.
55 Fenton KN, Heinemann MK, Hickey PR, et al. Inhibition of the fetal stress response improves cardiac output and gas exchange after fetal cardiac bypass. J Thorac Cardiovasc Surg. 1994;107:1416–1422.
56 Skillman CA, Plessinger MA, Woods JR, Clark KE. Effect of graded reductions in uteroplacental blood flow on the fetal lamb. Am J Physiol. 1985;249:H1098–H1105.
57 Baker BW, Hughes SC, Shnider SM, et al. Maternal anesthesia and the stressed fetus: effects of isoflurane on the asphyxiated fetal lamb. Anesthesiology. 1990;72:65–70.
58 Kjellmer I, Dagbjartsson A, Hrbek A, et al. Maternal beta-adrenoceptor blockade reduces fetal tolerance to asphyxia: a study in pregnant sheep. Acta Obstet Gynecol Scand Suppl. 1984;118:75–80.
59 Okado N, Kakimi S, Kojima T. Synaptogenesis in the cervical cord of the human embryo: sequence of synapse formation in a spinal reflex pathway. J Comp Neurol. 1979;184:491–518.
60 Rabinowicz T, de Courten-Myers GM, Petetot JM, et al. Human cortex development: estimates of neuronal numbers indicate major loss late during gestation. J Neuropathol Exp Neurol. 1996;55:320–328.
61 Smith S. Commission of inquiry into fetal sentience. London: CARE; 1996. Available at http://www.care.org.uk
62 Crick F. The astonishing hypothesis. London: Simon & Schuster Ltd; 1994.
63 Barker DJ. In utero programming of cardiovascular disease. Theriogenology. 2000;53:555–574.
64 Kosaka Y, Takahashi T, Mark LC. Intravenous thiobarbituate anesthesia for cesarean section. Anesthesiology. 1969;31:489–506.
65 Daillard P, Cockshott ID, Lirzin JD, et al. Intravenous propofol during cesarean section: placental transfer, concentrations in breast milk and neonatal effects: a preliminary study. Anesthesiology. 1989;71:827–834.
66 Warren TW, Datta S, Ostheimer GW, et al. Comparison of the maternal and neonatal effects of halothane, enflurane and isoflurane for cesarean delivery. Anesth Analg. 1983;62:516–520.
67 Biehl DR, Coté J, Wade JD, et al. Uptake of halothane by the foetal lamb. Can Anaesth J. 1983;30:24–27.
68 Biehl DR, Yarnell R, Wade JG, et al. The uptake of isoflurane by the foetal lamb in utero: effect on regional blood flow. Can Anaesth Soc J. 1983;30:581–586.
69 Southern EM. Fetal anoxia and its possible relation to changes in the prenatal electrocardiogram. Am J Obstet Gynecol. 1957;73:233–247.
70 Murray HG. The fetal electrocardiogram: current clinical developments in Nottingham. J Perinatol Med. 1986;14:399–404.
71 Lee KH, Blackwell R. Observations on the configuration of the fetal electrocardiogram before and during labor. J Obstet Gynaecol Br Commonw. 1974;81:61–69.
72 Murray HG, Evaluation of the fetal electrocardiogram (ECG). Thesis, Nottingham, England, University of Nottingham, 1992.
73 Widmark C, Linddecrantz K, Murray H, Rosen KG. Changes in the PR, RR intervals and the ST waveform of the fetal electrocardiogram with acute hypoxemia. J Dev Physiol. 1992;18:99–103.
74 Mohajer MP, Sahota DS, Reed NN, et al. Cumulative changes in the fetal electrocardiogram and biochemical indices of fetal hypoxemia. Eur J Obstet Gynecol Reprod Biol. 1994;55:63–70.
75 van Wijngaarden WJ, Sahota DS, James DK, et al. Improved intrapartum surveillance with PR interval analysis of the fetal electrocardiogram: a randomized trial showing a reduction in fetal blood sampling. Am J Obstet Gynecol. 1996;174:1295–1299.
76 Peat S, Booker M, Lanigan C, Ponte J. Continuous intrapartum measurement of fetal oxygen saturation. Lancet. 1988;332:213.
77 Johnson N, Lilford RJ. Continuous intrapartum measurement of fetal oxygen saturation. Lancet. 1988;332:517.
78 Yam J, Chua S, Arulkumaran S. Intrapartum fetal pulse oximetry: II. Clinical application. Obstet Gynecol Surv. 2000;55:173–183.
