Chapter 31 Fertility, preconception care and pregnancy
PRECONCEPTION CARE
There is solid scientific evidence that infant health is inextricably linked to the health of the women who bear them, especially regarding preconception care.1 Preconception care takes place prior to conception and focuses on the reduction of conception-related risk factors and increasing healthy behaviours. It can be said that preconception care epitomises the naturopathic principle to address the cause, not just the symptom, of illness. By ensuring health issues are addressed in both partners prior to conception, the aim is to improve the health of the infant at birth in a way that even early prenatal care can not.2 Ideally, preconception care involves both partners as some risk factors affect both males and females. Furthermore, involving both partners may help promote equal involvement in the preparation for a major life transition. As with all naturopathic treatments, preconception care incorporates a holistic approach and, as such, supports the physical and psychological health of both partners.
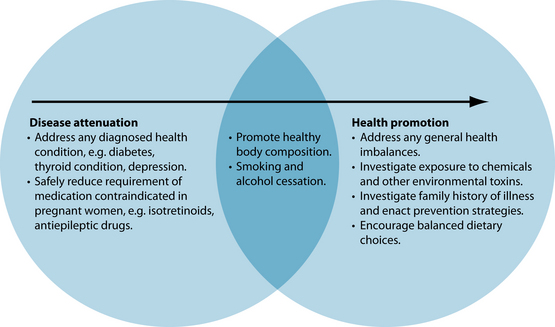
The nature of a preconception care plan will differ between couples. For ease of understanding, preconception care can be categorised into two broad categories: health promotion and disease attenuation. Health promotion preconception care describes couples who have not yet attempted conception and have no diagnosed illnesses, but would like to ensure optimum health before their baby is conceived. Disease attenuation preconception care, in contrast, applies to couples with current diagnosed health conditions, or who have already had unsuccessful attempts to conceive. There may be some crossover between these two categories and, once disease attenuation has been addressed, it is quite common to incorporate health promotion into the plan prior to conception (see Figure 31.1). However, these are general guides only and the approach to the treatment plan should always be patient-centred, with the time and level of intervention required for each category determined based on couples’ needs. As such, it is important to remind couples that, although many achieve conception soon after they commence attempting, for others patience is required.
Infertility and subfertility
Impaired fertility affects approximately one in six couples.3 In young, healthy couples, the probability of conception in one reproductive cycle is typically 20 to 25%, and in 1 year it is approximately 90%; however, this success rate can decline rapidly due to various age-related or health factors.3
Reproductive specialists use strict definitions of infertility.4 Clinical infertility in a couple is defined as the inability to become pregnant after 12 months of unprotected intercourse. However, consensus is building that the diagnosis of clinical infertility should also be considered after six cycles of unprotected sex in women over 35 years of age.5 Clinical infertility may also be considered when the female is incapable of carrying a pregnancy to full term. At this time further investigation becomes warranted to establish whether there are physical conditions hindering conception and, if so, what intervention may be appropriate. Infertility is not necessarily analogous to subfertility, which is often caused by other underlying conditions such as endometriosis or polycystic ovarian syndrome.
Causes of infertility and subfertility
Infertility can be considered to be primary or secondary. Couples with primary infertility have never been able to conceive, while secondary infertility is defined as difficulty conceiving after already having conceived (and either carried the pregnancy to term or had a miscarriage).4 Secondary infertility is not considered as a diagnosis if there has been a change of partners.4
Infertility may also be more broadly grouped into categories of sterility or relative infertility. Sterility can arise from various predominantly non-treatable underlying disorders involving lack of eggs (menopause, radiation damage or some autoimmune diseases); lack of sperm (infectious causes or immature sperm); fallopian tube obstruction (endometriosis, surgical or due to infection such as chlamydia) or hysterectomy. In contrast, infertility may be caused by other factors (see Table 31.1). Male causes of infertility include defective sperm production and/or insemination difficulties.6 Female causes include ovulation factors (anovulation or infrequent ovulation), tubal damage, uterine factors such as adhesions, and cervical mucus ‘hostility’ (commonly due to an immunological defect).6
Table 31.1 Common causes of infertility in males and females
| MALE | FEMALE |
|---|---|
| Low sperm count | Non-specific immune factors |
| Low percentage of progressively motile sperm | Irregular ovulation (e.g. polycystic ovarian syndrome) |
| Disorders of sperm morphology | Steroid hormone imbalance (may be influenced by insulin, thyroid function, stress, adiposity or exposure to hormone disrupting compounds) |
| High degree of abnormality on sperm | Hostile endometrial environment (may be influenced by hormonal imbalance, structural abnormalities, fibroids, infection or immunological factors) |
| Chromosome fragmentation | Genetic variations (such as MTHFR polymorphism) |
Source: Adapted from Speroff and Fritz 20054
CONVENTIONAL TREATMENT
The conventional approach to preconception care does not differ greatly from the naturopathic approach. The focus is on increasing the general level of health and ceasing unhealthy behaviours. The factors identified as areas of concern for preconception care include chronic diseases, infectious diseases, reproductive issues, genetic/inherited conditions, medications and medical treatment, and personal behaviours or exposures.2 Of these issues, a number have proposed clinical practice guidelines. Folic acid supplementation, for example, is considered essential to reduce the incidence of neural tube defects in the fetus, and thus supplementation ideally begins 3 months prior to conception.2 Prevention of congenital defects due to rubella infection is also recommended through rubella vaccination, and a similar approach is taken to hepatitis B due to the potential for vertical transmission to infants and resulting organ damage.2 Management of chronic diseases such as diabetes and hypothyroidism is also considered important in pregnancy to reduce the effects on the developing fetus. Likewise, conditions managed with medication such as isotretinoids and anti-epileptic medication need to be approached with lower dosages or alternative medication as these drugs are teratogens and as such can cause birth defects.2
If a couple have been attempting to conceive for at least 12 months, then initial assessment of hormone levels, ovulation, weight/body composition and semen analysis is undertaken. In the longer term, gynaecological examination to check for physical factors interfering with conception (e.g. scarring from previous STI or endometriosis) is conducted.
Once the diagnosis of infertility has been made, the conventional treatment approach varies depending on the diagnosed reason for the infertility. If the diagnosis is male infertility, then the treatment will depend on the seminal analysis. If azoospermia (absence of sperm) is diagnosed, then conception relies upon donor insemination.6 However, if there is severe oligospermia (fewer than 5 million sperm), then a single spermatozoon is recovered from the epididymis and microinjected in the ovum. This has a 30% success rate.6 There have been some attempts to increase the sperm count of men with oligospermia using hormonal therapy (testosterone analogues and antioestrogens) with limited documented benefit.6 Another alternative in this situation is in vitro fertilisation.6
Alternatively, infertility may be due to female reproductive pathophysiology. Anovulation is managed by encouraging the woman to aim for an appropriate body composition, and use of an antioestrogen drug (clomifene), which has resulted in a 70% conception rate in amenorrhoeic women.6 If tubal damage has been diagnosed, there are really only two options available: microsurgery to attempt to repair the fallopian tubes, or in vitro fertilisation. With a diagnosis of cervical hostility, the traditional conventional approach is to encourage the couple to use condoms for 6 months in the hope that the antibodies attacking the sperm will be eliminated. Other, more invasive approaches include ingestion of oral corticoids by the male in the first 10 days of the woman’s cycle, the use of washed sperm, or in vitro fertilisation or gamete intrafallopian transfer techniques.6
RISK FACTORS
Cigarette smoking adversely affects fertility in both males and females.7–10 Smoking affects sperm production, motility, morphology and incidence of DNA damage in males;11 this may be explained by increased reactive oxygen species, which has been linked with lowered sperm concentration, motility and morphology.12 Cigarette smoking in females may affect the follicular microenvironment, and may cause alteration of hormone levels in the follicular phase.11 Both active and passive smoking have been demonstrated to increase zona pellucida thickness; this may make it more difficult for sperm to penetrate.13 In active smokers, the effect of delayed conception is increased with the number of cigarettes smoked.9 Despite these statistics, more than 10% of pregnant women continue to smoke cigarettes.14
Caffeine intake may also adversely affect fertility outcomes.15 Some research has found that coffee and/or tea intake greater than six cups a day is associated with reduced fertility.10 However, other researchers assert that coffee and tea consumption associated with reduced fertility rates in males and females is not dose related, and that constituents other than caffeine may also have an effect.8 Other drug use, such as recreational drugs and alcohol, may also contribute to certain subtypes of infertility.15
Another lifestyle factor that may affect fertility is diet and its associated nutritional status. A range of dietary constituents have been linked with various aspects of infertility including trans-fatty acids,16 iron,17,18 antioxidants,19,20 selenium21 and zinc.22 Increasing intake of vegetable protein and replacing animal protein may also reduce the ovulatory infertility risk.23 Similarly, a high glycaemic load diet and overall high dietary carbohydrates have also been associated with increased ovulatory infertility.24
Psychological stress is an added risk factor for reduced fertility in females25 and males.26 Depression in males has been correlated with decrease in sperm concentration and poor coping mechanisms have been associated with increased occurrence of early miscarriage.26
Both maternal and paternal age have a bearing on the fertility level of a couple. Older women experience more difficulty achieving and maintaining pregnancy, and are less likely to deliver a healthy infant than younger women.27 In females spontaneous cumulative pregnancy rates begin to decline as early as 31–35 years of age.27 One-third of women aged 35–39 years of age will experience difficulty achieving pregnancy, and half of women aged 40–44 years will have an impaired ability to reproduce.27 Increasing maternal age results in a decreased number of oocytes, decreased oocyte quality, uterine age-related changes affecting endometrial receptivity and neuroendocrine system ageing.27
Another general risk factor to consider when approaching preconception care is the presence of underlying disease. Women with a chronic disease such as diabetes have an increased risk of congenital abnormalities in their offspring, but are known to have improved birth outcomes when they plan their pregnancies and use preconception care.28 Coeliac disease is another condition which is known to incur higher miscarriage rates, increased fetal growth restriction and lower birth weights.29 Although not a disease, obesity may also affect fertility for both males30 and females.31 Sexually transmitted infections, particularly chlamydia and gonorrhoea, may lead to infertility.15 Infection of any nature may be associated with reduced sperm motility.32 Other conditions may affect fertility but, rather than the disease being detrimental, it is the medication used to manage the condition which is problematic. Several different types of medications, including hormones, antibiotics, antidepressants, pain-relieving agents, and aspirin and ibuprofen when taken in the middle of the cycle, have been reported to affect female fertility.4 With this in mind, it is important to address any underlying health issues, resolving them where possible, to reduce reliance on medication. Alternatively, where the condition cannot be resolved, exploration of substitute medication may be necessary.
