23 FERTILIZATION, PLACENTATION, AND LACTATION
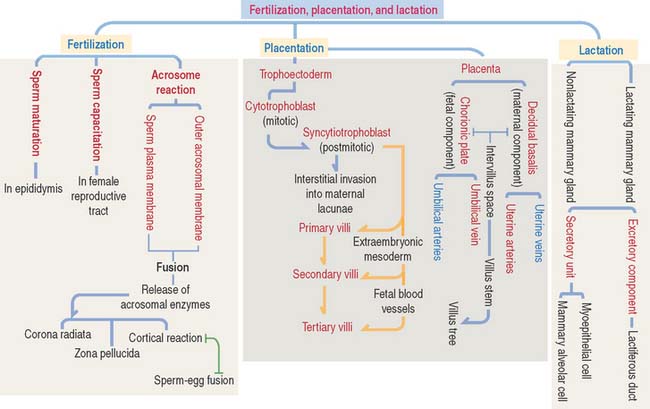
FERTILIZATION
The condensed nucleus consists of DNA surrounded by very basic protamines. Nucleosomes are not present because somatic histones have been replaced by protamines that protect and stabilize the DNA during fertilization.
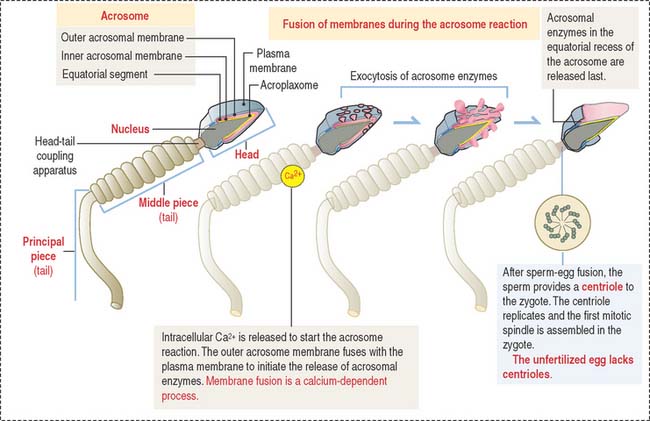
The acrosomal sac is formed by three constituents (Figure 23-1): (1) the outer acrosomal membrane, (2) the inner acrosomal membrane, and (3) hydrolytic enzymes (mainly hyaluronidase and acrosin, the latter derived from the precursor proacrosin). The thin portion of the acrosomal sac, extending toward the tail, is the equatorial segment.
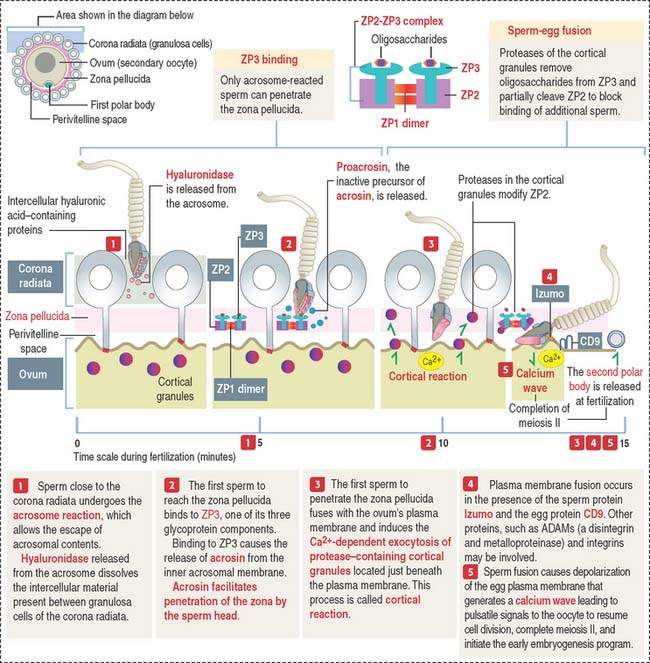
The three main events during fertilization are, sequentially the acrosome reaction, sperm binding to ZP3, a glycoprotein of the zona pellucida (ZP), and sperm-egg fusion (Figure 23-2).
In the proximity of the ovum, and in the presence of Ca2+, the sperm plasma membrane fuses with the outer acrosomal membrane. This event is known as the acrosome reaction. Small openings created by membrane fusion facilitate the release of hydrolytic enzymes (see Figures 23-1 and 23-2). The equatorial region of the acrosome does not participate in the plasma membrane-outer acrosome membrane fusion process. Male infertility may occur when the acrosome reaction fails to occur or takes place before the sperm reaches the egg.