79 Gardosi JO, Damianou D, Schram C. Artifacts in fetal pulse oximetry: incomplete sensor-to-skin contact. Am J Obstet Gynecol. 1994;170:1169–1170.
80 Lutkus AK, Dudenhausen JW. Fetal pulse oximetry. Baillieres Clin Obstet Gynaecol. 1996;10:295–306.
81 Johnson N, Johnson VA, Bannister J, Lilford R. The effect of caput succedaneum on oxygen saturation measurements. Br J Obstet Gynaecol. 1990;9:493–498.
82 Schram CMH, Gardosi JO. The effect of caput succedaneum on oxygen saturation measurements. Br J Obstet Gynaecol. 1991;98:113–114.
83 Johnson N. Development and potential of fetal pulse oximetry. Contemp Rev Obstet Gynecol. 1991;3:1–12.
84 Gardosi JO, Schram C, Symonds M. Adaptation of pulse oximetry for fetal monitoring during labor. Lancet. 1991;337:1265–1267.
85 Montague I, Johnson N, Comparing the oxygen saturation of the breech with cephalic presentation. Presented at the Blair Bell Research Society. London, 1993.
86 Knitza R, Rall G, Mainz S, et al. Fetale Geburtsuberwachung durch Oxykardiographie (OCTG). Gerbertshilfe Frauenheilkd. 1993;53:849–853.
87 Luttkus AK, Dimer JA, Dudenhausen JW. Are pulse oximetry findings in the breech consistent with fetal physiology? Am J Obstet Gynecol. 1998;178:48S.
88 Mannheimer PD, Casciani JR, Fein ME, et al. Wavelength selection for low-saturation pulse oximetry. IEEE Trans Biomed Eng. 1997;447:148–158.
89 Chua S, Yeong SM, Razvi K, et al. Fetal oxygen saturation during labour. Br J Obstet Gynaecol. 1997;104:1080–1083.
90 Myers LB, Bulich LA, Mizrahi A, et al. Ultrasonographic guidance for location of the trachea during the EXIT procedure for cervical teratoma. J Pediatr Surg. 2003;38E:12–14.
91 Wladimiroff JW, Tonge HM, Stewart PA. Doppler ultrasound assessment of cerebral blood flow in the human fetus. Br J Obstet Gynaecol. 1986;93:471–475.
92 Sutterlin MW, Seelbach-Gobel B, Oehler MK, et al. Doppler ultrasonographic evidence of brain-sparing effect in fetuses with low oxygen saturation according to pulse oximetry. Am J Obstet Gynecol. 1999;181:216–220.
93 Jeronima MA, Teixeira MD, Glover V, Fisk NM. Acute cerebral redistribution in response to invasive procedures in the human fetus. Am J Obstet Gynecol. 1999;181:1018–1025.
94 Kunzel W. [Fetal shock syndrome]. Z Geburtshilfe Perinatol. 1986;190:177–184.
95 Rosen MA. Management of anesthesia for the pregnant surgical patient. Anesthesiology. 1999;91:1159–1163.
96 Archer GW, Marx GF. Arterial oxygen tension during apnea in parturient women. Br J Anaesth. 1974;46:358–360.
97 Norris MC, Kirkland MR, Torjman MC, Goldberg ME. Denitrogenation in pregnancy. Can J Anaesth. 1989;36:523–525.
98 Palahniuk RJ, Shnider SM, Eger EI. Pregnancy decreases the requirement of inhaled anesthetic agents. Anesthesiology. 1974;41:82–83.
99 Thornburg KL, Jacobson SL, Giraud GD, Morton MJ. Hemodynamic changes in pregnancy. Semin Perinatol. 2000;24:11–14.
100 Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:1060–1065.
101 Hunter S, Robson SC. Adaptation of the maternal heart in pregnancy. Br Heart J. 1992;68:540–543.
102 Milsom I, Forssman L. Factors influencing aortocaval compression by the uterus in late human pregnancy: an arteriographic study. Am J Obstet Gynecol. 1968;100:203–217.
103 Ngan Kee WD, Khaw KS, Tan PE, Ng FF, Karmakar MK. Placental transfer and fetal metabolic effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2009;111:506–512.
104 Cogan R, Spinnato JA. Pain and discomfort thresholds in late pregnancy. Pain. 1986;27:63–68.
105 Dawson-Basoa M, Gintzler AR. Gestational and ovarian sex steroid antinociception: synergy between spinal kappa and delta opioid systems. Brain Res. 1998;794:61–67.