KEY TREATMENT PROTOCOLS
A key naturopathic principle to be considered when supporting couples with fertility issues is to treat the whole person. It is vital that the approach to the development of a treatment plan for such couples is patient-centred, and does not make assumptions about their individual needs without diligent exploration of their health history and current health complaints. Such exploration must go beyond reproductive health, as a number of conditions not directly linked to the reproductive system have been associated with infertility. Examples of such conditions are inflammatory bowel disease,33 thyroid disease34 and type 1 diabetes.35 Other conditions more directly associated with the reproductive system which may need to be addressed include endometriosis36 and polycystic ovarian syndrome.37
Underlying conditions aside, preconception care will still benefit many couples by promoting health. Many lifestyle factors dramatically affect fertility, birth success and infant health.11 Preconception care must address these factors in order to promote fertility, conception and healthy pregnancy outcome. A study found that 81% of couples previously classified as infertile were able to conceive within 2 years of commencing an individualised preconception program.38
In general, due to the individual nature of preconception care, the treatment interventions used will vary significantly between couples; however, there are some remedies which are more commonly used. Common herbal medicines that may be useful when supporting couples during preconception care include Vitex agnus-castus and Tribulus terrestris. Vitex agnus-castus, or chaste berry, is used traditionally in fertility disorders, particularly for women with progesterone deficiency or luteal phase defects. No large studies have explored this role; however, a randomised, placebo-controlled trial (RCT) with 96 women with various fertility disorders (secondary amenorrhoea, luteal insufficiency and idiopathic insufficiency) taking Vitex agnus-castus for 3 months resulted in women with secondary amenorrhoea and luteal insufficiency achieving pregnancy twice as often as those in the placebo group.39 Previous smaller trials show similar results.40,41 Tribulus terrestris has also been associated with improving conception outcomes in women with endocrine sterility.42
Window of fertility
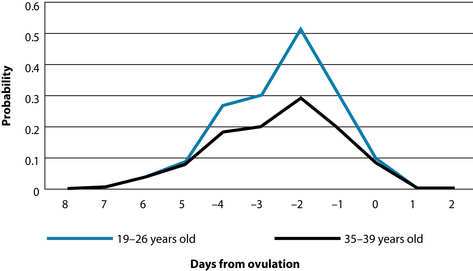
The first priority when approaching preconception care and couples with fertility issues is to establish the window of fertility. The window of fertility is probably best defined as the period in the 6 days leading up to ovulation, when in theory the oocytes and sperm should have maximum viability and survivability.43,44 However, in an individual clinical setting this can be more accurately garnered through analysis of intermenstrual intervals, cervical mucus and basal body temperature charts (see Chapter 20 on polycystic ovarian syndrome). Intercourse is most likely to result in pregnancy when it occurs within the 3 days prior to ovulation.
Although certainly not a prerequisite for pregnancy to occur, the probability of conception is highest when cervical mucus (vaginal secretions) is slippery and clear (see Figure 31.2).45–47 When combined with basal body temperature charts these simple and cheap analyses are able to predict peak fertility far better than menstrual charts alone. Cervical mucus analysis alone has been demonstrated to better predict peak fertility than either basal body temperature charts or biochemical ovulation detection kits based on LH.48
Monitoring cervical mucus may have other practical benefits as water-based vaginal lubricants can inhibit sperm motility by 60–100% in vitro.50 Mineral oil, canola oil or hydroxyethylcellulose-based lubricants do not seem to have this effect.
Diet
Dietary change is an important intervention in any preconception plan and, although the focus is on a general healthy diet for couples, some specific dietary choices have been found to have direct benefits for fertility. Replacing animal protein with vegetable protein, for example, has been found to be beneficial in women seeking to get pregnant.51 Similarly, low-fat dairy products have been connected with higher rates of anovulatory infertility, and higher dietary intake of trans-unsaturated fats have been linked with increased risk
of ovulatory infertility.52 Organic food may also be of benefit by reducing the potential exposure to environmental chemicals. Ultimately, the consensus seems to be that encouraging healthier eating habits more broadly improves fertility outcomes. As such, a healthy eating plan that includes foods with high levels of nutrients should be encouraged. High levels of brightly coloured fruit and vegetables to provide antioxidants plus good protein sources (meat if eaten, cheese, eggs, tofu if vegetarian; vegans need to be particularly careful with protein levels) and good-quality carbohydrates (wholemeal and wholegrain) should be routinely recommended (see Appendix 3, ‘Food sources of nutrients’).
Body composition
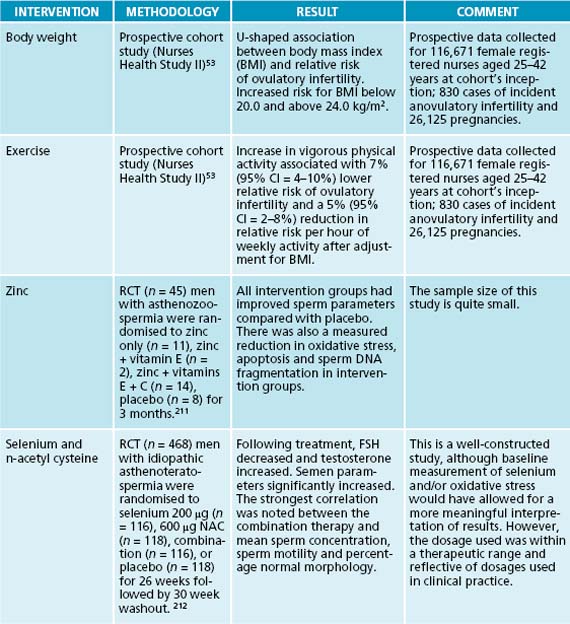
Overweight and obese women are less likely to conceive than those of normal weight.15 These women also experience increased risk of pregnancy complications and adverse pregnancy outcomes in comparison to women who weigh less. Conversely, women who are very underweight may also experience problems conceiving.15 Reproductive function can be affected by both obesity and low body weight, due to hormone imbalances and ovulatory dysfunction.11 Overall, the relative risk of ovulatory infertility is increased for body mass index (BMI) below 20.0 kg/m2 or above 24.0 kg/m2.53 There appears to be a 7% increase in the rate of fetal anomaly for each unit of BMI above 25.54 Obesity affects fertility in ways that are complex and not well understood; however, the association with functional hyperandrogenism and hyperinsulinaemia is thought to play an important role.55 Abdominal obesity in women with polycystic ovarian syndrome (PCOS) is considered to be co-responsible for the development of hyperandrogenism and chronic anovulation through mechanisms involving decreased concentrations of sex-hormone-binding globulin in the blood and insulin-mediated overstimulation of ovarian steroidogenesis.55 Obesity may also contribute to reduced outcomes of IVF/ICSI procedures by promoting resistance to clomiphene and gonadotrophin-induced ovulation.55 It has been demonstrated that weight loss in obese women can improve fertility through the recovery of spontaneous ovulation, and that others will have improved responses to ovarian stimulation in infertility treatment.56,57
Attenuating the hormonal imbalances resulting from high body fat can be achieved through both diet and exercise (as discussed in Chapter 20 on polycystic ovarian syndrome). Even after 12 weeks of dietary and exercise intervention, favourable menstrual and metabolic outcomes conducive to conception could be gained in infertile, overweight women.58 In fact, lifestyle modification proved more effective than fertility drugs in inducing ovulation in women with anovulatory disorders.59 However, it is important to note that weight loss needs to be approached responsibly, as rapid weight loss is understood to lower progesterone levels, slow follicular growth and inhibit the luteinising hormone surge, disallowing ovulation.60
Lifestyle activity
Maintaining an active lifestyle is beneficial in promoting both male and female fertility; however, moderation is very important. While moderate exercise may improve the chances of conceiving spontaneously or through fertility treatment,11 excessive physical exercise is associated with a spectrum of reproductive dysfunctions in both males and females. Female fertility issues associated with excessive exercise range from luteal-phase defects to anovulation to infertility and finally to amenorrhoea.53 Increase in vigorous activity (but not moderate activity) is associated with reduced relative risk of ovulatory infertility,53 and has been linked to poor IVF outcomes.61 This concern has also been found to affect male fertility, through subclinical changes in their reproductive hormone profile and semen parameters.62 For example, male endurance runners have been found to have a reduction in total and free testosterone, alterations in luteinising hormone release, and in pituitary responses to gonadotrophin-releasing hormone.62 Furthermore, there has been evidence of a change in the semen parameters of some endurance athletes, such as low normal sperm count, decreased motility and various morphological changes.62
This apparent contradiction between the benefits and risks of exercise can be best explained by the role of exercise in preventing and managing conditions that detrimentally effect fertility, such as polycystic ovarian syndrome and obesity.63 In contrast, any level of activity which induces metabolic stress will interfere with the hypothalamus–pituitary–gonadal axis, and therefore affect fertility.64 Overall, the focus when supporting couples prior to conception should be on moderate exercise that does not place undue stress on their systems.