Two membrane proteins have been shown to be essential for sperm-egg fusion, Izumo in the sperm and CD9 in the egg. Izumo is a sperm-specific membrane protein of the immunoglobulin superfamily that localizes to the sperm plasma membrane after the acrosomal reaction. CD9 is a member of the tetraspanin super family of transmembrane proteins (see Box 23-A). Izumo and CD9 may be involved in the organization or stabilization of plasma membrane protein complexes essential for the sperm-egg fusion reaction. Other proteins, such as ADAMs (a disintegrin and metalloprotease), may participate in this reaction. We discussed in Chapter 1, Epithelium, how the disintegrin domain of ADAMs participates in the shedding of the ectoplasmic portion of transmembrane proteins.
Box 23-A Tetraspanins
Zona pellucida during fertilization
The plasma membrane of mammalian eggs is surrounded by a 6- to 7-μm-thick zona pellucida (plural zonae pellucidae), a glycoprotein coat produced mainly by the primary oocyte during folliculogenesis, as early as during the primary follicle stage. The zona pellucida has important roles in fertilization and implantation of the embryo in the endometrium. In vitro fertilization overcomes most forms of infertility (see Box 23-B)
Box 23-B In vitro fertilization
The zona pellucida is composed of three glycoproteins (see Figure 23-2): ZP1, a dimer of 200 kd; ZP2, 120 kd; and ZP3, 83 kd. ZP2 and ZP3 interact to form a long filament complex interconnected by ZP1 dimers at regular intervals.
PLACENTATION
Cell diversity is achieved in the blastocyst, when the trophectoderm and inner cell mass are recognized. In the late blastocyst, the trophectoderm is referred to trophoblast and is distributed in two regions: in direct contact with the inner cell mass, the polar trophoblast, and surrounding the blastocyst cavity, the mural trophoblast. The blastocyst hatches from its zona pellucida at 6 to 7 days and the the differentiation of the inner cell mas proceeds.
Implantation of the blastocyst
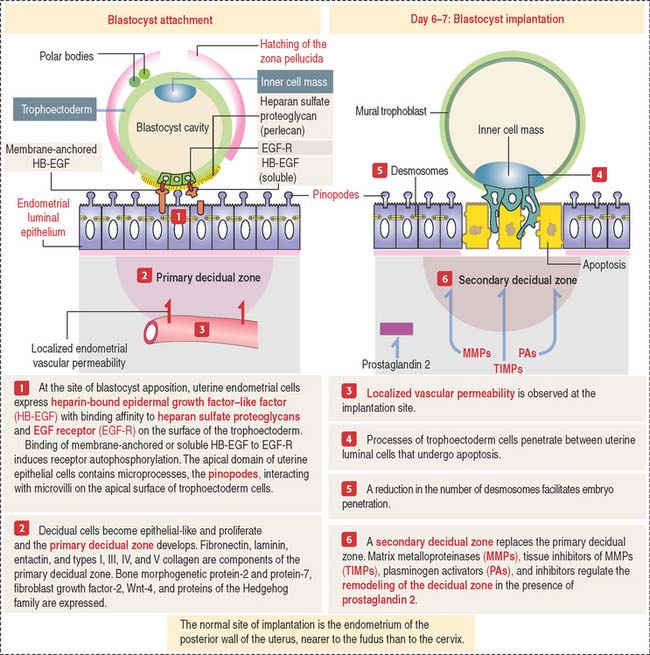
The implantation of the blastocyst into a nurturing endometrium involves (1) the initial unstable adhesion of the blastocyst to the endometrial surface, called apposition, followed by a stable adhesion phase and (2) the decidualization of the endometrial stroma (Figure 23-3).
The timing of preimplantation and implantation is extremely precise (see Box 23-C). So is the preparation of the implantation site.
Box 23-C Timetable of implantation
On day 4 of pregnancy, the embryo—at the blastocyst stage—is within the uterine cavity. The coordinated effect of ovarian estrogens and progesterone has already conditioned the endometrium for implantation, including an increase in endometrial vascular permeability at the implantation site.
At implantation (see Figure 23-3), cytoplasmic processes of trophoblastic cells interact with small processes on the apical surface of the endometrial epithelial cells, called pinopodes, and penetrate the intercellular spaces of the endometrial cells. Penetration is facilitated by a decrease in the number of desmosomes linking the endometrial epithelial cells that undergo apoptosis.
As you recall, the endometrial lamina propria has undergone a decidual transformation during the secretory phase of the menstrual cycle. This primary decidual zone is remodeled by the action of metalloproteinases (see Figure 23-5), and a secondary decidual zone houses the implanting embryo.
Role of the decidual cells during implantation
The decidua provides an immune-protective environment for the development of the embryo. The decidual reaction involves (1) the production of immunosuppressive substances (mainly prostaglandins) by decidual cells to inhibit the activation of natural killer cells at the implantation site and (2) infiltrating leukocytes in the endometrial stroma that secrete interleukin-2 to prevent maternal tissue rejection of the implanting embryo. Syncytiotrophoblastic cells do not express major histocompatibility complex class II. Therefore, the syncytiotrophoblast cannot present antigens to maternal CD4+ T cells.