106 Chan MT, Mainland P, Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology. 1996;85:782–786.
107 Evans SE, Crawford JS, Stevens ID, et al. Fluid therapy for induced labour under epidural analgesia: biochemical consequences for mother and infant. Br J Obstet Gynaecol. 1986;93:329–333.
108 Ames C, Cobbold S, Maddock J. Lactic acidosis complicating treatment of ketosis of labour. BMJ. 1975;4:611–613.
109 Gin T, Mainland P, Chan M, et al. Decreased thiopental requirements in early pregnancy. Anesthesiology. 1997;86:73–78.
110 Sanchez-Alcaraz A, Quintana MB, Laguarda M. Placental transfer and neonatal effects of propofol in caesarean section. J Clin Pharm Ther. 1998;23:19–23.
111 Baraka A, Louis F, Dalleh R. Maternal awareness and neonatal outcome after ketamine induction of anaesthesia for caesarean section. Can J Anaesth. 1990;37:641–644.
112 Karsli B, Kaya T, Cetin A. Effects of intravenous anesthetic agents on pregnant myometrium. Pol J Pharmacol. 1999;51:505–510.
113 Leighton BL, Cheek TG, Gross JB. Succinylcholine pharmacodynamics in peripartum patients. Anesthesiology. 1986;64:202–205.
114 Shnider SM. Serum cholinesterase activity during pregnancy, labor and the puerperium. Anesthesiology. 1965;26:335.
115 Jan GS, Tong WN, Chan AM, et al. Recovery from mivacurium block with or without anticholinesterase following continuous infusion in obstetric patients. Anaesth Intensive Care. 1996;24:585–589.
116 Khuenl-Brady KS, Koller J, Mair P, et al. Comparison of vecuronium-and atracurium-induced neuromuscular blockade in postpartum and nonpregnant patients. Anesth Analg. 1991;72:110–113.
117 Guay J, Grenier Y, Varin F. Clinical pharmacokinetics of neuromuscular relaxants in pregnancy. Clin Pharmacokinet. 1998;34:483.
118 Pan PH, Moore C. Comparison of cisatracurium-induced neuromuscular blockade between immediate postpartum and nonpregnant patients. J Clin Anesth. 2001;13:112–117.
119 Gin T, Chan MT. Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology. 1994;81:829–832.
120 Bravermann D, Johnson M, Kern F. Effects of pregnancy and contraceptive steroids on gallbladder function. N Engl J Med. 1980;302:3624.
121 Kern F, Everson G, DeMark B, et al. Biliary lipids, bile acids, and gallbladder function in the human female: effects of pregnancy and the ovulatory cycle. J Clin Invest. 1981;68:1229–1242.
122 Carter J. Liver function in normal pregnancy. Aust N Z J Obstet Gynaecol. 1990;30:296–302.
123 Naftalin NJ, McKay DM, Phear WPC, et al. The effects of halothane on pregnant and nonpregnant human myometrium. Anesthesiology. 1977;46:15–19.
124 Munson ES, Embro WJ. Enflurane, isoflurane, and halothane and isolated human uterine muscle. Anesthesiology. 1977;46:11–14.
125 Turner RJ, Lambrost M, Holmes C, et al. The effects of sevoflurane on isolated gravid human myometrium. Anaesth Intensive Care. 2002;30:591–596.
126 Langer JC, Harrson MR, Schmidt KG, et al. Fetal hydrops and death from sacrococcygeal teratoma: rationale for fetal surgery. Am J Obstet Gynecol. 1989;160:1145–1150.
127 Bouchard S, Johnson MP, Flake AW, et al. The EXIT procedure; experience and outcomes in 31 cases. J Pediatr Surg. 2002;37:418–426.
128 Stevens GH, Schoot BC, Smets MJ, et al. The ex utero intrapartum treatment (EXIT) procedure in fetal neck masses: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2002;100:246–250.
129 Liu YL, Nwosu UC, Rice PJ. Relaxation of isolated human myometrial muscle by beta2-adrenergic receptors but not beta1-adrenergic receptors. Am J Obstet Gynecol. 1998;179:895–898.
130 Macones GA, Marder SJ, Clothier B, Stamilio DM. The controversy surrounding indomethacin for tocolysis. Am J Obstet Gynecol. 2001;184:264–272.