Reduce risk factors
Factors such as smoking, caffeine intake and alcohol consumption may adversely affect fertility outcomes and should be reduced. Even if fertility is not yet a concern for a couple, these risk factors will still need to be addressed as they all have negative effects on the developing fetus and infant health. Maternal smoking during pregnancy, for example, has been linked to increased risk of wheezing in infants up to 2 years old65 and reduced fetal brain development,66 and may increase the infant’s risk of adult development of diabetes, hypertension and metabolic syndrome.67 Similarly, high alcohol consumption during pregnancy puts the developing fetus at risk of fetal alcohol syndrome.68 Even lower-level intake can affect the neuroendocrine and behavioural functions of the offspring.69
Stress
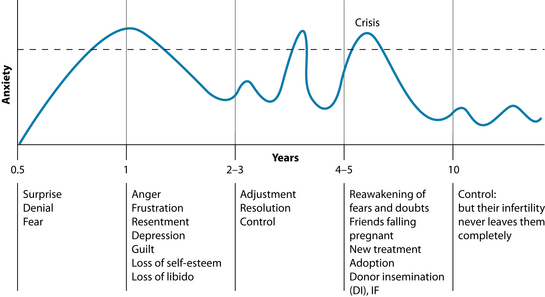
The emotional journey of a subfertile couple is complex. Seemingly innocuous events such as friends falling pregnant, family events and birthdays may trigger underlying anxiety issues (see Figure 31.3).
The process of undergoing infertility treatment itself can also be stressful and exacerbate anxiety, depression and stress, often enough to negatively affect pregnancy outcomes.72,73 This may be due to increased cortisol secretion, resulting from a normal stress response, down-regulating the hypothalamus–pituitary–gonadal (HPG) axis. It has been postulated that this may occur by inhibiting gonadotrophin-releasing hormone’s (GnRH) release of follicle-stimulating hormone (FSH) and luteinising hormone (LH) from the pituitary.74
As such, counselling or psychological support, particularly interventions which focus on stress management and coping-skills training, should be strongly recommended throughout this process.75 It is equally as important for the infertile couple to build a support network. Both attending support sessions and using cognitive behavioural interventions were equally effective in reducing the emotional aspects of infertility and improving the chances of pregnancy.76 Music therapy has also been associated with positive pregnancy outcomes.77 Overall, couples should be encouraged to take part in stress reduction activities at all stages of preconception and pregnancy. Anecdotal stories of previously infertile couples conceiving after ceasing trying or while on holiday are not to be ignored.78
Environmental concerns
Exposure to herbicides, fungicides, pesticides and other chlorinated hydrocarbons has been associated with decreased fertility and a higher risk of spontaneous miscarriage.79,80 Further to this, it should be noted that, although over 140,000 chemicals are in common use in today’s society, evaluation of the effects on reproduction of common physical and chemical agents has occurred in only 5% of substances.81 With this in mind, it is important to investigate potential exposure to environmental chemicals such as pesticides, herbicides, household chemicals, paint and paint thinners, and plastics. Paradoxically many couples will subject themselves to high levels of environmental toxins during ‘nesting’ activities while trying to conceive or during pregnancy. While preparation for the child is certainly important, activities that include exposure to dust, paint or other chemicals and substances that release toxins, such as home renovations, may adversely affect pregnancy outcomes and should be considered carefully.
If exposure is identified, and particularly if it is occupational (for example, factory workers, tradesmen, farmers and horticulturalists), then protective measures must be taken. Such measures include appropriate occupation health and safety interventions like wearing protective clothing and masks.82 Beyond this, the preconception treatment plan needs to incorporate suitable detoxification protocols (see the box on liver detoxification in Chapter 19 on endometriosis).
Immune dysfunction
Immune system imbalances may adversely affect fertility outcomes through a number of ways, including high generalised inflammation and antibodies targeted to key tissues. High levels of inflammatory prostaglandins, for example, may reduce uterine receptivity to fertilised embryos,83 possibly by affecting the regulation of genes necessary for human endometrial receptivity.84 Chronic inflammation may also contribute to the development of anatomic abnormalities such as pelvic adhesions and occluded fallopian tubes, as well as premature ovarian failure.85 Causes of inflammation in reproductive tissues vary and may include sexually transmitted infections such as Chlamydia trachomatis,
INFLAMMATION AND HEALTHY REPRODUCTION
Inflammation is often approached as an undesirable adversary in the human body. In fact, inflammation is a mechanism necessary for the normal and healthy reproductive process. As the luteinising hormone (LH) surge occurs prior to ovulation, LH stimulates granulosa cells to secrete inflammatory mediators (prostaglandins and cytokines) and progesterone. These compounds all trigger the secretion of matrix metalloproteinases, which break down the extracellular matrix, thereby allowing for follicular rupture and ovulation.85 As such, indiscriminate use of anti-inflammatory interventions in preconception care should be avoided.
endometriosis and autoimmune conditions.85 Autoimmune conditions which can contribute to infertility may be non-specific, such as type 1 diabetes mellitus and Hashimoto’s thyroiditis, or specific, such as antibodies that target FSH and LH and their receptors.86,87 Another such example is antibodies that target ovaries and sperm.84 It is worth noting, however, that the inflammatory response is also an important mechanism within healthy, normal reproductive function (see the box on inflammation and healthy reproduction). With this in mind, various measures to reduce inflammation systemically and specifically can be found in other relevant chapters.
Nutritional medicines
The primary conventional focus of nutrient supplementation in preconception care is on the role of folic acid in preventing neural tube defect.88 The benefits attributed to folic acid in the prevention of this condition require maternal sufficiency in the first 28 days of gestation, before many women know they are pregnant.88 It is this knowledge that has led to public health interventions such as folate fortification of bread flour and further supplementation of 400 μg/day for women of reproductive age.88
Folic acid is not the only nutrient required in preconception and the early stages of gestation. A recent longitudinal study89 observed the effect of pregnancy on the micronutrient status of the mothers. It was noted that, while folate levels decreased slightly during pregnancy and remained decreased up to 6 weeks after delivery, vitamin B12 progressively declined throughout gestation and reached marginal or deficient levels.89 This is of particular concern, as vitamin B12 has been overlooked as an important nutrient for preconception supplementation. Low maternal vitamin B12 status has been associated with a threefold risk of neural tube defect.90 This deviates from the previous approach to neural tube defect prevention, which has been firmly focused on folic acid supplementation and fortification of food. In fact, the focus on folic acid fortification of food, such as bread flour, may be contributing to a masking of vitamin B12 deficiency and an increased risk of neural tube defect91 (see the box on vitamin B12 and folate).
