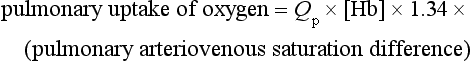FETAL CARDIOVASCULAR SYSTEM AND CONGENITAL HEART DISEASE
Introduction
Though the main functions of the fetal cardiovascular system are the same as in the adult, i.e. the distribution of oxygen and nutrients to the body and the transfer of carbon dioxide and waste products to the organ of excretion, there are some fundamental differences. Before birth the lungs are collapsed and filled with fluid and the organ of oxygenation, nutrient delivery and waste excretion is the placenta. The fetus is essentially a parasite and must extract oxygen and nutrients from the maternal circulation. Despite adaptations, such as an increased affinity of fetal haemoglobin (HbF) for oxygen compared with adult haemoglobin (HbA), the fetus exists in a state of relative hypoxia. Haemoglobin in the blood returning from the placenta is generally around 80% saturated with oxygen compared to above 98% in the pulmonary venous blood of a child or adult (see Chapter 1). Carbon dioxide levels are correspondingly elevated in the fetus. It is important to remember that the fetus is adapted to tolerate these conditions and aspects of this adaptation initially persist after birth.
As in adult life the brain, after the heart, is the dominant oxygen demanding organ. In contrast to the adult, in the fetus the oxygen supply comes from the placenta and so the most highly oxygenated blood arrives at the heart in the inferior vena cava rather than in the pulmonary veins. This blood is partially desaturated at around 80% despite the high affinity of HbF for oxygen. In order to ensure optimum supply of oxygen to the brain the fetal circulation has two major structural differences compared to that in the adult, the ductus arteriosus and the foramen ovale. Figure 12.1 summarizes the fetal circulation.
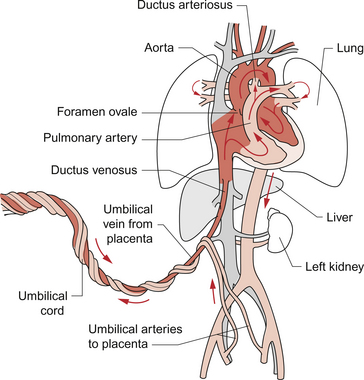
Fig. 12.1 The fetal circulation.
The division of blood flow at the level of the right atrium has an important impact on the flow of blood around the aortic arch. Since the major recipient of the relatively well oxygenated blood is the brain, only a small proportion (about 10%) of the left ventricular output passes through the section of the aortic arch between the origin of the left subclavian artery and the opening of the ductus arteriosus.
To re-cap, the main features of the fetal circulation compared with the adult circulation are as shown in Table 12.1.
Table 12.1
Main features of the fetal circulation compared with the adult circulation
| Fetus | Adult |
| Newly oxygenated, but still partially desaturated, blood leaves the placenta and arrives at the right atrium in the inferior vena cava | Blood which is nearly fully saturated with oxygen arrives at the left atrium via the pulmonary veins |
| Pressure in right atrium is higher than that in the left atrium | Left atrial pressure is higher than that in the right atrium |
| Right-to-left shunting of blood occurs across the foramen ovale | There is no shunt at atrial level |
| The right ventricle supplies blood to the systemic circulation | The right ventricle only supplies blood to the pulmonary circulation |
| The right ventricle output predominantly passes through the ductus arteriosus into the aorta | The right ventricle output passes to the lungs |
| Resistance to blood flow is low in the placenta and this leads to a low peripheral resistance overall | Peripheral resistance to blood flow is high |
| Stiff, fluid-filled, lungs have high resistance to flow | Expanded, air-filled lungs, have low resistance to blood flow |
Sometimes transition from fetal to adult circulation at birth is incomplete, leading to so-called persistent fetal circulation. An example of a case history is shown in Case 12.1:1.
How does the transition from fetal to adult circulation occur?