Formation of primary, secondary, and tertiary villi
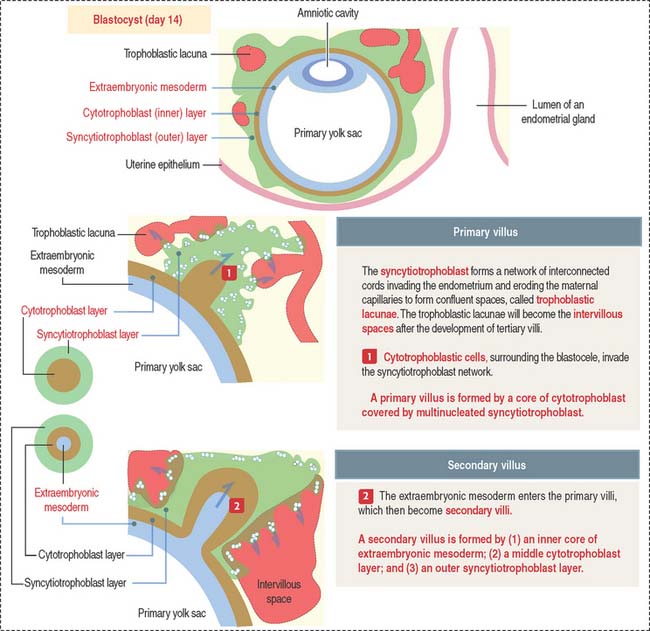
At the end of the second week, cytotrophoblastic cells proliferate under the influence of the extraembryonic mesoderm, and extend into the syncytiotrophoblast mass, forming the primary villi (Figure 23-4).
Early in the third week, the extraembryonic mesoderm extends into the primary villi, forming the secondary villi (see Figure 23-4). Secondary villi cover the entire surface of the chorionic sac. In cross section, a secondary villus is formed by a core of extraembryonic mesoderm surrounded by a middle cytotrophoblast layer and an outer layer of syncytiotrophoblast.
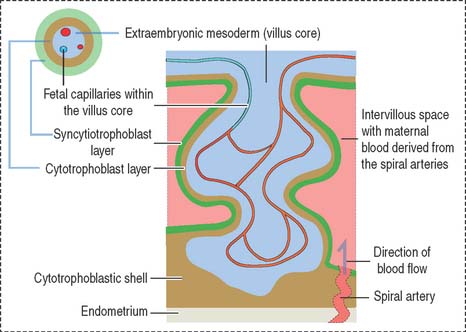
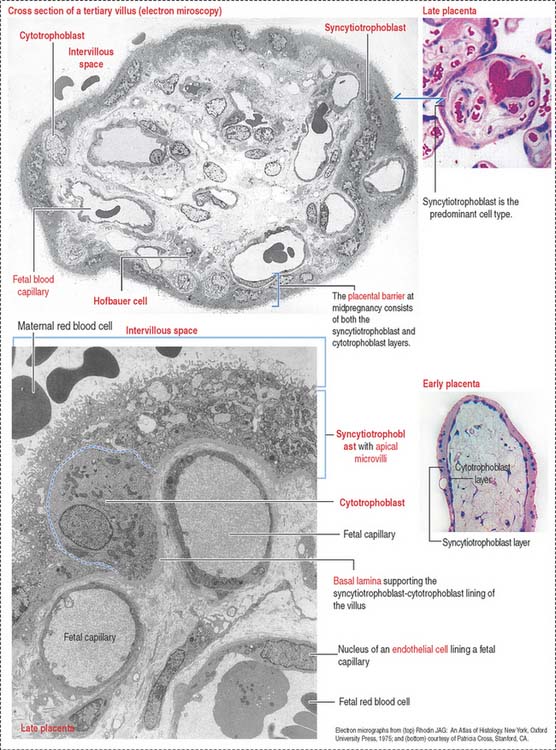
Soon after, cells of the extraembryonic mesoderm differentiate into capillary and blood cells, forming the tertiary villi (Figure 23-5). The difference between the secondary and tertiary villi is the presence of capillaries in the latter. The capillaries in the tertiary villi interconnect to form arteriocapillary networks leading to the embryonic heart.