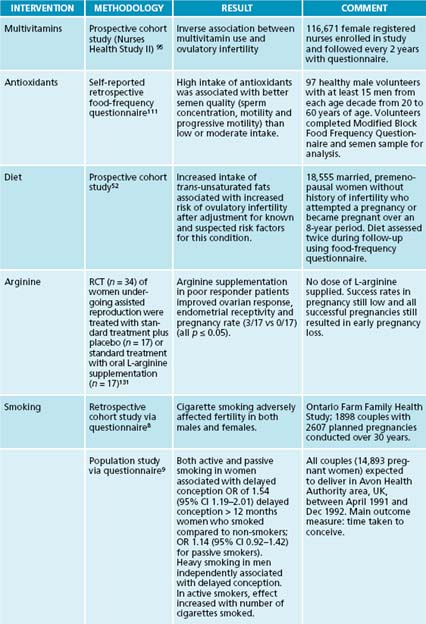
Various multivitamin and antioxidant nutritional supplements have improved pregnancy rates in those undergoing assisted reproduction92 or lowered time to conception in couples seeking preconception care.92,93 Preconception multivitamin use has also been associated with a higher incidence of multiple births for unknown reasons.94 Folate needs to be taken at least 3 months prior to conception for optimal benefit in reducing neural tube defects or leukaemia development in the fetus. However, it is also associated with decreased incidence of ovulatory infertility more generally.95 Vitamin C
VITAMIN B12 OR FOLIC ACID?
Folic acid has been used for a number of years to prevent neural tube defect;88 however, recent research has identified that vitamin B12 is also important in preventing this condition.90 With this in mind, the most predictable course of action may be to incorporate vitamin B12 supplementation into standard preconception care approaches alongside folic acid. Unfortunately, just as some concerns regarding the risks of folic acid supplementation masking vitamin B12 deficiency have been raised,91 excess vitamin B12 intake, resulting in potential cobalt toxicity,97 may also be a concern. To avoid this, and to stay true to the naturopathic patient-centred approach, assessing the most appropriate nutrients required for supplementation and the relevant dosages are vital. Folic acid is found predominantly in legumes and green leafy vegetables, while vitamin B12 is found in its most bioavailable form in animal products.98 As such, an assessment of a patient’s diet will provide an initial indication as to whether supplementation of folic acid and/or vitamin B12 is required. In general, though, it is important to remember that the absorption of vitamin B12 is an incredibly complex process that relies on healthy gastric, pancreatic and intestinal function, and that dysfunction in any one of these organs can compromise B12 status.98 A more thorough assessment of sufficiency of these nutrients can be gleaned through testing. The most accurate test to determine folic acid status is red blood cell folate, not the commonly used serum folate, which does not correlate with tissue stores.99 Vitamin B12 status can be assessed using serum cobalamin, which is more specific and stable compared with serum folate; however, both pregnancy and folate deficiency can result in false low readings. A more accurate assessment, which is independent of both of these conditions, is that of methylmalonic acid. Unfortunately, this test is much more expensive and technically demanding.100
supplementation has also had improved fertility outcomes in women with luteal phase defects.96
The male partner
It is important to realise that in 20% of infertile couples males are the sole cause of infertility and are an important contributing factor in a further 20–40% of infertile couples.101 Although many infertile men may have physical or structural conditions that require surgical intervention, many may have reversible issues that can be corrected with non-invasive measures. Men also experience declining fertility as they age—most profoundly after the age of 55 years but even men over the relatively young age of 35 years have half the chance of successfully inseminating as men under the age of 25 years.102
A decline in male fertility has been reported over the past few decades in a number of countries, though this has been controversial.103 It has been suggested that environmental and lifestyle factors such as increased occupational chemical and pesticide exposure are at least partly responsible for this decline.104–106 Oestrogen-like products are thought to be partly responsible. The fact that organic farmers have higher sperm counts than regular farmers or other exposed occupational groups lends further credence to this theory.107 Other environmental and lifestyle factors that may be affecting fertility include wearing tight-fitting clothing, using hot baths and spas and having occupations that require long periods of sitting down, as these behaviours all increase scrotal temperature.108 Dietary intake must also be considered, as it may affect semen quality. Men consuming diets high in meat and dairy products109 and soy protein110 have compromised semen parameters, whereas diets high in fruits and vegetables show benefit.109 The advantage in a fruit- and vegetable-rich diet may be attributed to an increased antioxidant intake.111
Beyond diet and lifestyle, some specific nutrients have been identified to improve fertility in men. For example, there is evidence that coenzyme Q10 supplementation can improve semen parameters in men,112,113 while vitamin C, vitamin E, beta carotene, folate and zinc are important for semen quality.4 A similar trial that identified increased pregnancy rates in couples with severe male infertility when taking an antioxidant supplement containing ascorbic acid, zinc, vitamin E, folate, lycopene, garlic oil and selenium has been conducted.114 In contrast, selenium has been demonstrated to improve sperm quality and motility in subfertile men, but not those diagnosed with infertility,115–117 or conversely with normal testicular selenium levels.118,119 Similarly, L-carnitine has been associated with increased semen quality and sperm concentration, particularly in groups with lower baseline levels,120–123 though one trial suggested that this may be true only in those with normal mitochondrial function.124
Assisted fertility procedures
Assisted reproductive technologies encompass a spectrum of methods and are valid options for infertile couples (see Table 31.2). However, the usefulness of these therapies needs to be considered by any prospective couple in the context of the costs and risks. For example, a systematic review of studies measuring the prevalence of birth defects in infants conceived using assisted reproductive technologies found a 30–40% increased risk.125 Furthermore, the average cost of IVF for Australian women is $32,903,126 while the success rate is 10% for a single IVF procedure, and increases to 40% if the procedure is repeated five times.6 Finally, the process of IVF requires constant emotional adjustment through each phase of the process,127 and can be debilitating for the woman in particular. To support this, a questionnaire study128 found that financial burden (23%), psychological stress (36%) and lack of success (23%) were the most predominant reasons couples discontinued IVF programs. In particular, a combination of lack of success and psychological stress was noted in 18% of participants.
| TYPE | PROCEDURE | PREGNANCY RATE∗ |
|---|---|---|
| Assisted insemination with husband’s sperm (AIH) | Sperm are transferred by catheter into uterus or fallopian tube. | Up to 15% per cycle |
| In vitro fertilisation (IVF) | Fertilised eggs are transferred in to the uterus or fallopian tube. | 10–25% per cycle; depends on maternal age |
| Gamete intrafallopian transfer (GIFT) | Unfertilised eggs and sperm are transferred into one or both fallopian tubes using laparoscopy or transvaginal ultrasound. | Up to 30–40% per cycle |
| Intracytoplasmic sperm injection (ICSI) | Sperm is injected into the egg. | More than 50% per cycle |
| Zygote intrafallopian transfer (ZIFT), tubal embryo stage transfer (TEST) | Zygote or early embryo is transferred into the fallopian tube using laparoscopy or transvaginal ultrasound the day after egg pick-up. | Up to 30–40% per cycle |
∗ Adapted from Oats and Abraham 20056 Note that pregnancy rate is not the same as live birth rate. Naturopathic treatment of couples undergoing assisted reproductive techniques should not cease once these interventions have resulted in a successful pregnancy.
Often this course of action is used as a symptomatic approach to infertility and does not have the added benefit of preparing the body for a healthy pregnancy or allowing for improved success of subsequent births. In one study 65% of couples who had previously undergone multiple IVF cycles were able to conceive within 2 years of a preconception program.38 However, there will be instances where referral to this procedure will be appropriate.
Most patients attending assisted reproduction will be using some form of complementary therapy and are likely to be consulting a complementary therapist; they are perhaps using several options concurrently.129 Therefore it may be prudent to identify the broad scope of treatment the patient is undertaking so as to reduce the risk of negative interactions. Acupuncture on the day of embryo transfer is demonstrated to have a beneficial effect on live births.130 L-arginine supplementation can improve the response rates of poor responder women undergoing assisted reproduction.131
DIETARY REQUIREMENTS
Dietary requirements in pregnancy encompass nutrients that must be included and foods that should be avoided. Additional energy is needed in pregnancy and lactation to cover the needs of the growing fetus, the placenta and expanding maternal tissues, and additional maternal effort at rest and in physical activity, as well as the production of breast milk during lactation. Nothing additional over pre-pregnancy requirements is needed in the first trimester, though in the second trimester an extra 1.4 MJ/day and in the third trimester an extra 1.9 MJ/day over pre-pregnancy levels should be consumed.132 Protein requirements also increase to 1.1 g/kg of body weight, as does the recommended daily intake of a number of nutrients including folic acid, vitamin C, iron, zinc and calcium (see Table 31.3).
