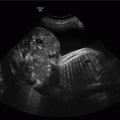
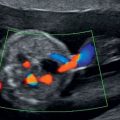
Exomphalos with stomach within peritoneal sac.
The incidence is 1 in 4,000 births. There is a high association with chromosomal abnormalities (approximately 33–61% depending on gestational age) mainly trisomy 18 (the most common) and trisomy 13; 30–50% will have some form of cardiac abnormality. Paradoxically, the larger the lesion the less likely there is to be a chromosomal abnormality(1–3).
Diagnosis
The diagnosis is usually made after 12 weeks and is often detected at the first trimester dating scan in places where nuchal translucency (NT)-based screening is in place. It is best observed in the sagittal and axial views. The umbilical cord will insert onto the anterior part of the peritoneal sac and blood flow can be seen passing around the sac. In places where the triple or quadruple blood test is offered for screening, a raised alpha-fetoprotein (AFP) should prompt a search for an anterior abdominal wall abnormality.
The sac may contain bowel, stomach and liver. The bladder and stomach are often “pulled” towards the sac. In large lesions, the heart may also be shifted towards the defect. As the abdominal anatomy is distorted it is often difficult to obtain a true abdominal circumference, which may lead to inaccuracies in estimation of fetal weight or the misdiagnosis of fetal growth restriction.
Common chromosomal abnormalities associated with exomphalos are trisomy 18 and trisomy 21. Other syndromes associated with an exomphalos are Beckwith–Weidemann syndrome (macrosomia and macroglossia with renal cysts), OEIS (omphalocoele, bladder exstrophy, imperforate anus and spinal defects) and pentalogy of Cantrell (exomphalos, congenital diaphragmatic hernia, ectopia cordis, sternal cleft and intracardiac defect, usually a ventricular septal defect). A repeat anatomic survey should focus on these areas as well as the heart and features of trisomy 18 and 13. Cloacal and bladder extrophy can be associated with an exomphalos, and identification of the bladder is mandatory to exclude these conditions. Both are thought to be due to a defect in the development of the cloacal membrane.
Management
Karyotyping should be offered due to the high rate of chromosomal abnormalities, particularly trisomy 18. A full fetal echocardiogram should also be performed as approximately 30–50% will have some form of cardiac abnormality. Associated structural and/or chromosomal abnormalities worsen the prognosis. Counseling regarding the prognosis should be done in association with neonatalogists and pediatric surgeons.
The fetus should be monitored every 4 weeks with growth scans, but the true abdominal circumference may be difficult to measure, and so fetal growth restriction may be difficult to diagnose.
Delivery is best organized in a tertiary center with neonatal surgical facilities. A vaginal delivery is safe and cesarean section is not essential; cesarean section may be considered in certain cases where the exomphalos is very large. In general, the outcome is good with an 80–90% survival rate if there are no associated chromosomal or structural issues, but this depends upon the size of the lesion.
Neonatal management
A neonatal pediatrician should be present at the delivery of the baby. Initial management is focused on stabilization of the baby and the avoidance of trauma to the sac. The sac should have a moist covering to prevent evaporative heat and water loss. Neonatal management is directed at fluid balance and blood sugar monitoring.
Repair depends upon firstly the exclusion of other structural abnormalities that may not have been detected antenatally, and the size of the peritoneal sac.
If the sac is small enough, then primary repair can be performed with excision of the sac and closure of the fascia and the abdominal wall. If the exomphalos is large, then a silastic pouch may be used to reduce the viscera into the abdominal cavity and closure performed when the sac is smaller.
Gastroschisis
Gastroschisis can be defined as herniation of the abdominal wall contents medial to the umbilical cord with no surrounding membrane. It occurs in approximately 1 in 4,000 births. The exact cause of gastroschisis is unknown but is believed to be caused by a disruption of the blood supply to the developing abdominal wall from the omphalomesenteric duct artery by the 8th week of gestation. There are no known genetic associations, and so karyotyping is not indicated[1–3].