The following events occur as the chorionic tertiary villi continue to develop:
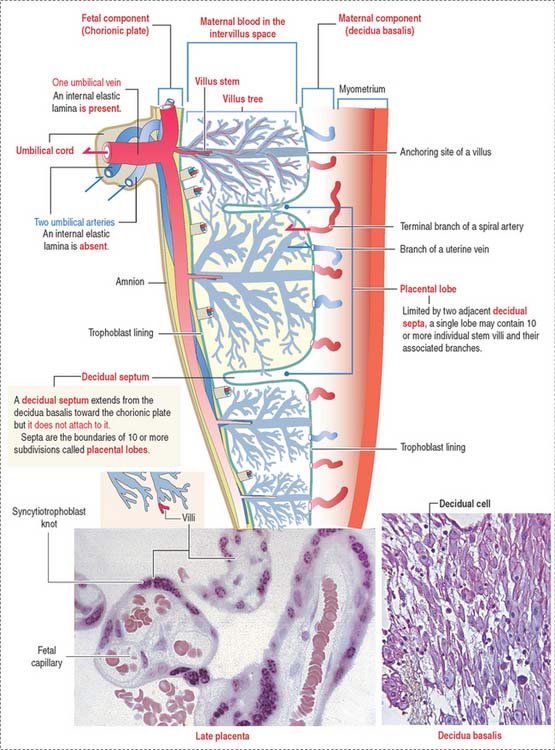
Histologic features of the placenta
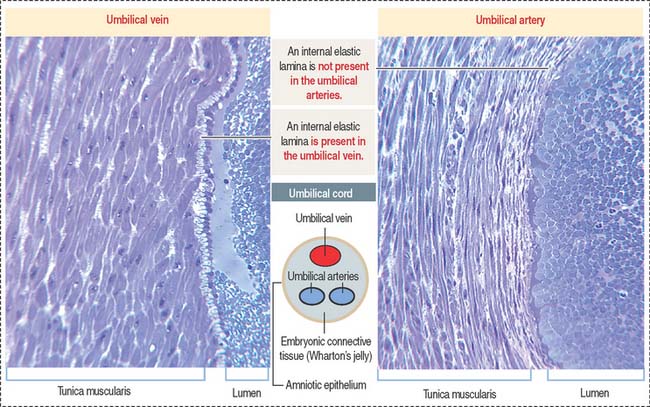
Each lobe contains 10 or more stem villi and its branches. The 50- to 60-cmlong and 12-mm-thick and twisted umbilical cord is attached to the chorionic plate and contains two umbilical arteries (transporting deoxygenated blood) and one umbilical vein (transporting oxygen-rich blood). The umbilical vessels (Figure 23-6) are embedded in embryonic connective tissue, called Wharton’s jelly (see Chapter 4, Connective Tissue). The cord is lined by amniotic epithelium.
Maternal and fetal components
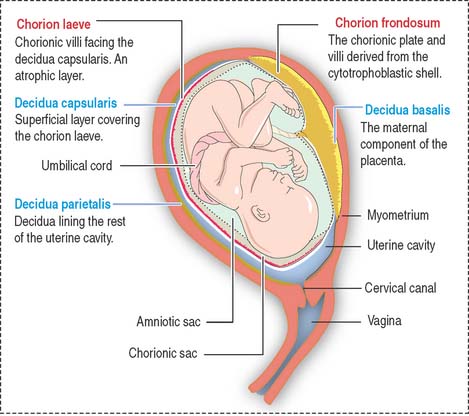
The placenta consists of a maternal and a fetal component (Figure 23-7). The maternal component is represented by the decidua. The decidua (Latin deciduus, falling off; a tissue shed at birth) is the endometrium of the gravid uterus.
There are three regions of the decidua, named according to their relation to the developing fetus:
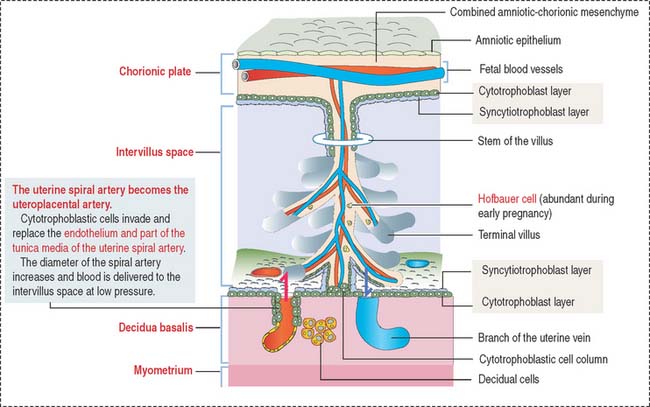
The intervillous space between the maternal and fetal components contains circulating maternal blood (Figures 23-8 and 23-9). Arterial blood, derived from the open ends of the spiral arteries, flows into the intervillous space and moves blood into the uterine veins. A plug of cytotrophoblastic cells and the contraction of the smooth muscle wall of the artery control the flow of blood.
Placental blood circulation
Placental blood circulation has two relevant characteristics: (1) the fetal blood circulation is closed (within blood vessels). (2) The maternal blood circulation is open (not bound by blood vessels). Maternal blood enters the intervillous space under reduced pressure, regulated by the cytotrophoblastic cell plugs, and leaves through the uterine veins after exchanges occur with the fetal blood in the terminal branched villi (see Box 23-D).