Changes at birth
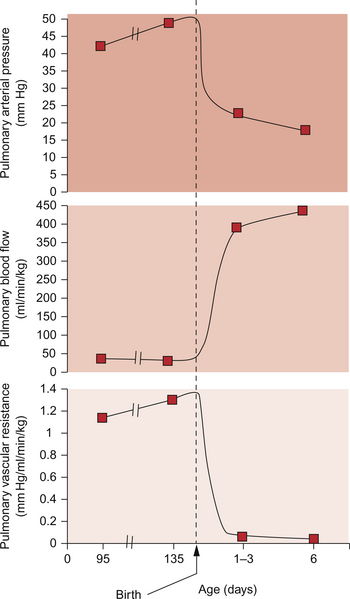
The first breath opens the airways and allows gas exchange across the alveolar membrane. The lungs expand and blood vessels open. This initiates a drop in the pulmonary vascular resistance (see Figure 12.5). Combined with the sudden rise in peripheral resistance resulting from the loss of the low-resistance placenta, there is a profound alteration in haemodynamics in the newborn infant. The blood leaving the right ventricle is preferentially directed through the now low-resistance lungs and returns to the left atrium. This increase in venous flow into the left atrium raises the left atrial pressure. At the same time there is a fall in right atrial pressure because venous return to this chamber has been reduced following the loss of the placental circulation. As left atrial pressure is now higher than right atrial pressure the foramen ovale, a flap valve, closes. Thus, in the newborn any detectable flow across the atrial septum is predominantly left to right. With the changes in pressures and the establishment of lung ventilation there is a sharp increase in blood oxygen content. This improved tissue oxygenation reduces the acidaemia to which the fetus has been adapted. Rising pH and Pao2 inhibit the synthesis of prostaglandins in the ductus arteriosus. This leads to closure of the ductus arteriosus separating the pulmonary circulation from the systemic circulation. Though generally the duct has closed within 24 hours of birth, early echocardiography demonstrates that a small left to right ductal shunt persists in a large minority of newborns for some days or even weeks. However this shunt is not usually of clinical significance.
Longer term changes in the heart during childhood and adulthood
Table 12.2 summarizes the normal variation of HR and BP with age. An estimate of the normal systolic BP, for a given age in children, can be made from the formula: systolic BP = 80 + (2 × age in years).
Table 12.2
Heart rate and BP variations with age
| Age (yr) | HR (bpm) | Systolic BP (mm Hg) |
| <1 | 110–160 | 70–90 |
| 1–2 | 100–150 | 80–95 |
| 2–5 | 95–140 | 80–100 |
| 5–12 | 80–120 | 90–110 |
| >12 | 60–100 | 100–120 |
The variation in heart rate reflects the relatively small size of the infant heart compared with its body mass and the relatively low compliance (increased stiffness) of the ventricles. The stiff ventricles mean that the stroke volume of the heart responds less dramatically to increasing venous return (preload effects) compared to an adult (see Chapter 4). In order to meet the demand for increased cardiac output the newborn heart rate must rise substantially, even at rest. Thus it is not unusual to see heart rates in newborns varying from 90 bpm when deeply asleep to 160 bpm when only slightly active. In the sick newborn under stress heart rates over 200 bpm may be seen with the heart still in sinus rhythm.
The normal ECG in childhood
Broadly speaking the ECG of the child has the same components as that of the adult, i.e. P waves, QRS complexes and T waves (see Chapter 7). However the relative contribution of the right and left ventricles to the shape of the ECG trace is different in children compared to adults. There is therefore a progressive change in the pattern of the ECG through childhood which reflects the declining contribution of the muscle mass of the right ventricle compared to the left ventricle. The large muscle mass of the newborn right ventricle affects the axis of the QRS complex in the frontal plane. The axis of the frontal QRS vector in the infant may be well past the upper limit of normal for the adult with the normal range being as high as +180°. Again, with time and the involution of the right ventricle, the axis ‘swings’ leftwards until it reaches the normal range for adults −30° to +90° (see Chapter 7). This change over time means that the acceptable axis for children in the frontal plane is very wide ranging. Broadly speaking, the axis should be inferior, i.e. between 0° and 180°. Deviation above the horizontal axis should be considered abnormal in the absence of evidence to the contrary. In the presence of physical evidence to support it a rightwards axis more extreme than usual may be suggestive of a residual load on the right ventricle. Put simply if the QRS vector is positive (i.e. pen deflection is upwards) in lead aVF then the vertical plane axis of the heart is probably normal.
The chest leads are particularly informative. In V1 the QRS complexes are initially dominantly positive with a dominant R wave reflecting depolarization in the relatively hypertrophied septum. T waves in V1 may be positive in the first 2 weeks of life but then become negative and remain inverted up until late childhood. The initial solely, or dominantly, positive deflection in V1 slowly becomes equiphasic and by adulthood will be purely negative in the majority of normal ECGs. Figure 12.2 shows the typical variation.