131 Jeyabalan A, Caritis SN. Pharmacologic inhibition of preterm labor. Clin Obstet Gynecol. 2002;45:99–113.
132 Hearne AE, Nagey DA. Therapeutic agents in preterm labor: tocolytic agents. Clin Obstet Gynecol. 2000;43:787–801.
133 Wheeler AS, James FM, III., Meis PJ, et al. Effects of nitroglycerin and nitroprusside on the uterine vasculature of gravid ewes. Anesthesiology. 1980;52:390–394.
134 DiFederico EM, Burlingame JM, Kilpatrick SJ, et al. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–933.
135 Papatsonis DN, Van Geijn HP, Ader HJ, et al. Nifedipine and ritodrine in the management of preterm labor: a randomized multicenter trial. Obstet Gynecol. 1997;90:230–234.
136 King JF, Flenady VJ, Papatsonis DN, et al. Calcium channel blockers for inhibiting preterm labour. Cochrane Database Syst Rev. 2003. CD002255
137 Guinn DA, Goepfert AR, Owen J, et al. Terbutaline pump maintenance therapy for prevention of preterm delivery: a double-blind trial. Am J Obstet Gynecol. 1998;179:874–878.
138 Wenstrom KD, Weiner CP, Merrill D, Niebyl J. A placebo-controlled randomized trial of the terbutaline pump for prevention of preterm delivery. Am J Perinatol. 1997;14:87–91.
139 Sydorak RM, Albanese CT. Minimal access techniques for fetal surgery. World J Surg. 2003;27:95–102.
140 Adzick NS, Harrison MR, Crombleholme TM, et al. Fetal lung lesions: management and outcome. Am J Obstet Gynecol. 1998;179:884–889.
141 DiFederico EM, Burlingame JM, Kilpatrick SJ, et al. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of nitroglycerine tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–933.
142 MacLennan FM, Thomson MA, Rankin R, et al. Fatal pulmonary oedema associated with the use of ritodrine in pregnancy. Br J Obstet Gynaecol. 1985;92:703–705.
143 Yeast JD, Halberstadt C, Meyer BA, et al. The risk of pulmonary edema and colloid osmotic pressure changes during magnesium sulfate infusion. Am J Obstet Gynecol. 1993;169:1566–1571.
144 Palahniuk RJ, Shnider SM. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462–472.
145 Chavkin Y, Kupfersztain D, Ergaz Z, et al. Successful outcome of idiopathic nonimmune hydrops fetalis treated by maternal digoxin. Gynecol Obstet Invest. 1996;42:137–139.
146 Yournis JS, Granat M. Insufficient transplacental digoxin transfer in severe hydrops fetalis. Am J Obstet Gynecol. 1987;157:1268–1269.
147 Adzick NS, Harrison MR, Crombleholme TM, et al. Fetal lung lesions; management and outcome. Am J Obstet Gynecol. 1998;179:884–889.
148 Adzick NS, Harrison MR. Management of the fetus with a cystic adenomatoid malformation. World J Surg. 1993;17:342–349.
149 Nicolini U, Cerri V, Groli C, et al. A new approach to prenatal treatment of extralobar pulmonary sequestration. Prenat Diagn. 2000;20:758–760.
150 Miller RK, Sieber WK, Yunis EJ. Congenital cystic adenomatoid malformation of the lung: a report of 17 cases and review of the literature. Pathol Annu. 1980;1:387–407.
151 Stocker JJ, Medwell JE, Drake RM. CCAM of the lung: classification and morphologic spectrum. Hum Pathol. 1977;8:155.
152 Leninger BJ, Haight C. Congenital cystic adenomatoid malformation of the left lower lobe with compression of the remaining lung. Clin Pediatr. 1973;12:182–186.
153 Hernanz-Schulman M, Stein SM, Neblett WW, et al. Pulmonary sequestration: diagnosis with color Doppler sonography and a new theory of associated hydrothorax. Radiology. 1991;180:817–821.
154 Gerle RD, Jaretzki A, III., Asheley CA, et al. Congenital bronchopulmonary foregut malformation: pulmonary sequestration with the gastrointestinal tract. N Engl J Med. 1968;278:1413–1419.
155 Levi A, Findler M, Dolfin T, et al. Intrapericardial extralobar pulmonary sequestration in a neonate. Chest. 1990;98:1014–1015.
156 Harrison MR. Fetal surgery. Am J Obstet Gynecol. 1996;174:1255–1264.
157 Bianchi DW, Crombleholme TM, D’Alton ME. Fetology: diagnosis and management of the fetal patient. New York: McGraw-Hill; 2000. p. 959
158 Ross MG, Ervin MG, Leake RD, et al, Fetal lung fluid response to maternal hyperosmolality. Scientific abstract presented before the 32nd meeting of the Society of Gynecologic Investigation. Phoenix: March 20-3, 1985.