Box 23-D Trophoblastic cells
The umbilical vein has a subendothelial elastic lamina; the two umbilical arteries lack an elastic lamina (see Figure 23-6). The umbilical vein carries 80% oxygenated fetal blood. Although the partial pressure of oxygen in fetal blood is low (20 to 25 mm Hg), the higher cardiac output in organ blood flow, higher hemoglobin concentration in fetal red blood cells, and higher oxygen saturation provide adequate oxygenation to the fetus. The umbilical arteries return deoxygenated fetal blood to the placenta.
Structure of the chorionic villus
The mesenchymal core contains two major cell types:
The mesenchymal core is covered by two cell types:
Several important structural characteristics define the cytotrophoblast and syncytiotrophoblast:
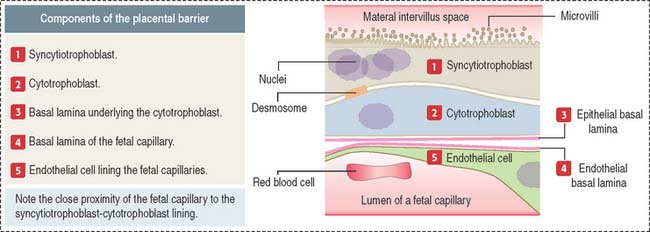
Fetal vessels are separated from maternal blood in the intervillous space by the placental barrier (Figure 23-11), which is formed by (1) endothelial cells and basal lamina of the fetal blood capillaries and (2) the cytotrophoblast and syncytiotrophoblast and supporting basal lamina.
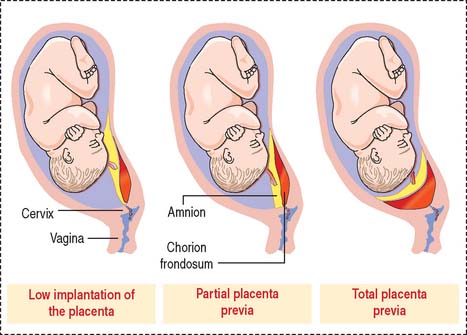
Placenta previa (second half of pregnancy)
There are three types of placenta previa (Figure 23-12): (1) low implantation of the placenta, when the margin of the placenta lies close to the internal cervical os (marginal placenta previa); (2) partial placenta previa, when the edge of the placenta extends across part of the internal ostium; and (3) total placenta previa, when the placenta covers the internal cervical ostium.
Placental abruption, or abruptio placentae (second half of pregnancy)
Spontaneous painful bleeding and uterine contractions are typical symptoms.
Placenta accreta
Penetration of the placenta into the uterine muscle is called placenta increta.
Extensive invasion of the placenta through the thickness of the uterine muscle is known as placenta percreta.
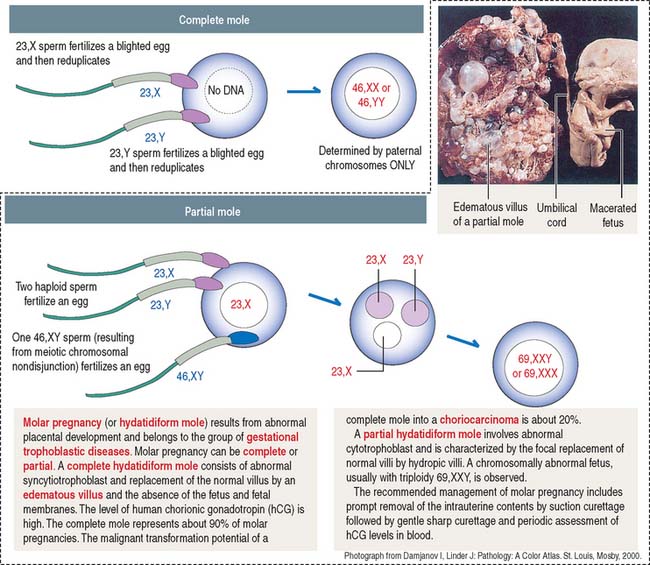
Clinical significance: Gestational trophoblastic disease
Complete moles are of paternal origin and result from the fertilization of a blighted (empty) ovum by a haploid sperm that reduplicates within the egg (Figure 23-13). The frequent karyotype of a complete mole is 46,XX, and no fetus is observed.
Choriocarcinoma is observed in about 20% of patients with molar pregnancies.
Clinical significance: Functions of the placenta
The main function of the placenta is the regulation of the fetal-maternal exchange of molecules, ions, and gases. This function is accomplished at specialized areas of the syncytiotrophoblast adjacent to fetal capillaries. The transfer of molecules across the placental barrier can follow intercellular and transcellular pathways. Figure 23-14 illustrates the main functional aspects of the placenta that are of clinical and physiologic relevance.