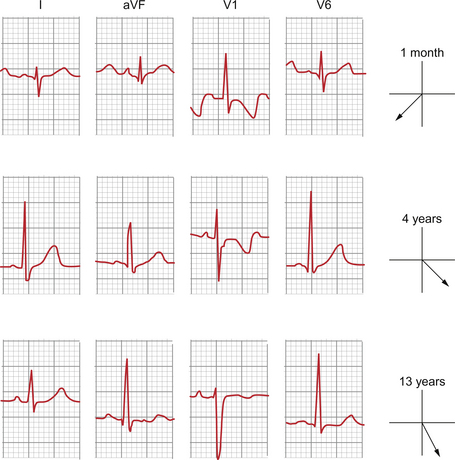
Fig. 12.2 ECG pattern—variation with age.
Broadly, the normal range for the intervals between successive components of the ECG for children are shorter than for adults and generally the younger the child the shorter the interval. This largely reflects the physical size of the heart, as a wave of electrical activity spreading through a smaller heart has a shorter distance to travel and so takes less time. This generalization applies for everything but the index referred to as the corrected QT interval (QTc). This is defined as the QT interval/square root (preceding R–R interval). Generally this falls with increasing age. Identification of the QTc has become clinically more important with increasing understanding of the role that abnormal repolarization plays in sudden death in young people. This may be evident in prolongation of the QTc above the normal range for an adult on the resting ECG.
Congenital heart disease
Clearly‘genetic programming’ is crucial to certain forms of congenital heart disease. As genetic knowledge advances more and more syndromes are linked with specific genetic lesions. For example, complete atrioventricular septal defect (AVSD) accounts for around 4% of all cardiac defects in the general population. However in children with Down syndrome (trisomy 21) it accounts for around 40% of the heart defects which are relatively common in this condition. About 40% of children with Down’s syndrome have some form of congenital heart disease. Tetralogy of Fallot (see p. 146) and some related cardiac defects (pulmonary atresia with ventricular septal defect, truncus arteriosus, interrupted aortic arch) are associated with deletion in the q11 region of chromosome 22. However not all children with tetralogy of Fallot have the 22q11 deletion and usually those children with this deletion have substantial non-cardiac problems in addition to their cardiac defects. These include cleft palate, speech and learning difficulties, hypocalcaemia and immunological problems. However the q11 region of chromosome 22 contains several hundred genes and research is underway to see if some specific genes account for the cardiac problems associated with the large DNA deletion. One candidate is the TBX-1 (‘TBOX-1’) gene. In mice in which TBX-1 has been blocked or disabled cardiac defects similar to those found in 22q11 deletion syndromes in humans are found.
A second important element in the pathogenesis of congenital heart disease is how the growth of structures is dependent on the way they function during fetal development. Thus for vascular structures to grow appropriately they must have a blood flow which encourages them to develop correctly. For example, obstruction on the left side of the heart is frequently associated with problems at multiple levels. This may explain the association between stenosis of the aortic valve and coarctation of the aorta (narrowing of the aorta adjacent to the insertion of the ductus arteriosus into the aorta). As described earlier in this chapter, in the fetus only about 10% of the cardiac output traverses the isthmus—that part of the aortic arch between the origin of the left subclavian artery and the insertion of the duct. If stenosis of the aortic valve reduces the output from the left side of the heart—possibly only marginally—then flow across this critical region of blood vessel may be significantly reduced and normal development of the aorta discouraged.
Presentation of congenital heart disease
Murmurs
Murmurs are extra sounds caused by turbulent flow of blood during the cardiac cycle (see Chapter 8). Murmurs can initially be divided on the basis of cause; innocent or functional murmurs have no underlying structural cause whereas pathological murmurs result from specific abnormalities of cardiac structures.
Innocent murmurs are generally characterized by:
• lack of radiation (can only be heard at one point)
• frequently vary with body position or state, e.g. only audible when sitting, when ill or after exercise
Typical examples of innocent murmurs are:
• flow murmur—ejection murmur typically heard in the pulmonary area when the cardiac output is high
• vibratory (Still’s) murmur—systolic murmur with buzzing musical quality localized to left sternal edge
• venous hum—continuous murmur heard at the base of the neck; typically abolished by lying down or pressing on the jugular vein
• carotid bruit—audible in the neck and thought to arise from blood flow into the brachiocephalic artery.