| NUTRIENT | EFFECT | COMMENT |
|---|---|---|
| DHA | Accumulates in the developing brain, and is important for prenatal and postnatal neurological development. | Can be easily converted via desaturases from α-linolenic acid. |
| Vitamin A | Important for the regulation of gene expression and for cell differentiation and proliferation. | Direct studies of vitamin A status are lacking, but excess retinol is a known teratogen. The threshold risk is still unclear, but the upper intake level is 3000 μg/day. |
| Folate | Required for normal cell division, and methylation during nucleotide synthesis. Associated with prevention of neural tube defect. | Supplementation still needs to be approached judiciously as the upper limit is only 1000 μg/day and some women already have folate-rich diets. |
| Vitamin B12 | Supports methylation of nucleotides in conjunction with folate. Also essential for neurological function. Absorption decreases during pregnancy. | Although vitamin B12 can be stored in adults long term, only newly absorbed vitamin B12 is readily transported across the placenta.Vegan women will need to supplement. |
| Biotin | Animal studies imply that deficiency is teratogenic. | More evidence relating specifically to pregnant women is required to make confident clinical decisions. |
| Calcium | Required for bones, teeth, vascular contraction, vasodilation, muscle contraction, nerve transmission and glandular secretion. | Most fetal accretion occurs in the third trimester, and this is lower when maternal intake is low. |
| Chromium | Potentates the action of insulin. | Chromium is depleted throughout pregnancy and fetal tissue levels decline after birth, suggesting the need for deposition during pregnancy. |
| Iodine | Required for thyroid hormones, and therefore associated with myelination of the central nervous system and general metabolism. Most active in perinatal periods. | Deficiency is damaging to the developing brain and includes mental retardation, hypothyroidism and goitre. |
| Iron | Required for haem proteins and other iron-dependent enzymes. | Deficiency in pregnancy is associated with increased perinatal maternal and infant mortality, premature delivery and low birth weight |
| Zinc | A cofactor to nearly 100 enzymes, with catalytic, structural and regulatory functions. | Maternal zinc deficiency may lead to prolonged labour, intrauterine growth retardation, teratogenesis and embryonic or fetal death. Lower dietary intakes can lead to a higher incidence of premature deliveries. |
Source: Adapted from Turner 2006135
A number of dietary practices should be avoided or limited.133 Alcohol consumption during pregnancy is linked to a spectrum of disorders in the infant ranging from fetal alcohol syndrome through to alcohol-related birth defects or alcohol-related neurodevelopmental disorders.133 There is no safe level of alcohol intake during pregnancy, and as such pregnant women should be discouraged from any consumption (see the box on ethanol-based herbal extracts and pregnancy). Fish consumption must also be approached with care in pregnancy due to the risks associated with fetal exposure to methylmercury. In general, this compound accumulates from industrial pollution (although it also occurs naturally) in some of the larger, longer-lived fish, and those that consume other fish.133 Examples include shark, swordfish, king mackerel and tuna.133 In contrast, sardines and white fish have lower mercury levels and as much as 360 g can be safely consumed per week.133 Another risk is food contamination with (listerosis) which can cause spontaneous abortion, stillbirth and fetal infection Listeria monocytogenes.133 To prevent this illness, pregnant women should avoid unpasteurised milk, undercooked or raw animal products, refrigerated smoked food, pâtés or meat spreads, soft cheeses, and unwashed fruit and vegetables.133 Caffeine consumption must also be approached with caution during pregnancy, as it has been connected with fetal growth restriction and low birth weight infants.134 One of the concerns surrounding caffeine is that the enzyme responsible for caffeine clearance, CYP1A2, is not present in fetal tissue, although caffeine can easily pass through the fetoplacental barrier.134 For this reason, it is important that if the pregnant woman is going to consume caffeine their own phase 1 detoxification pathway is functioning at its optimum. This should be addressed in preconception treatment, however, not during pregnancy. It has been recommended that women should not consume more than 200 mg/day of caffeine throughout gestation.134
Appropriate weight gain
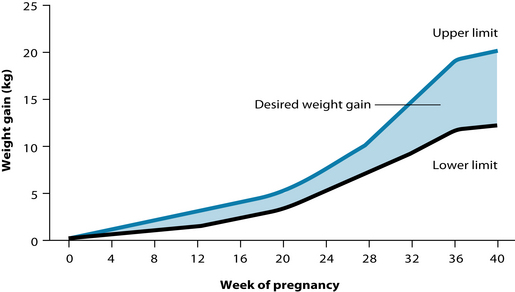
There should be relatively little maternal weight gain until the second and third trimesters, with the bulk of the weight gain in the third trimester (see Figure 31.4). Increased weight gain may lead to an increased risk of gestational diabetes, which has significant health implications for both mother and child.4 High blood-sugar levels are used as an energy source by the growing baby and will therefore lead to increased birth weight. Although there are several negative health consequences for the baby associated with
ETHANOL-BASED HERBAL EXTRACTS AND PREGNANCY
It is recommended that all pregnant women minimise their alcohol consumption and abstain if possible.133 But where does that leave the prescription of ethanol-based herbal extracts? If possible, other forms of herbal products should be prescribed to pregnant women to keep alcohol consumption to a minimum. This can include preformulated tablets, infusions, decoctions or glycetracts. However, the value in an individualised and extemporaneously dispensed formula of ethanol-based herbal extracts is well known to most practising naturopaths. If it is deemed that the best treatment for the individual is in the form of an ethanol-based herbal extract, then a useful approach is the addition of hot water to the tincture prior to each dose. This encourages evaporation of the alcohol and, although it may not eliminate the alcohol completely, it will reduce the amount remaining in the tincture.
Another potential cause of inappropriate weight gain is oedema, which may be linked to preeclampsia. Preeclampsia is a form of hypertension that occurs only in pregnancy, and is accompanied by proteinuria and excessive oedema.133 Although obesity does increase the risk of developing preeclampsia,133 it should not be assumed that weight gain is simply fat gain. Thorough dietary and physical assessment are needed to determine if fluid retention is an issue, or whether a high glycaemic, hypercaloric diet is the concern.
Anaemia
Maternal iron requirements increase in pregnancy because of the demands of the developing fetus, the formation of the placenta and the increasing maternal red cell mass.136 Fetal iron requirements seem to come at the expense of maternal stores if there is insufficient intake. Even moderate iron deficiency is associated with a twofold risk of maternal death.136 However, routine iron supplementation in women with normal haemoglobin is not associated with improved pregnancy outcomes. Furthermore, supplementation with high levels of iron increases the risk of oxidative stress, and should be approached with caution. With this in mind, one study137 found that taking an iron supplement (60 mg iron, 200 μg folic acid and 1 μg B12) daily was no more beneficial than taking two tablets once per week. This may be an approach to reduce the risk of oxidative damage and still ensure iron sufficiency.
Safety in pregnancy
As the aim of pregnancy care is generally to move towards optimal health rather than treatment of particular disease states, herbal medicines and large doses of specific nutrients should generally be avoided during pregnancy (see Table 31.4). Even seemingly innocuous herbal medicines with hormonal activity or uterine activity are best avoided during pregnancy. Although uterine tonics may have a role to play in preparation for labour, even they need to be avoided at early stages of pregnancy.
Table 31.4 Herbs contraindicated during pregnancy (bold indicates common herbs more likely to be encountered regularly in clinical practice)141–144
There is still a high use of many different herbal and nutritional medicines by pregnant women, and nearly three-quarters of these women do not discuss this use with their conventional physician.138,139 This may be due to the fact that specialist obstetricians generally have less favourable attitudes towards complementary medicines than women’s non-obstetric physicians.140
To assist naturopaths to determine the safety of herbal medicines, a classification system141 based on Therapeutic Goods Administration (Australia) and Food and Drug Administration (USA) categories for prescription medicines in pregnancy has been developed. Contraindicated herbs fit into categories D and X in this system (see Table 31.5). However, it is recommended that most herbal medicines be avoided during pregnancy unless absolutely necessary.