Rh (D antigen) isoimmunization
Maternal antibodies against D antigen (present in the Rh system of fetal red blood cells) cause hemolytic disease (erythroblastosis fetalis). The fetus is Rh-positive (Rh D antigen received from the father), but the mother lacks the D antigen (she is Rh-negative). Isoimmunization refers to maternal exposure and sensitization to fetal Rh+ red blood cells, mainly during delivery. In a subsequent pregnancy, antibodies to D antigen (IgG) cross the placenta and cause hemolysis of fetal red blood cells (see Chapter 6, Blood and Hematopoiesis).
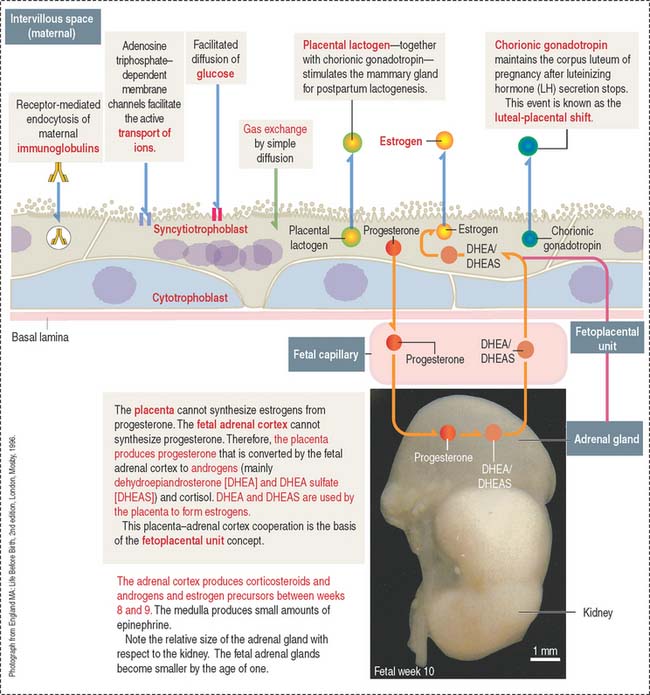
Steroid hormone production: The fetoplacental unit
A fetal-maternal cooperation—known as the fetoplacental unit—enables the transport of placental progesterone to the adrenal cortex and its conversion to dehydroepiandrosterone (DHEA), which can be sulfated to form DHEA sulfate (DHEAS) (see Figure 23-14).
When DHEA and DHEAS are transported to the syncytiotrophoblast, the conversion to estrone (E1) and estradiol (E2) occurs. DHEA can be hydroxylated in the liver and serves as a substrate for the synthesis of estriol (E3) by the syncytiotrophoblast.
LACTATION
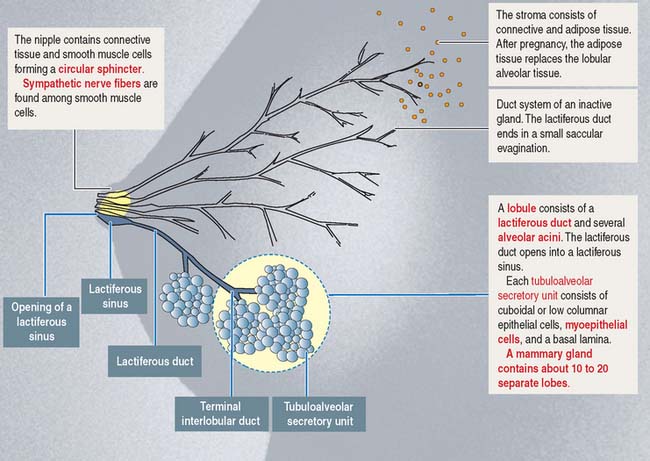
The mammary glands
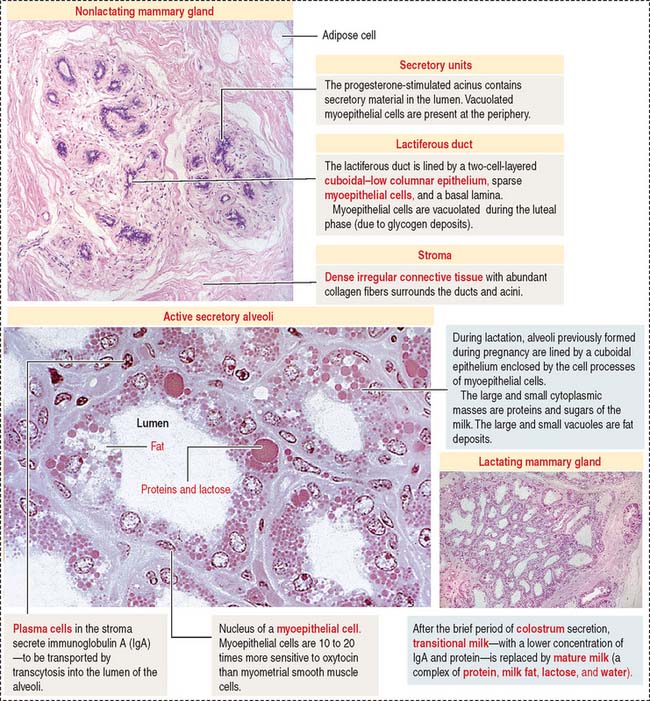
Like most branched (compound) glands, the mammary gland contains a duct system, lobes, and lobules (Figure 23-15). Each lobe contains a branching lactiferous duct that extends into the fibroadipose tissue of the breast. Each lactiferous duct is lined by a simple columnar or cuboidal epithelium and a discontinuous outer layer of myoepithelial cells. Each duct is surrounded by loose connective tissue and a capillary network.