159 Adzick NS, Harrison MR, Flake MR, et al. Fetal surgery for cystic adenomatoid malformation of the lung. J Pediatr Surg. 1993;28:806–812.
160 Myers LB, Cohen D, Galinkin J, et al. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569–578.
161 Boat A, Mahmoud M, Michelfelder EC, et al. Supplementing desflurane with intravenous anesthesia reduces fetal cardiac dysfunction during open fetal surgery. Paediatr Anaesth. 2010;20:748–756.
162 Ioscovich A, Shen O, Sichel JY, et al. Remifentanil-nitroglycerin combination as an anesthetic support for ex utero intrapartum treatment (EXIT) procedure. Clin Anesth. 2011;23:142–144.
163 Fink RJ, Allen TK, Habib AS. Remifentanil for fetal immobilization and analgesia during the ex utero intrapartum treatment procedure under combined spinal-epidural anaesthesia. Br J Anaesth. 2011;106:851–855.
164 McNamara H, Johnson N. The effect of uterine contractions on fetal oxygen saturation. Br J Obstet Gynecol. 1995;102:664–667.
165 Finster MM, Ralston DH, Pedersen H. Perinatal pharmacology. In: Shnider SM, Levinson G, eds. Anesthesia for obstetrics. Baltimore: Williams & Wilkins; 1993:71–82.
166 Boue DR, Smith GA, Krous HF. Lingual bronchogenic cyst in a child; an unusual site of presentation. Pediatr Pathol. 1994;14:201–205.
167 Motoyama EK, Rivard G, Acheson F, et al. Adverse effect of maternal hyperventilation on the fetus. Lancet. 1966;1:286–288.
168 Motoyama EK, Rivard G, Acheson F, et al. The effect of changes in maternal pH and Pco2 on the Po2 of fetal lambs. Anesthesiology. 1967;28:891–903.
169 Rivard G, Motoyama E, Acheson F, et al. The relation between maternal and fetal oxygen tensions in sheep. Am J Obstet Gynecol. 1967;97:925–930.
170 DiFederico EM, Harrison M, Matthay MA. Pulmonary edema in a woman following fetal surgery. Chest. 1996;109:1114–1117.
171 Creasy R. Mirror syndromes. In: Goodlin RC, ed. Care of the fetus. New York: Masson; 1979:48–50.
172 Gaiser RR, Kurth CD. Anesthetic considerations for fetal surgery. Semin Perinatol. 1999;23:507–514.
173 Myers LB, Cohen D, Galinkin J, et al. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569–578.
174 Gaiser RR, Cheek TG, Kurth CD. Anesthetic management of cesarean delivery complicated by ex-utero intrapartum treatment of the fetus. Anesth Analg. 1997;84:1150.
175 Quinn TM, Adzick NS. Fetal surgery. Obstet Gynecol Clin North Am. 1997;24:143–157.
176 Castracane VD. Endocrinology of preterm labor. Clin Obstet Gynecol. 2000;43:717–726.
177 Gerle RD, Jaretzki A, III., Asheley CA, et al. Congenital bronchopulmonary foregut malformation: pulmonary sequestration with the gastrointestinal tract. N Engl J Med. 1968;278:1413–1419.
178 Dembinski J, Kaminski M, Schild R, et al. Congenital intrapulmonary bronchogenic cyst in the neonate–prenatal management. Am J Perinatol. 1999;16:509–514.
179 Adzick NS, Harrison MR, Flake MR, et al. Fetal surgery for cystic adenomatoid malformation of the lung. J Pediatr Surg. 1993;28:806–812.
180 Schiffer MA, Greenberg E. Sacrococcygeal teratoma in labor and the newborn. Am J Obstet Gynecol. 1956;72:1054–1062.
181 Abbott PD, Bowman A, Kantor HI. Dystocia caused by sacrococcygeal teratoma. Obstet Gynecol. 1966;27:571–579.
182 Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma. American Academy of Pediatrics Surgical Section Survey 1973. J Pediatr Surg. 1974;9:389–398.
183 Gross RE, Clatworthy HW, Meeker IA. Sacrococcygeal teratomas in infants and children. Surg Gynecol Obstet. 1951;92:341–354.
184 Mahour GH, Woolley MM, Trinedi SN, et al. Sacrococcygeal teratoma: a 33 year experience. J Pediatr Surg. 1975;10:183–188.