Table 31.5 Examples of herbs classified for use in pregnancy141
| CATEGORY | CATEGORY DEFINITION | RELEVANT HERBS |
|---|---|---|
| Category A | Drugs which have been taken by a large number of pregnant women and women of childbearing age without any proven increase in the frequency of malformations or other direct or indirect harmful effects on the fetus having been observed. | |
| Category B1 | Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed.Studies in animals have not shown evidence of an increased occurrence of fetal damage. | |
| Category B2 | Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals are inadequate or may be lacking, but available data show no evidence of an increased occurrence of fetal damage. | Barosma betulina |
| Category B3 | Drugs which have been taken by only a limited number of pregnant women and women of childbearing age, without an increase in the frequency of malformation or other direct or indirect harmful effects on the human fetus having been observed. Studies in animals have shown evidence of an increased occurrence of fetal damage, the significance of which is considered uncertain in humans. | Andrographis paniculata |
| Category C | Drugs which, owing to their pharmacological effects, have caused or may be suspected of causing harmful effects on the human fetus or neonate without causing malformations. These effects may be reversible. Accompanying texts should be consulted for further details. | |
| Category D | Drugs which have caused, are suspected to have caused or may be expected to cause an increased incidence of human fetal malformations or irreversible damage. These drugs may also have adverse pharmacological effects. Accompanying texts should be consulted for further details. | |
| Category X | Drugs which have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy. |
Partus preparator
Rubus idaeus has long been traditionally used as a ‘partus preparator’—preparing the uterus for delivery and to facilitate labour.145 Animal studies have suggested that Rubus idaeus may increase the regularity and decrease the frequency of uterine contractions.146 Although no clinical studies have been conducted in humans, retrospective studies have demonstrated that R. idaeus use is associated with a decreased rate of medical interventions required in childbirth.147,148 One study found that R. idaeus shortened labour times and reduced the incidence of pre- and postterm labour,149 while another suggested it reduced only the duration of second stage of labour. A recent literature review concluded that the evidence for the use of R. idaeus was scarce, and more research is needed.150
Chronic miscarriage
Conservative estimates suggest that 10% of first trimester pregnancies end in spontaneous abortion or miscarriage.4 Viburnum prunifolium was traditionally used by the eclectic physicians of North America to prevent miscarriage,151 and was embraced by obstetricians in the late 1800s for the same purpose.152–155 Dioscorea villosa and Chamaelirium luteum have also been traditionally used in threatened miscarriage.142 Unfortunately, more recent research into the efficacy of these herbs has not been conducted; furthermore, concerns over the accurate identification of the herb used in earlier interventions have been raised.156
Other underlying factors may need to be considered and treated in cases of recurrent or threatened miscarriage. For example, an increased risk of miscarriage in both naturally conceived pregnancies and following fertility treatment has been associated with extremes of BMI,157 and interventions that have addressed elevated BMI have been found to reduce the incidence of miscarriage in high-risk women.158
Nausea and vomiting
Up to 80% of all pregnant women experience some nausea and vomiting,159 commonly referred to as ‘morning sickness’. It has been noted, however, that one of the possible causes of nausea and vomiting in early pregnancy is elevated prostaglandin E2, stimulated by human chorionic gonadotrophin.160 Due to the important functions PgE2 performs in early stages of pregnancy, treatment of morning sickness in the first trimester needs to be tempered with respect for the natural process of gestation.
The most common naturopathic treatment for nausea and vomiting in pregnancy is Zingiber officinale. Z. officinale has been demonstrated to be an effective treatment for nausea and vomiting in early pregnancy according to a Cochrane review.161 Since this review, other studies have also demonstrated positive effects,162–164 but some authorities have expressed concern at the high levels of Z. officinale in commercial herbal supplements.165 Ginger tea and candied ginger are also suitable therapeutic sources in the pregnant patient. Z. officinale is also effective for postoperative nausea associated with childbirth.166
Several large trials have demonstrated vitamin B6 to be an effective treatment for the nausea and vomiting of pregnancy.167–169 The optimum dose for this is thought to be between 30 and 75 mg daily.161
Preeclampsia
Obesity and stress are both associated with increased risk of preeclampsia.170,171 Exercise can help reduce the incidence of preeclampsia.172 Insufficient protein, magnesium, calcium, iron, pyridoxine (B6), vitamin C, vitamin E, essential fatty acids and folic acid have all been directly indicated in the pathogenesis of preeclampsia.173 Rather than focusing on one particular nutrient, consensus is moving towards nutritional education more generally as a preventive measure.
Urinary tract infections
Women experience urinary tract infections more frequently during pregnancy. Vaccinium macrocarpon is an effective naturopathic treatment with a documented safety profile in pregnancy and therefore offers a valid therapeutic choice.174,175
Another potentially beneficial treatment option is the use of probiotics. The most direct route to increase the Lactobacillus spp. colony in vaginal mucosal tissue is through insertion of encapsulated probiotics, and should result in improved populations within 3 days.176 If oral administration is preferred then 10 × 109 colony forming units are recommended, and will require 28–60 days to normalise vaginal colonies.176 (see Chapter 27 on recurrent urinary tract infections.)
Childbirth
Childbirth is a culmination of between 37 and 42 weeks’ gestation, and providing support to women at this important moment in time can prevent unnecessary interventions at later stages. Education and empowerment of women to trust their body and the birth process are paramount before labour begins.177 This can be achieved successfully through group psychoeducation and support work to release fear surrounding the birth process.178 It is also important that the woman feels supported by sensitive and nurturing birth companions at the time of birth.177 Birth companions such as midwives179–182 and doulas183–186 have been associated with improved birth outcomes for women seeking low-intervention births.
Reducing interventions associated with birth not only benefits the birth experience of the woman if she desires a low-intervention birth, but may also benefit the health of the infant. For example, an induced labour frequently results in a cascade of interventions such as the use of intravenous lines, enforced bed rest, continuous electronic fetal heart monitoring, amniotomy, increased pain and discomfort, epidural analgesia, operative (caesarean) delivery and prolonged hospital stay.187 Postbirth health risks associated with induction and the potentially resulting caesarean delivery included maternal depression and neonatal respiratory illness,188 as well as longer term risks to the infant of atopic diseases such as allergic rhinitis,189 eczema and asthma190 (see Chapter 25 on inflammatory skin disorders). Interventions such as induction and operative delivery may still be indicated in high risk circumstances, but the importance lies not so much in avoiding the intervention as ensuring women are educated and empowered to feel in control of their birth process.191
Outside of the medical model, there are some low-intervention therapies which may benefit child birth. For example, acupressure has been used effectively to reduce pain or delivery time in labour.192 A case report has also been published promoting the use of homoeopathic Caulophyllum in conjunction with nipple stimulation to induce and augment labour,193 and a small randomised controlled-trial found that a combination of homoeopathic Arnica and Bellis Perennis resulted in an apparent reduction in postpartum blood loss.194 Even less invasive models such as muscle relaxation techniques and lower back massage have been associated with reduced labour pain.195
Postnatal support
Lactation
The mammary glands develop during pregnancy, but the levels of progesterone and oestrogen secreted by the placenta prevent lactation occurring until 30–40 hours after birth.196 Healthy and adequate lactation provides extensive health benefits to infants both at birth and later in life, and promoting efficient suckling and successful breastfeeding begins with timely skin-to-skin contact between mother and infant.197 Furthermore, promotion of good health practices through preconception and pregnancy education reduces the risk of breastfeeding complications.198 In contrast, delayed contact between mother and infant, washing the mother or infant prior to contact or the use of a pacifier before 6 weeks of age have all been shown to interfere with effective and successful breastfeeding.197
A number of herbs have been used traditionally to encourage lactation: Trigonella foenum-graecum, Galega officinalis, Foeniculum vulgare, Pimpinella anisum, Cnicus benedictus, Silybum marianum, Asparagus racemosus (shatavari) and Urtica dioica.199,200
Unfortunately, recent research into the efficacy and physiological activity of these herbs is scarce. Based on experimental data, increased milk production can generally be expected 24–72 hours after consumption of F. vulgare,201 and A. racemosus’s traditional Ayurvedic use as a galactagogue has also been confirmed in a clinical trial.202
Formula feeding
Soy proteins have been used as an alternative protein source for infants with allergies or food intolerances, although there is little evidence to support their use. A Cochrane review of five studies found only one study comparing soy to cow’s milk formula noted a reduction in childhood allergy, asthma and allergic rhinitis.203 The other four studies that fit the inclusion criteria reported no significant benefit for any allergy or food intolerance. Many infants allergic to cow’s milk may also be allergic to soy milk,204 suggesting a deeper underlying immunological issue. Furthermore, intestinal permeability is higher in infants fed formula than those fed breast milk;205 this may contribute to the risk of the development of atopic disease (see Chapter 25 on inflammatory skin disorders). In these circumstances, colostrum supplementation in the initial feeding of formula-fed infants may offer some protection.206
Postnatal depression
Some women develop a severe depression after childbirth. Sleep deprivation and general tiredness may worsen these symptoms.207,208 Recent research has also acknowledged that in 50% of couples, if women are depressed, their partners are depressed also.209 Unfortunately, current family health systems do not effectively balance the postnatal support to both members of the parenting team.209 If either partner is experiencing fatigue, promoting adequate sleep is important and may simply require sleeping when the baby sleeps. If there is difficulty sleeping during these odd hours, sleeping aids may be considered (see Chapter 14 on insomnia). Omega-3 essential fatty acids are also indicated in general postnatal depression (see Chapter 12 on depression). Other underlying issues, particularly those associated with the development of menstrual disorders, should be investigated, as women with a history of postnatal depression are more likely to develop menstrual difficulties and perimenstrual symptoms when menstruation recommences.210
INTEGRATIVE MEDICAL CONSIDERATIONS
Traditional Chinese medicine
Acupuncture on the day of embryo transfer is demonstrated to have a beneficial effect on live births.130 Acupuncture has been demonstrated to be a safe and effective treatment tool for pelvic and back pain associated with pregnancy.213 Similarly, acupressure, a less invasive therapy similar to acupuncture, has been associated with
reduced pain and shorter delivery time in labour.192 Moxibustion is a method used in traditional Chinese medicine as a method for cephalic version of breech babies;214 however, due to methodological issues randomised controlled trials have not been completed.215
Antenatal classes
Antenatal classes can provide appropriate supervision and advice on antenatal exercises, back care, labour pain relief, relaxation skills and posture. An observational study involving 9004 women found that women who attended antenatal classes had a much lower risk of caesarean section and were half as likely to bottle feed in hospital, as well as being more satisfied with the experience of childbirth.216 Furthermore, group psychoeducation classes, which focus on releasing the fear surrounding the birth process, can improve a woman’s pain tolerance and coping mechanisms in childbirth.178 Similarly, fathers attending antenatal classes felt they were more prepared for the birth and for their role as a support person.217
Homoeopathy
Individualised homoeopathic treatment in 45 subfertile men was found to improve semen parameters (sperm count and motility in addition to general health parameters) equal to conventional treatment.218 Caulophyllum is a commonly used homoeopathic remedy for third trimester cervical ripening and induction of labour. A Cochrane review219 evaluating this remedy identified two trials involving 133 women, but the results of the review were inconclusive due to a lack of information about the methodology used in the studies. Although a lower level of evidence, a case report has also been published promoting the use of this remedy.193
Aromatherapy
A pilot randomised-controlled feasibility study which took an individualised approach to the prescription of aromatherapy oils in childbirth found that the intervention group rated a lower pain perception, and a higher proportion of the control group had their infants transferred to the neonatal intensive care unit.220
Example treatment
The initial treatment for this case focused on supporting her nervous system and reproductive hormones, while further exploring her glucose tolerance. Due to the effects of physiological responses to stress on the reproductive hormones, anxiolytic, sedative and antidepressant herbs, such as Matricaria recutita, Hypericum perforatum, Melissa officinalis and Verbena officinalis were included in the formula.200 Asparagus racemosus was also included as a general nervine tonic, and for its capacity to support libido and conception.221 The nervous system was also supported by the use of an individualised flower essence formula. (Note: Although popular in pregnancy and preconception, energetic medicines often require further evidentiary support.) The effect of her polycystic ovarian disease on potential fertility and capacity to carry to term was also acknowledged. She had already begun to modify her diet following the diagnosis 5 months ago, and reduced her dietary carbohydrate intake, with a focus on low glycaemic load carbohydrates, prior to her first appointment. It was recommended that, to support these changes, she resume regular exercise and aim for 20–30 minutes, three or four times per week. She had also begun weekly acupuncture treatment following the miscarriage, and was encouraged to continue. Prior to more aggressive treatment of her insulin sensitivity, a glucose-insulin tolerance test (GITT) was ordered. It was also recommended for her partner to join her for the next consultation.