In the resting, nonlactating state, the mammary glands consist of lactiferous ducts, each ending in a group of blind, saccular evaginations (see Figure 23-15).
Development of the mammary glands
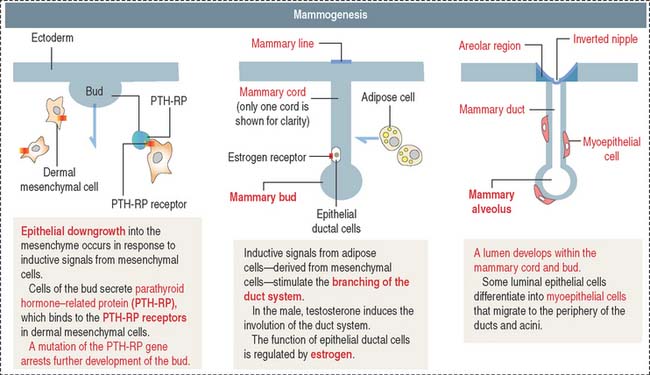
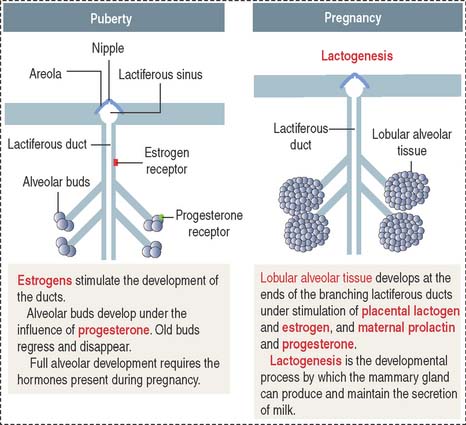
Placental lactogen and estrogen stimulate the development of the mammary gland. The development involves epithelial-mesenchymal interactions and consists of two phases (Figure 23-16): (1) the formation of the nipple and (2) the development of the mammary gland.
During development of the mammary gland, an ectodermic epithelial cell bud, the mammary bud, enters the underlying mesoderm. Epithelial buds sprout during the first trimester to give rise to 15 to 25 solid epithelial mammary cords. During the second trimester, the mammary cords become hollow, and alveoli develop by the end of the third trimester (see Figure 23-16). The mammary ducts become lactiferous ducts.
The mesoderm differentiates into a connective and adipose stroma as well as into the smooth muscle of the nipple. Luminal epithelial cells of ducts and alveoli are precursors of the myoepithelial cells, which migrate to the basal region of the lining epithelium. The epithelial-myoepithelial conversion also occurs in the mature mammary gland.
At puberty (Figure 23-17), circulating estrogen (in the presence of prolactin) stimulates the development of the lactiferous ducts and the enlargement of the surrounding fat tissue.
During pregnancy (see Figure 23-17), prolactin and placental lactogen, in the presence of estrogen, progesterone, and growth factors, stimulate the development of lactiferous ducts and secretory alveoli at the ends of the branched ducts.
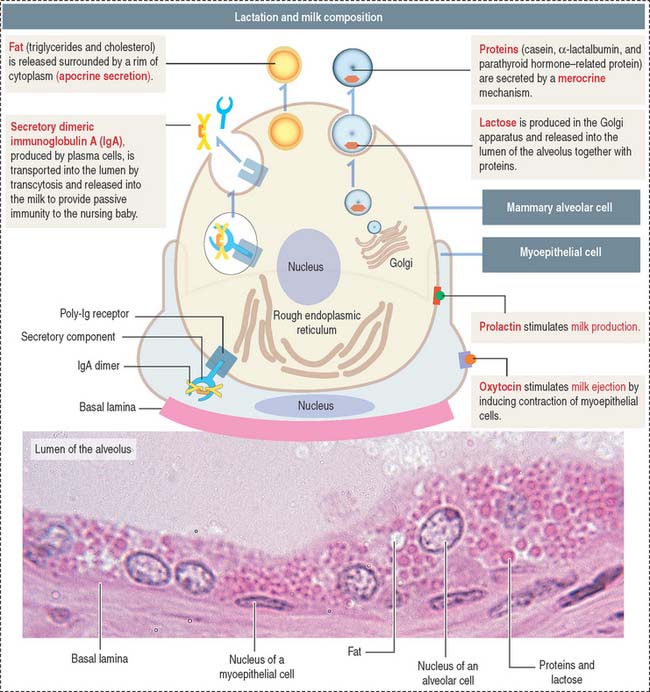
During lactation, the lactiferous duct system and the lobular alveolar tissue are fully developed and functional (Figure 23-18). Prolactin stimulates secretion by alveolar cells.