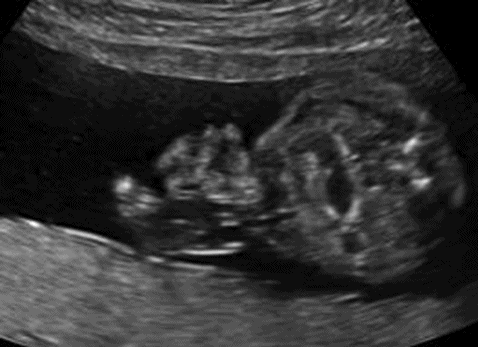
Gastroschisis in axial view with dilated internal bowel.
Diagnosis
Gastroschisis can be diagnosed at the 11–14 week scan, and as there is an open defect the AFP levels are commonly raised. However, serum screening tests including AFP are not offered in most places, so the opportunity to find a raised AFP may not arise. The exposure of the bowel to the amniotic fluid leads to thickening of the bowel wall. As in exomphalos, the bladder and heart can be pulled towards the lesion distorting the internal anatomy.
The main issue is that of bowel atresias and necrosis that need to be resected after delivery, which if severe can lead to “short bowel” syndrome where long-term parenteral nutrition is required. Unfortunately, there do not seem to be any strong prognostic indicators. Bowel thickness (>2mm), hyperperistalsis, mesenteric arterial blood flow and bowel dilatation have all been evaluated. but none seem to be particularly good at predicting the need for surgery or the length of bowel that requires resection[4]. A recent review suggested that the only predictive factor was internal bowel dilatation greater than 14mm[5].
However, a ruptured exomphalos is a possibility, so a careful secondary survey is always necessary, in particular looking for a free-floating membrane in the amniotic fluid. There are other conditions that may appear to be a gastroschisis. Limb-body wall complex or body stalk anomaly, a large anterior thoracoabdominal wall abnormality with an associated myelomeningocoele. The contents of the abdomen, pelvis and chest lie outside the body cavities and can be attached to the uterine wall or placenta. This condition is lethal. Amniotic band syndrome, due to early amnion rupture, may cause disruption of the anterior abdominal wall and a gastroschisis.
Management
Gastroschisis is associated with fetal growth restriction and serial measurements of fetal biometry with umbilical cord Dopplers are required at least every 4 weeks, with delivery at around 37–38 weeks due to the risk of stillbirth. Ultrasonography underestimates the fetal abdominal circumference, and therefore there may be a false positive for diagnosis of fetal growth restriction.
Ultrasound evaluation of the bowel includes the measurement of internal and external bowel dilatation, the presence of intrabdominal peristalsis (which may indicate an area of stenosis) and blood flow within the extra-abdominal bowel. However, the only feature that may be predictive of bowel atresia or infarction is inflammatory bowel disease.
Multidisciplinary management and counseling are essential and should include pediatric surgeons and neonatalogists.
Delivery should occur at a facility with the appropriate neonatal surgical expertise, and vaginal delivery is not contraindicated because of the presence of a gastroschisis alone.
Neonatal management
In general, the outcome is good with a greater than 90% survival. A pediatrician should be present at the delivery, and immediate care involves keeping the bowel moist and covered, and careful fluid balance. Repair involves either immediate closure of the defect or delayed closure if there is a large amount of extra-abdominal bowel. If surgery cannot be performed immediately, the bowel is surrounded by a silastic (a plastic bag) to allow reduction of the bowel into the abdominal cavity. The baby may have intestinal ischemia with necrosis, and if required, may need either resection and primary anastomosis or the formation of temporary stomas. Following repair, the neonate requires 3 weeks of parenteral nutrition via central venous access.
Gastrointestinal atresias
Atresia can occur anywhere in the bowel. In general, proximal atresias are easier to detect as the proximal bowel will fill up with fluid, and there will be a dilated segment and associated polyhydramnios.