The intensity of a pathological murmur is largely dependent on the size of the hole through which blood must pass and the pressure gradient across the narrowest point. These are the factors which determine the velocity of flow and hence the likelihood of turbulence occurring (see Chapter 8). Thus with stenotic valves where the whole of the cardiac output must pass through the valve the intensity and length of the murmur increase with the degree of stenosis. However, for ventricular septal defects (VSD) the relationship between size and flow is complex as larger defects have a greater flow but a lower pressure gradient. Thus there is a peaked relationship between the intensity of the murmur and the size of the defect with very large defects generating little or no murmur and very small defects usually quieter murmurs because the flow is low despite maximal pressure difference. As the defect becomes vanishingly small the murmur disappears.
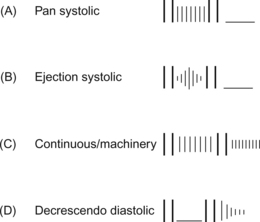
The time course of a murmur is very important in assessing its origin. Murmurs arise from jets of blood passing between chambers or vessels. The timing of the start and finish of the pressure gradient which generates the jet helps define its quality. For example, in the case of a VSD there is no pressure gradient during diastole as both ventricles fill. However almost immediately after the start of systole when the pressure in both ventricles has risen to the point where the atrioventricular valves have closed, i.e. immediately after the first heart sound, the pressure in the left ventricle is greater than that in the right. Thus the flow of blood through the VSD commences at the first heart sound and continues through into early diastole when the semilunar valves are heard to shut, i.e. the second heart sound. Thus the typical VSD murmur occurs throughout systole and is described as being pan- or holo-systolic (Fig. 12.3A). This may be compared with the murmur of aortic valve stenosis. Initially the pressure in the left ventricle is lower than in the aorta so there is a period between the first heart sound (closure of the mitral valve) and the opening of the aortic valve in which there is no blood flow out of the ventricle. The pressure in the ventricle rises to a peak during early systole at which time the volume of blood ejected from the ventricle is maximal and so the murmur reaches its maximum intensity. Then there is a period of reduced ejection of blood during which the pressure in the ventricle begins to fall. The intensity of the murmur falls to zero at the time the semilunar valves close. A recording of heart sounds, a phonocardiogram, has a typical diamond-shaped pattern for ejection systolic murmurs (Fig 12.3B).
Continuous murmurs can only occur where there is a persistent pressure difference between two vessels throughout the cardiac cycle. Apart from the innocent venous hum the commonest cause of continuous murmur is a patent ductus arteriosus (PDA). Since the aorta is always at a higher pressure than the pulmonary vasculature, even in diastole, the murmur occurs both in systole and diastole. However, since the pressure difference is greater in systole the quality of the sound varies between systole and diastole. Thus the murmur is continuous with two phases—it is often described as a machinery murmur (Fig. 12.3C). Large arteriovenous malformations are another cause of continuous murmurs.
Diastolic murmurs are often more difficult to hear because the pressure gradients are less than for systolic murmurs. The loudest diastolic murmurs tend to be caused by leakage (incompetence) in the semilunar valves. These typically occur in early diastole and are decrescendo in character. The typical decrescendo intensity profile reflects the fact that extracardiac vascular pressures fall during diastole whilst intraventricular pressures rise during diastole (Fig. 12.3D). Diastolic murmurs may also occur where there is stenosis of the atrioventricular (AV) valves, though these are likely to be difficult to hear. Classically described as ‘rumbling’ in quality the murmur has a low pitch rather different to high pressure murmurs. Increased flow through the AV valves may cause a murmur because of a functional stenosis.