Suckling during lactation
A neural stimulus at the nipple resulting from suckling determines:
Milk contains (Figure 23-19; see Box 23-E):
Box 23-E Lactation
In addition, plasma cells present in the stroma surrounding the alveolar tissue secrete dimeric IgA. Dimeric IgA is taken up by alveolar cells and transported to the lumen by a mechanism similar to that discussed in Chapter 16, Lower Digestive Segment.
Clinical significance: Androgen insensitivity syndrome
In normal males, the lactiferous ducts undergo rapid involution by an inductive mechanism mediated by the mammary mesenchyme. Lactiferous ducts developing in the absence of testosterone or a functional androgen receptor, as in the androgen insensitivity syndrome, assume a female pattern of development.
Clinical significance: Benign breast diseases and breast cancer
Fibroadenoma, the second most common form of benign breast disease, occurs in young women (20 to 30 years old). Fibroadenomas are slow-growing masses of epithelial and connective tissues and are painless.
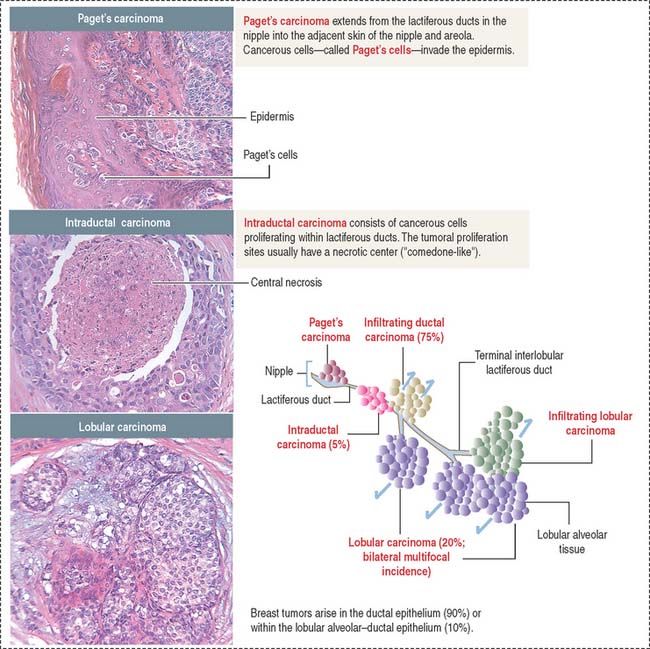
About 80% of breast cancers originate in the epithelial lining of the lactiferous ducts (Figure 23-20). Epithelial cells lining the lactiferous ducts have estrogen receptors and about 50% to 85% of breast tumors have estrogen receptors.
Estrogen-replacement therapy in postmenopausal women has been implicated as a risk factor for breast cancer. In premenopausal women, the ovaries are the predominant source of estrogen. In postmenopausal women, estrogen derives predominantly from aromatization of adrenal (see Adrenal Gland in Chapter 19, Endocrine System) and ovarian androgens in the liver, muscle, and adipose tissue.
Essential concepts | Fertilization, Placentation, and Lactation
The acrosome and the condensed nucleus are components of the sperm head. As discussed in Chapter 21 (Spermatogenesis), the sperm tail is attached to the head by a head-tail coupling apparatus derived from the centrosome (organized by the proximal and distal centrioles and pericentriolar matrix). The tail consists of a middle piece, a principal piece, and an end piece. The major components of the middle piece are the axoneme and the surrounding outer dense fibers and mitochondrial helical sheath. The major components of the principal piece are the axoneme surrounded by outer dense fibers and the concentric ribs of the fibrous sheath anchored to longitudinal columns.
The trophoblast differentiates into an inner cell layer, the mitotically dividing cytotrophoblast, and an outer cell layer, the postmitotic syncytiotrophoblast. Proteolytic enzymes released by the syncytiotrophoblast erode the branches of the spiral uterine arteries, forming lacunae. This event, called endovascular invasion, initiates the uteroplacental circulation. Lacunae represent the starting point of the future intervillous space of the placenta.
Breast cancer originates in the epithelial lining of the lactiferous ducts (80%). Estrogen receptors and the tumor suppressor genes BRCA1 and BRCA2 play an important role in breast tumors. The most frequent breast tumors are the infiltrating duct carcinoma (originating in lactiferous ducts) and lobular carcinoma (derived from the lobular alveolar tissue).