185 Chervenak FA, Isaacson G, Touloukian R, et al. Diagnosis and management of fetal teratomas. Obstet Gynecol. 1985;66:666–671.
186 Seeds JW, Mittlestaedt CA, Cefalo RC, et al. Prenatal diagnosis of sacrococcygeal teratoma: an anechoic caudal mass. J Clin Ultrasound. 1982;10:193–195.
187 Uchiyama M, Iwafuchi M, Naitoh M, et al. Sacrococcygeal teratoma: a series of 19 cases with long-term follow-up. Eur J Pediatr Surg. 1999;3:158–162.
188 Langer JC, Harrison MR, Schmidt KG, et al. Fetal hydrops and death from sacrococcygeal teratoma: rationale for fetal surgery. Am J Obstet Gynecol. 1989;160:1145–1150.
189 Tworetzky W, McElhinney DB, Reddy VM, et al. Improved surgical outcome after fetal diagnosis of hypoplastic left heart syndrome. Circulation. 2001;103:1269–1273.
190 Bonnet D, Coltri A, Butera G, et al. Detection of transposition of the great arteries in fetuses reduces neonatal morbidity and mortality. Circulation. 1999;99:916–918.
191 Maxwell D, Allan L, Tynan MJ. Balloon dilation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991;65:256–258.
192 Allan LD, Maxwell DJ, Carminati M, Tynan MJ. Survival after fetal aortic balloon valvuloplasty. Ultrasound Obstet Gynecol. 1995;5:90–91.
193 Kohl T, Sharland G, Allan LD, et al. World experience of percutaneous ultrasound-guided balloon valvuloplasty in human fetuses with severe aortic valve obstruction. Am J Cardiol. 2000;15:1230–1233.
194 Rychik J, Rome JJ, Collins MH, et al. The hypoplastic left heart syndrome with intact septum: atrial morphology, pulmonary vascular histopathology and outcome. J Am Coll Cardiol. 1999;34:554–560.
195 Tweddell JS, Hoffman GM, Mussatto KA, et al. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–I89.
196 Pollard JB. Cardiac arrest during spinal anesthesia: common mechanisms and strategies for prevention. Anesth Analg. 2001;92:252–256.
197 Tame JD, Abrams LM, Ding XY. Level of postoperative analgesia is a critical factor in regulation of myometrial contractility after laparotomy in the pregnant baboon: implications for human fetal surgery. Am J Obstet Gynecol. 1999;180:1196–1201.
198 Fauza DO, Berde CB, Fishman SJ. Prolonged myometrial blockade prevents preterm labor after fetal surgery in a leporine model. J Pediatr Surg. 1999;34:540–542.
199 Naftalin NJ, McKay DM, Phear WPC, et al. The effects of halothane on pregnant and nonpregnant human myometrium. Anesthesiology. 1977;46:15–19.
200 Miller JR, Stoelting VK, Stander RW, et al. In vitro and in vivo responses of the uterus to halothane anesthesia. Anesth Analg. 1966;45:583–589.
201 Gilbert RD. Control of fetal cardiac output during changes in blood volume. Am J Physiol. 1980;238:1180–1186.
202 Reddy VM, Liddicoat JR, Klein JR, et al. Fetal cardiac bypass using an in-line axial flow pump to minimize extracorporeal surface and avoiding priming volume. Ann Thorac Surg. 1996;62:393–400.
203 Reddy VM, Liddicoat JR, Klein JR, et al. Long-term fetal outcome after fetal cardiac bypass: fetal survival to full term and organ abnormalities. J Thorac Cardiovasc Surg. 1996;111:536–544.
204 Kohl T, Westphal M, Strumper D, et al. Multimodal fetal transesophageal echocardiography for fetal cardiac intervention in sheep. Circulation. 2001;104:1757–1760.
205 Kohl T, Strumper D, Witteler R, et al. Fetoscopic direct fetal cardiac access in sheep: an important experimental milestone along the route to human fetal cardiac intervention. Circulation. 2000;102:1602–1604.
206 Kohl T, Szabo Z, Suda K, et al. Fetoscopic and open transumbilical fetal cardiac catheterization in sheep: potential approaches for fetal cardiac intervention. Circulation. 1997;18:1048–1053.
207 Tworetzky W, Marshall AC. Balloon valvuloplasty for congenital heart disease in the fetus. Clin Perinatol. 2003;30:541–550.