Shunts
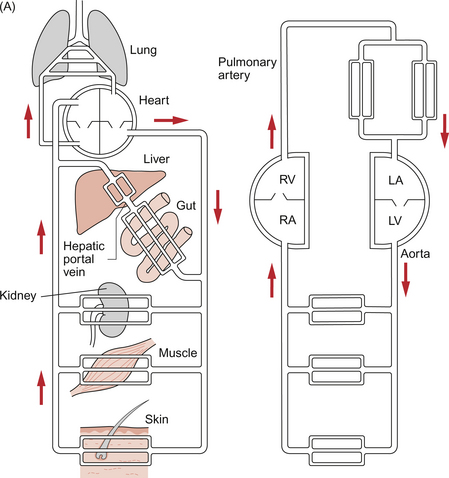
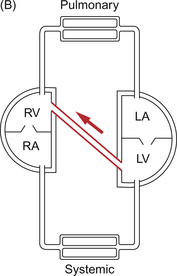
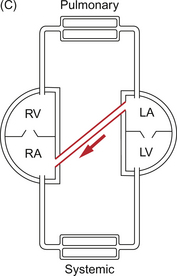
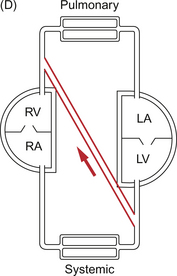
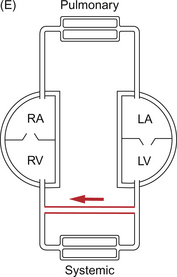
The normal ex utero circulation places the right and left ventricles in series with the pulmonary and systemic vascular beds between them (Fig. 12.4A). Though both pumps are combined in a single organ, the heart, they could be separate organs. This would make controlling the balanced output of each ventricle more difficult but not impossible. When blood passes from one side of the heart to the other bypassing the relevant end organs this is known as a shunt. Shunts may be intracardiac (e.g. VSD (Fig. 12.4B) or ASD (Fig. 12.4C)) or extracardiac (e.g. PDA (Fig. 12.4D) or arteriovenous malformation (Fig. 12.4E)). The main effect of a shunt is to volume load one or other side of the heart. Persistently high pulmonary blood flows associated with high driving pressure will eventually produce irreversible pulmonary hypertension. In the extreme case, systemic to pulmonary shunts may reverse, producing a flow of desaturated blood from right to left with consequent peripheral desaturation. This is known as Eisenmenger’s syndrome.
The presence of a shunt will affect cardiac performance. For example a PDA will generally cause blood to pass from the aorta into the pulmonary circulation which is at lower pressure. This extra blood, the shunt flow (S), is added to the flow already passing through the lungs from the right ventricle. Since the amount of blood returning to the right ventricle is equal to the amount passing through the systemic organs, the cardiac output (CO), the flow through the lungs is CO+S. The venous return to the left side of the heart from the lungs is equal to CO+S and may be considerably greater than the resting systemic requirement. This produces volume loading of the left side of the heart with a dilated left atrium and ventricle and is associated with exercise limitation. Although the left ventricle has the capacity in a normal adult to increase its output to about five times resting levels (see Chapter 4), in the presence of a substantial shunt it may already be preloaded by several times the resting cardiac output at rest. Thus, during exercise, the left ventricle soon reaches its maximum output and the individual becomes exercise limited. In children this is frequently seen as having to stop for a rest before any of their playmates. Babies may become out of breath whilst feeding and have to stop to ‘catch their breath’. In the case of premature infants the volume load on the left heart and high pulmonary blood flow may mean that they are unable to cope without mechanical ventilatory support.
Calculating shunts
where [Hb] represents the concentration of haemoglobin in the blood in g/L and 1.34 is the volume (mL/gHb) of oxygen carried by a gram of haemoglobin when saturated (see Chapter 1). Since, at equilibrium, pulmonary uptake must equal systemic extraction then it follows that:
and therefore
Where the size of a VSD is small the amount of blood passing through it adds little to the workload of the left ventricle and so is of little significance. However left to right shunts in which the pulmonary blood flow is above twice the systemic flow may be sufficient to produce symptoms. As flow becomes relatively greater so do symptoms. Initially, the most telling sign of a large shunt in a baby is breathlessness, particularly when feeding, their most energy demanding activity. The other major energy demand after homeostasis is growth. Feeding behaviour and weight gain are important markers of cardiac function in the infant. Babies with large shunts feed slowly, become more breathless during feeding and often need calorie supplements or mechanical feeding support such as nasogastric feeds. Tissue oedema leads to relative tissue hypoxia because of impaired diffusion of oxygen. This reduces the efficiency with which nutritional calories can be utilized. Pulmonary oedema occurs because the high blood flow and high blood pressure lead to fluid outflow from the pulmonary capillaries at a rate greater than the lung lymphatics can cope with (see Chapter 11). Oedematous lungs are both stiff and less efficient for gas transfer. Thus the work of breathing is greatly increased due to decreased compliance of the lungs. This increase in energy demand also contributes to the poor weight gain.