Expected outcomes and follow-up treatments
KEY POINTS
Atrash H., et al. Preconception care: a 2008 update. Curr Opin Obstet Gynecol. 2008;20(6):581-589.
Derbyshire E. Dietary factors and fertility in women of childbearing age. Nutr Food Sci. 2007;37(2):100-104.
Gleicher N., Barad D. Unexplained infertility: does it really exist? Hum Reprod. 2006;21(8):1951-1955.
Oats J., Abraham S. Fundamentals of obstetrics and gynaecology. Philadelphia: Elsevier Mosby; 2005.
Pairman S., editor. Midwifery: preparation for practice. Sydney: Elsevier Churchill Livingstone, 2006.
1. Johnson K., et al. Recommendations to improve preconception health and health care—United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. 2006;55:1-23. MMWR Recomm Rep
2. Atrash H.K., et al. Preconception care for improving perinatal outcomes: the time to act. Matern Child Health J. 2006;10(Suppl 1):S3-S11.
3. Gnoth C., et al. Time to pregnancy: results of the German prospective study and impact on the management of infertility. Hum Reprod. 2003;18:1959-1966.
4. Speroff L., Fritz M. Clinical gynecologic endocrinology and infertility, 7th edn. Philadelphia: Lippincott Williams & Wilkins; 2005.
5. Gnoth C., et al. Definition and prevalence of subfertility and infertility. Hum Reprod. 2005;20:1144-1147.
6. Oats J., Abraham S. Fundamentals of obstetrics and gynaecology. Philadelphia: Elsevier Mosby; 2005.
7. Practice Committee of American Society for Reproductive Medicine. Smoking and infertility. Fertil Steril. 2008;90(5 Suppl):S254-S259.
8. Curtis K., et al. Effects of cigarette smoking, caffeine consumption and alcohol intake on fecundability. Am J Epidemiol. 1997;146(1):32-41.
9. Hull M., et al. Delayed conception and active and passive smoking. Fertil Steril. 2000;74(4):725-733.
10. Hassan M., Killick S. Negative lifestyle is associated with a significant reduction in fecundity. Fertil Steril. 2004;81(2):384-392.
11. Homan G., et al. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13(3):209-233.
12. Agarwal A., et al. Reactive oxygen species as an independent marker of male factor infertility. Fertil Steril. 2006;86(4):878-885.
13. Shiloh H., et al. The impact of cigarette smoking on zona pellucida thickness of oocytes and embryos prior to transfer into the uterine cavity. Hum Reprod. 2004;19(1):157-159.
14. Martin J., et al. Births: final data for 2002. Natl Vital Stat Rep. 2003;52:1-113.
15. Silva P., et al. Impact of lifestyle choices on female infertility. J Reprod Med. 1999;44(3):288-296.
16. Chavarro J.E., et al. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85(1):231-237.
17. Chavarro J.E., et al. Iron intake and risk of ovulatory infertility. Obstet Gynecol. 2006;108:1145-1152.
18. Physicians F. Iron supplements may reduce risk for ovulatory infertility CME/CE. Obstet Gynecol. 2006;108:1145-1152.
19. Ruder E.H., et al. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14(4):345-357.
20. Verit F.F., et al. Association of increased total antioxidant capacity and anovulation in nonobese infertile patients with clomiphene citrate–resistant polycystic ovary syndrome. Fertil Steril. 2007;88(2):418-424.
21. Boitani C., Puglisi R. Selenium, a key element in spermatogenesis and male fertility. Molecular Mechanisms in Spermatogenesis. 2008:65.
22. Yuyan L., et al. Are serum zinc and copper levels related to semen quality? Fertil Steril. 2008;89(4):1008-1011.
23. Chavarro J.E., et al. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(2):210.
24. Chavarro J.E., et al. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Human Reproduction. 2007;22(5):1340-1347.
25. Hjolland N., et al. Distress and reduced fertility: a follow up study of first-pregnancy planners. Fertil Steril. 1999;72(2):47-53.
26. Zorn B., et al. Psychological factors in male partners of infertile couples: relationship with semen quality and early miscarriage. J Andrology. 2007;31(6):557-564.
27. Fitzgerald C., et al. Aging and reproductive potential in women. Yale J Biol Med. 1998;71:367-381.
28. Ray J., et al. Preconception care and the risk of congenital anomalies in the offspring of women with diabetes mellitus: a meta-analysis. QJM. 2001;94:435-444.
29. Eliakim R., Sherer D.M. Celiac disease: fertility and pregnancy. Gynecol Obstet Invest. 2001;51:3-7.
30. Sallmen M., et al. Reduced fertility among overweight and obese men. Epidemiology. 2006;17(5):520-523.
31. Zain M.M., Norman R.J. Impact of obesity on female fertility and fertility treatment. Women’s Health. 2008;4(2):183-194.
32. Diemer T., et al. Urogenital infection and sperm motility. Andrologia. 2003;35(5):283-287.
33. Mahadevan U. Fertility and pregnancy in the patient with inflammatory bowel disease. Br Med J. 2006;55(8):1198.
34. Poppe K., et al. Thyroid disease and female reproduction. Clin Endocrinol. 2007;66(3):309-321.
35. Jonasson J.M., et al. Fertility in women with type 1 diabetes. Diabetes Care. 2007;30(9):2271-2276.
36. Kim H.O., et al. Are IVF/ICSI outcomes of women with minimal to mild endometriosis associated infertility comparable to those with unexplained infertility? Fertil Steril. 2007;88:215.
37. Franks S. Polycystic ovary syndrome. N Engl J Med. 1995;333(13):853-861.
38. Ward N. Preconceptual care questionnaire research project. J Nutr Environ Med. 1995;5:205-208.
39. Gerhard I., et al. Mastodynon(R) bei weiblicher Sterilität. Forsch Komplementarmed. 1998;5(6):272-278.
40. Bubenzer R. Therapy with agnus castus extract (Strotan®). Therapiewoche. 1993;43:32-33.
41. Bergmann J., et al. The efficacy of the complex medication Phyto-Hypophyson L in female, hormone-related sterility: a randomized, placebo-controlled clinical double-blind study. Forsch Komplementarmed Klass Naturheilkd. 2000;7:190-199.
42. Tabakova P., et al. Clinical study of Tribestan® in females with endocrine sterility. Sofia: Bulgarian Pharmaclogy Group; 2000.
43. Wilcox A., et al. Timing of sexual intercourse in relation to ovulation-effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517-1521.
44. Brosens I., et al. Managing infertility with fertility-awareness methods. Sex Reprod Menopause. 2006;4(1):13-16.
45. Scarpa B., et al. Cervical mucus secretions on the day of intercourse: an accurate marker of highly fertile days. Eur J Obstet Gynaecol Reprod Biol. 2006;125:72-78.