208 DiFederico EM, Burlingame JM, Kilpatrick SJ. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925–933.
209 DiFederico EM, Harrison MR, Natthay MA. Pulmonary edema in a woman following fetal surgery. Chest. 1996;109:1114–1117.
210 McGregor M, Davenport HT, Jegier W, et al. Cardiovascular effects of halothane in normal children. Br J Anaesth. 1958;30:398–408.
211 Lichtor JL, Beher BE, Ruschhaupt DG. Myocardial depression during induction in infants [Abstract]. Anesthesiology. 1983;59:A452.
212 Neerhof MG, Haney EI, Silver RK, et al. Lamellar body counts compared with traditional phospholipid analysis as an assay for evaluating fetal lung maturity. Obstet Gynecol. 2001;97:305–309.
213 Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–525.
214 Bellotti M, Rognoni G, de Gasperi C, et al. Controlled fetal bloodletting of the recipient twin as a new method for the treatment of severe twin-twin transfusion syndrome: preliminary results. Ultrasound Obstet Gynecol. 2001;18:666–668.
215 Mahieu-Caputo D, Muller F, Joly D, et al. Pathogenesis of twin-twin transfusion syndrome: the renin-angiotensin system hypothesis. Fetal Diagn Ther. 2001;16:241–244.
216 van Gemert MJ, Umur A, Tijssen JG, et al. Twin-twin transfusion syndrome: etiology, severity and rational management. Curr Opin Obstet Gynecol. 2001;13:193–206.
217 Sherer DM. Adverse perinatal outcome of twin pregnancies according to chorionicity: review of the literature. Am J Perinatol. 2001;18:23–37.
218 Seng YC, Rajadurai VS. Twin-twin transfusion syndrome: a five year review. Arch Dis Child Fetal Neonat Ed. 2000;83:F16–F70.
219 Quintero RA, Comas C, Bornick PW, et al. Selective versus non-selective laser photocoagulation of placental vessels in twin-twin transfusion syndrome. Ultrasound Obstet Gynecol. 2000;16:230–236.
220 Ville Y, Hecher K, Gagnon A, et al. Endoscopic laser coagulation in the management of severe twin-twin transfusion syndrome. Br J Obstet Gynecol. 1998;105:446–453.
221 Hubinont C, Bernard P, Pirot N, et al. Twin-twin transfusion syndrome: treatment by amniodrainage and septostomy. Eur J Obstet Gynecol Reprod Biol. 2000;92:141–144.
222 Galinkin JL, Gaiser RR, Cohen DE, et al. Anesthesia for fetoscopic surgery: twin-reverse arterial perfusion sequence and twin-twin transfusion syndrome. Anesth Analg. 2000;91:1394–1397.
223 Shnider SM, Levinson G. Anesthesia for obstetrics, 3rd ed. Baltimore: Williams & Wilkins; 1999.
224 Myers LB, Watcha M. Regional versus general anesthesia for twin-twin transfusion syndrome requiring fetal surgery. Fetal Diagn Ther. 2004;19:286–291.
225 Moore TR, Gale S, Benirschke K. Perinatal outcome of forty-nine pregnancies complicated by acardiac twinning. Am J Obstet Gynecol. 1990;163:907–912.
226 Ash K, Harman CR, Gritter H. TRAP sequence–successful outcome with indomethacin treatment. Obstet Gynecol. 1990;76:960.
227 Platt LD, DeVore GR, Bieniarz A, et al. Antenatal diagnosis of acephalus acardia: a proposed management scheme. Am J Obstet Gynecol. 1983;146:857.
228 Norris MC, Joseph J, Leighton BL. Anesthesia for perinatal surgery. Am J Perinatol. 1989;6:39–40.
229 Skarsgard ED, Chitkara U, Krane EJ, et al. The OOPS procedure (operation on placental support): in utero airway management of the fetus with prenatally diagnosed tracheal obstruction. J Pediatr Surg. 1996;31:826–828.
230 Bouchard S, Johnson MP, Flake AW, et al. The EXIT procedure; experience and outcomes in 31 cases. J Pediatr Surg. 2002;37:418–426.
231 Myers LB, Bulich LA, Mizrahi A, et al. Ultrasonographic guidance for the location of the trachea during the EXIT procedure for cervical teratoma. J Pediatr Surg. 2003;38:E12–E14.
232 Zerella JT, Finberg FJ. Obstruction of the neonatal airway from teratomas. Surg Gynecol Obstet. 1990;170:126–131.
233 Bianchi DW, Crombleholme TM, D’Alton ME, eds. Fetology: diagnosis and management of the fetal patient. New York: McGraw-Hill, 2000.