Cyanosis
The third common presentation of congenital heart disease is cyanosis which may occur in the newborn for several reasons. Peripheral cyanosis, caused by poor perfusion of tissues with blood, must be differentiated from central cyanosis, caused by reduced oxygenation of blood in the central arterial tree (see Chapter 1). Central cyanosis can be viewed as having two major causes, ‘respiratory’, in which the cyanosis is secondary to lung disease with impaired gas transfer, and ‘cardiac’ in which the cyanosis is due to the mixing of partially desaturated (venous) and normally saturated (arterial) blood (see Case 12.1:2).
The single major distinguishing feature between the pulmonary and cardiac causes of cyanosis is the lack of response to increasing the inhaled oxygen concentration. If cyanosis is due to impaired gas transfer then increasing Po2 in the inhaled gas mixture and hence increasing the concentration of O2 within the alveolus should improve the transfer of oxygen into the blood. However in the case of cyanotic heart disease the blood passing through alveolar capillaries is already nearly fully oxygenated and so little change results from increasing the oxygen content of inhaled gas. The cyanosis is due to mixing of this oxygenated sample with blood which has not been exposed to oxygen in the pulmonary vascular bed. The lack of respiratory distress is suggestive of a cardiac cause for cyanosis, as is the presence of a murmur or other physical sign linked with cardiac disease. However these are not 100% predictive as respiratory distress may occur secondary to the effects of heart failure or hypoxia whilst murmurs are frequently not present in cyanotic lesions particularly in the newborn period.
Ultimately management of cyanotic heart disease relies on surgical repair. This may be definitive, in which a normal circulation with two separate ventricles is achieved, or palliative in which stable saturations are achieved even though a normal circulation cannot be produced (see Blalock–Taussig shunt and Fontan circulation described on p. 147). Medical therapy may be important in stabilizing the child prior to surgery, e.g. the use of exogenous prostaglandin to maintain ductal patency prior to the insertion of a synthetic shunt to give a stable pulmonary blood flow (see Case 12.1:1).
Change from in utero to ex utero physiology: impact on physical signs
The change from in utero to ex utero physiology also has a profound influence on the presentation of congenital heart disease. Left-to-right shunts may be clinically undetectable at birth because of the high pulmonary pressure. As the right ventricular pressure falls (Fig. 12.5) VSD murmurs will become louder as the degree of left-to-right shunting increases. Parents frequently question the quality of newborn examination when significant defects are missed at the routine examination. However the paediatrician may take comfort in the fact that some murmurs may not be present in the first few days of life. This is a key feature differentiating obstructive lesions such as pulmonary stenosis or aortic stenosis from shunting lesions such as VSDs. In the former, since the cardiac output must pass through these valves at all times, a murmur will be audible from birth whereas the latter will depend upon the relative pressures between right and left ventricles which changes in the first few days of life. Initially, with right and left ventricular pressures equal, shunts from one side of the heart to the other will be minimal and in fact a mild degree of de-saturation may be observed reflecting right-to-left shunting.
By contrast, the residual presence of prenatal structures may obscure complex underlying lesions. For example it is common in transposition of the great arteries (TGA) for children to present at a few days of age with profound cyanosis or collapse. The underlying lesion in TGA is a complex failure of septation in which the aorta arises from the right ventricle and the pulmonary artery arises from the left ventricle. Thus there are essentially two separate circulations. Venous blood returns from the body to the right atrium, passes into the right ventricle and is pumped out round the body again. Fully saturated oxygenated blood returns from the lungs to the left atrium, passes into the left ventricle and is then pumped back to the lungs via the pulmonary artery. Clearly in a completely separate circulation oxygen would be extracted until the point where tissues would become hypoxic and die. In order to be compatible with life there must be some mixing of oxygenated and deoxygenated blood. This can occur if the foramen ovale remains open and is of a good size and if the ductus arteriosus remains patent. Mixing only occurs at the foramen ovale but the ductus arteriosus is crucial for maintaining left atrial pressure by filling the pulmonary circulation. As in the normal circulation the pulmonary pressure is lower than systemic pressure and so shunting at the duct is always left to right (systemic to pulmonary).