Usually the small bowel cannot be seen as separate loops unless there is dilatation. Large bowel can be seen, particularly in the third trimester as meconium within the lumen has a different echogenicity, but if dilated can be recognized by its haustral pattern.
Esophageal atresia and tracheoesophageal fistula
These are rare conditions with an incidence of approximately 1 in 5,000. The most common abnormality is an esophageal atresia with a distal tracheoesophageal atresia. When the esophagus does not connect to the stomach, the esophagus appears very dilated, the stomach is absent and there is marked polyhydramnios. In the cases of a fistula, the stomach may appear small or normal and there may be no polyhydramnios.
The most common associations are chromosomal (trisomy 21 or 18) or the VACTERL (vertebral, anorectal, cardiac, tracheoesophageal, renal and limb anomalies) syndrome and karyotyping is recommended.
Due to the issues with polyhydramnios, amniodrainage may be required to prevent preterm labor, and cervical scanning may be useful in predicting this.
In the absence of other anomalies or chromosomal problems, the outcome is generally good with a 95% survival rate, and delivery should take place in a tertiary center with the appropriate neonatal surgical facilities.
Duodenal atresias
Doudenal atresia has an approximate incidence of 1 in 10,000. There is a strong association (33%) with trisomy 21 and invasive testing should be offered. The diagnosis is made by the appearance of a “double bubble” – the dilated duodenum proximal to the lesion and the dilated stomach proximal to this. There is often associated polyhydramnios.
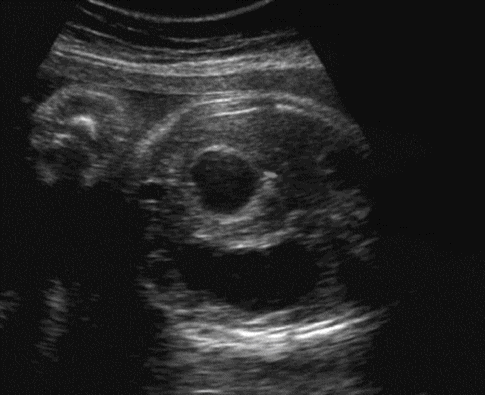
“Double bubble” of duodenal atresia.
Small bowel atresias
These occur in the jejunum or ileum and are most likely to occur due to vascular events or ischemic injury due to hypotension affecting the bowel wall or meconium ileus within the bowel lumen or secondary to cystic fibrosis.
On ultrasound, multiple dilated loops of bowel can be observed. Polyhydramnios is not as common as the more proximal lesions detailed above, as the bowel can dilate to accommodate the extra fluid.
In the absence of other abnormalities the prognosis is good.
Large bowel atresias
These are more difficult to detect as they do not appear on ultrasound until late in the third trimester due to the ability of the bowel to expand for the extra fluid. Polyhydramnios is rare because of this. Common causes are meconium ileus, Hirschprung’s disease (lack of nervous innervation prevents relaxation of the bowel, and therefore an obstruction) and imperforate anus.
Intrabdominal cysts
A variety of cysts can occur within the abdominal cavity. The cause depends on the location[6].
Gastrointestinal tract
Mesenteric cyst
Enteric duplication cyst
Liver
Simple cyst
Gall bladder
Choledochal cyst
Adrenal
Hemorrhage
Neuroblastoma
Ovarian – determination of fetal sex is important
Follicular cyst – may undergo torsion
Intra-abdominal calcification
Intra-abdominal calfication is rare but can be indicative of fetal infection, particularly cytomegalovirus (CMV) and toxoplasmosis. However, in the absence of infection the outcome is good with no cause found.
Echogenic bowel
The true definition is that the bowel should be as “bright as bone,” and there are multiple associations:
(1) chromosomal abnormalities, particularly trisomy 21, but in the context of screening this is only a minor marker of chromosomal anomalies
(2) first-trimester bleeding – the bloody amniotic fluid is swallowed and the iron from hemoglobin in the red blood cells precipitates in the bowel wall
(3) infection – CMV and toxoplasmosis