234 Stevens GH, Schoot BC, Smets MJ, et al. The ex utero intrapartum treatment (EXIT) procedure in fetal neck masses: a case report and review of the literature. Eur J Obstet Gynecol Reprod Biol. 2002;100:246–250.
235 Kelly MF, Berenholz L, Rizzo KA, et al. Approach for oxygenation of the newborn with airway obstruction due to a cervical mass. Ann Otol Rhinol Laryngol. 1990;99:179–182.
236 Richards DS, Yancey MK, Duff P, et al. The perinatal management of severe laryngeal stenosis. Obstet Gynecol. 1992;80:537–540.
237 Tournier G, Goossens M, Bessis R, et al. Diagnostic antenatal et maladies gentiques pneumologiques. Rev Mal Respir. 1988;5:231–238.
238 Watson WJ, Thorp JM, Miller RC, et al. Prenatal diagnosis of laryngeal atresia. Am J Obstet Gynecol. 1990;163:1456–1457.
239 DeCou JM, Jones DC, Jacobs HD, et al. Successful ex utero intrapartum treatment (EXIT) procedure for congenital high airway obstruction syndrome (CHAOS) owing to laryngeal atresia. J Pediatr Surg. 1998;33:1563–1565.
240 Belfar HL, Foley TP, Hill LM, et al. Sonographic findings in maternal hyperthyroidism: fetal hyperthyroidism/fetal goiter. J Ultrasound Med. 1991;10:281–284.
241 Hadi HA, Strickland D. Prenatal diagnosis and management of fetal goiter caused by maternal Graves disease. Am J Perinatol. 1995;12:240–242.
242 MacKenzie TC, Crombleholme TM, Flake AW. The ex-utero intrapartum treatment. Curr Opin Pediatr. 2002;14:453–458.
243 Myers LB, Cohen D, Galinkin J, et al. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569–578.
244 Gaiser RR, Cheek TG, Kurth CD. Anesthetic management of cesarean delivery complicated by ex utero intrapartum treatment of the fetus. Anesth Analg. 1997;84:1150–1153.
245 Gaiser RR, Kurth CD, Cohen D, et al. The cesarean delivery of a twin gestation under 2 minimum alveolar anesthetic concentration isoflurane: one normal and one with a large neck mass. Anesth Analg. 1995;81:90–95.
246 Morris LM, Lim FY, Elluru RG, et al. Severe micrognathia: indications for EXIT-to-airway. Fetal Diagn Ther. 2009;26:162–166.
247 Courtier J, Poder L, Wang ZJ, et al. Fetal tracheolaryngeal airway obstruction: prenatal evaluation by sonography and MRI. Pediatr Radiol. 2010;40:1800–1805.
248 Langer JC, Tabb T, Thompson P, et al. Management of prenatally diagnosed tracheal obstruction; access to the airway in utero prior to delivery. Fetal Diagn Ther. 1992;7:12–16.
249 Butwick A, Aleshi P, Yamout I. Obstetric hemorrhage during an EXIT procedure for severe fetal airway obstruction. Can J Anaesth. 2009;56:437–442.
250 Coleman BG, Adzick NS, Crombleholme TM, et al. Fetal therapy: state of the art. J Ultrasound Med. 2002;21:1257–1288.
251 Ertl-Wagner B, Lienemann A, Strauss A, et al. Fetal magnetic resonance imaging: indications, technique, anatomical considerations and a review of fetal abnormalities. Eur Radiol. 2002;12:1931–1940.
252 Farmer DL, Koch CS, Peacock WJ, et al. In utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. Arch Surg. 2003;138:872–878.
253 Bowman RM, McLone DG, Grant JA, et al. Spina bifida outcome: a 25-year prospective. Pediatr Neurosurg. 2001;34:114–120.
254 Hirose S, Meuli-Simmem C, Meuli M. Fetal surgery for myelomeningocele: panacea or peril? World J Surg. 2003;27:87–94.
255 Hirose S, Farmer DL, Albanese CT. Fetal surgery for myelomeningocele. Curr Opin Obstet Gynecol. 2001;13:215–222.
256 Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004.
257 Wilder RT, Flick RP, Sprung J, et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804.
258 Flood PD. Fetal anesthesia and brain development. Anesthesiology. 2011;114:479–480.
259 Wilder RT. Is there any relationship between long-term behavior disturbance and early exposure to anesthesia? Curr Opin Anaesthesiol. 2010;23:332–336.