A single anatomical abnormality may vary sufficiently to present with completely different clinical pictures. Take for example the anatomical variability of Fallot’s tetralogy. This is the combination of a large subaortic VSD which does not provide any obstruction to flow (not ‘restrictive’), and an overriding of the aorta across the ventricular septum, subpulmonary and pulmonary stenosis and right ventricular hypertrophy. At one end of the spectrum the pulmonary obstruction is mild and the pulmonary blood flow is high—remember the VSD is not restrictive. A murmur of pulmonary stenosis may be noted in the newborn period (the VSD is not restrictive and therefore does not produce a murmur). However saturations will be near normal with predominantly left-to-right shunt across the VSD though some mixing at the ventricular level will occur. As the pulmonary vascular resistance falls the left-to-right shunt may increase and the child may become breathless but systemic blood will remain almost fully saturated with oxygen. By comparison, where the pulmonary obstruction is severe, resistance to blood flow to the lungs is greater than that to the body and so desaturated blood preferentially enters the systemic circulation and the child may present with a murmur and cyanosis in the newborn period.
Early and late management of congenital heart disease
Palliative techniques such as Blalock–Taussig (BT) shunts (connection of the subclavian artery to the pulmonary artery, originally directly but now usually with a Gore-Tex tube) allow the maintenance of pulmonary blood flow in circumstances where this may be restricted such as in tetralogy of Fallot or pulmonary atresia. This stabilizes systemic saturation and encourages growth of the pulmonary arteries whilst protecting the lung vasculature from damage by high pressures and flows. The construction of the shunt is such that it provides a significant resistance between the systemic and pulmonary circulations, balancing adequate blood flow against damage to the pulmonary vasculature. Initially performed in 1944, this was one of the first palliative repairs for cyanotic heart diseases though now definitive repair is more common. Other forms of artificial shunt exist from the aorta to the pulmonary artery, though it is technically more difficult to obtain controlled results. More recent practice has involved the introduction of conduits from the right or, more rarely, left ventricle to pulmonary artery, the Sanno procedure. This was originally developed to avoid the problem of a reduction in coronary circulation during diastole which may occur with BT shunts. Since the pulmonary artery pressure is lower than systemic at all points of the cardiac cycle, diastolic steal can occur in which coronary perfusion is compromised by preferential flow into the pulmonary system during diastole. Remember that coronary perfusion occurs primarily during diastole (see Chapter 5).
The construction of a Fontan circulation is staged to allow for the changing haemodynamics of the pulmonary vasculature. Initially the pulmonary vasculature needs to be protected and pulmonary blood flow maintained. In situations such as tricuspid atresia and VSD with hypoplastic right ventricle this may require banding of the pulmonary artery to restrict pulmonary blood flow and lessen damage to the pulmonary vascular bed. It is critical to avoid long-term damage to the pulmonary vascular bed as elevated pulmonary artery pressures will prevent completion of the Fontan circulation. Alternatively, where there is inadequate pulmonary blood flow the creation of a shunt to augment the blood flow may be necessary. Between 4 months and 1 year of age, depending on the lesion and progress of the child, the next stage may be completed with connection of the superior vena cava (SVC) to the right pulmonary artery. Usually any arterial shunt is tied off at this time. Then, between 2 and 6 years of age, the venous connections may be completed with connection of the inferior vena cava (IVC) to the pulmonary arteries. Prior to the completion of the venopulmonary connections the blood of the child will be poorly saturated with oxygen. However, after completion of the surgery the systemic blood saturation with oxygen will generally be in the mid to high 90+% range. Various residual shunts, e.g. the venous drainage from the cardiac muscle itself which returns via the coronary sinus to the right atrium, will generally mean that full saturation can never be achieved.
Allen, H. D., Gutgesell, H. P., Clark, E. B., Driscoll, D. J. Moss and Adams Heart Disease in Infants, Children, Adolescents and Young Adult, sixth ed. Philadelphia: Lippincott/Williams & Wilkins; 2000.
Anderson, R. H., Baker, E. J., Macartney, F. J., Rigby, M. L., Shinebourne, E. A., Tynan, M. Paediatric Cardiology, third ed. Edinburgh: Churchill Livingstone; 2009.
Behrman, R. E., Kliegman, R. M., Jenson, H. B. Nelson Textbook of Pediatrics, eighteenth ed. Philadelphia: WB Saunders; 2007.
Campbell, A. G. M., McIntosh, N. Forfar and Arneils Textbook of Pediatrics, fifth ed. Edinburgh: Churchill Livingstone; 1998.
Park, M. K. The Paediatric Cardiology Handbook, third ed. St Louis: Mosby; 2002.
Tulzer, G. Fetal cardiology. Curr. Opin. Pediatr.. 2000; 12:492–496.