46. Stanford J., et al. Timing intercourse to achieve pregnancy: current evidence. Obstet Gynecol. 2002;100:1333-1341.
47. Stanford J., et al. Vulvar mucus observations and the probability of pregnancy. Obstet Gynecol. 2003;101:1285-1293.
48. Bigelow J., et al. Mucus observations in the fertile window: a better predictor of conception than timing of intercourse. Hum Reprod. 2004;19:889-892.
49. Stanford J., Dunson D. Effects of sexual intercourse patterns in time to pregnancy studies. Am J Epidemiol. 2007;165:1088-1095.
50. Kutteh W., et al. Vaginal lubricants for the infertile couple: effect on sperm activity. Int J Fertil Menopausal Stud. 1996;41:400-404.
51. Chavarro J., et al. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(2):210.
52. Chavarro J., et al. Dietary fatty acid intakes and the risk of ovulatory infertility. Am J Clin Nutr. 2007;85:231-237.
53. Rich-Edwards J., et al. Physical activity, body mass index, and ovulatory disorder infertility. Epidemiology. 2002;13(2):184-190.
54. Nelson S., Fleming R. The preconceptual contraception paradigm: obesity and infertility. Hum Reprod. 2007;22(4):912-915.
55. Pasquali R., et al. Obesity and reproductive disorders in women. Hum Reprod. 2003;9(4):359-372.
56. Norman R., et al. Improving reproductive performance in overweight/obese women with effective weight management. Hum Reprod Update. 2004;10(3):267-280.
57. Zain M., Norman R. Impact of obesity on female fertility and fertility treatment. Womens Health. 2008;4(2):183-194.
58. Miller P., et al. Effect of short-term diet and exercise on hormone levels and menses in obese, infertile women. J Reprod Med. 2008;53(5):315-319.
59. Karimzadeh M., Javedani M. An assessment of lifestyle modification versus medical treatment with clomiphene citrate, metformin, and clomiphene citrate-metformin in patients with polycystic ovary syndrome. Fertil Steril. 2009. In press
60. Wynn M., Wynn A. Slimming and fertility. Mod Midwife. 1994;4:17-20.
61. Morris S.N., et al. Effects of lifetime exercise on the outcome of in vitro fertilization. Obstet Gynecol. 2006;108(4):938-946.
62. Arce J.C., De Souza M.J. Exercise and male factor infertility. Sports med. 1993;15(3):146-169.
63. Nelson S.M., Fleming R.F. The preconceptual contraception paradigm: obesity and infertility. Hum Reprod. 2007;22(4):912-915.
64. Hill J.W., et al. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294(5):E827-E832.
65. Lannerö E., et al. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res. 2006;7(1):3.
66. Roza S.J., et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. Eur J Neurosci. 2007;25(3):611-617.
67. Hunt K.J., et al. Impact of parental smoking on diabetes, hypertension and the metabolic syndrome in adult men and women in the San Antonio Heart Study. Diabetologia. 2006;49(10):2291-2298.
68. Guerri C., et al. Foetal alcohol spectrum disorders and alterations in brain and behaviour. Alcohol Alcohol. 2009;44(2):108-114.
69. Weinberg J., et al. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20(4):470-488.
70. Murtagh J. General practice. Sydney: McGraw-Hill; 2007.
71. Craig S. A medical model for infertility counselling. Aust Fam Physician. 1990;19:491-500.
72. Burns L. Psychiatric aspects of infertility and infertility treatments. Psychiatr Clin North Am. 2007;30(4):689-716.
73. Champagne D. Should fertilization treatment start with reducing stress? Hum Reprod. 2006;21(7):1651-1658.
74. Damti O.B., et al. Stress and distress in infertility among women. Harefuah. 2008;147(3):256-260.
75. Cousineau T.M., Domar A.D. Psychological impact of infertility. Baillière’s best practice and research. Clin obstet gynaecol. 2007;21(2):293-308.
76. Domar A., et al. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. 2000;73(4):805-811.
77. Chang M., et al. Effects of music therapy on psychological health of women during pregnancy. J Clin Nurs. 2008;17(19):2580-2587.
78. Kotz D. Success at last. Couples fighting infertility might have more control than they think. US News World Rep. 2007;142(16):62-64.
79. Greenlee A., et al. Risk factors for female infertility in an agricultural region. Epidemiology. 2003;14(4):429-436.
80. Hruska K., et al. Environmental factors in infertility. Clin Obstet Gynecol. 2000;43(4):821-829.
81. Gold E., et al. Reproductive hazards. Occup Med. 1994;9(3):363-372.
82. Claman P. Men at risk: occupation and male infertility. Sexuality, Reproduction and Menopause. 2004;2(1):19-26.
83. Simon C., et al. Cytokines and embryo implantation. Reprod Immunol. 1998;39:117-131.
84. Weiss G., et al. Inflammation in reproductive disorders. Reprod Sci. 2009;16(2):216-219.
85. Weiss G., et al. Inflammation in reproductive disorders. Reprod Sci. 2009;16(2):216.
86. Tuohy V., Altuntas C. Autoimmunity and premature ovarian failure. Curr Opin Obstet Gynecol. 2007;19(4):366-369.
87. Altuntas C., et al. Autoimmune targeted disruption of the pituitary-ovarian axis causes premature ovarian failure. J Immunol. 2006;177(3):1988-1996.
88. Johnston R.B.Jnr. Folic acid: preventive nutrition for preconception, the fetus, and the newborn. Neo Rev. 2009;10(1):e10.
89. Cikot R., et al. Longitudinal vitamin and homocysteine levels in normal pregnancy. Br J Nutr. 2007;85(01):49-58.
90. Ray J.G., et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology. 2007;18(3):362-366.
91. Ray J.G., et al. High rate of maternal vitamin B12 deficiency nearly a decade after Canadian folic acid flour fortification. QJM. 2008;101(6):475-477.
92. Westphal L.M., et al. A nutritional supplement for improving fertility in women: a pilot study. J Reprod Med. 2004;49(4):289-293.
93. Czeizel A., et al. The effect of preconceptional multivitamin supplementation on fertility. Int J Vitam Nutr Res. 1996;66(1):55-58.
94. Czeizel A., et al. The higher rate of multiple births after periconceptional multivitamin supplementation: an analysis of causes. Acta Genet Med Gemellol. 1994;43(3–4):175-184.
95. Chavarro J., et al. Use of multivitamins, intake of B vitamins and risk of ovulatory infertility. Fertil Steril. 2008;89(3):668-676.
96. Henmi H., et al. Effects of ascorbic acid supplementation on serum progesterone levels in patients with luteal phase defect. Fertil Steril. 2003;80:459-461.
97. Karovic O., et al. Toxic effects of cobalt in primary cultures of mouse astrocytes: Similarities with hypoxia and role of HIF-1alpha. Biochem Pharmacol. 2007;73(5):694-708.
98. Kohlmeier M. Nutrient metabolism. San Diego: Academic Press; 2003.
99. Carmel R. Folic acid. In: Shils M.E., et al, editors. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2006:470-481.
100. Carmel R. Cobalamin (vitamin B12). In: Shils M.E., et al, editors. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2006:482-497.
101. Thonneau P., et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988–1989). Hum Reprod. 1991;6(6):811-816.
102. Ford W., et al. Increasing paternal age is associated with delayed conception in a large population of fertile couples: evidence for declining fecundity in older men. The ALSPAC study team (Avon Longitudinal Study of Pregnancy and Childhood). Hum Reprod. 2000;15:1703-1708.
103. Jorgensen N., et al. Regional differences in semen quality in Europe. Hum Reprod. 2001;16:1012-1019.
104. Oliva A., et al. Contribution of environmental factors to the risk of male infertility. Hum Reprod. 2001;16(8):1768-1776.
105. Kumar S. Occupational exposure associated with reproductive dysfunction. J Occupational Health. 2004;46(1):1-19.
106. Swan S., et al. Semen quality in relation to biomarkers of pesticide exposure. Environ Health Perspect. 2003;111(12):1478-1484.
107. Abell A., et al. High sperm density among members of organic farmers’ association. Lancet. 1994;343:1498.
108. Wang C., et al. Effect of increased scrotal temperature on sperm production in normal men. Fertil Steril. 1997;68(2):334-339.
109. Mendiola J., et al. Food intake and its relationship with semen quality: a case-control study. Fertil Steril. 2009;91(3):812-818.
110. Chavarro J., et al. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum Reprod. 2008;23(11):2584-2590.
111. Eskanzi B., et al. Antioxidant intake is associated with semen quality in healthy men. Hum Reprod. 2005;20(4):1006-1012.
112. Lewin A., Lavon H. The effect of coenzyme Q10 on sperm motility and function. Mol Aspects Med. 1997;18(Suppl):S213-S219.
113. Balercia G., et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81(1):93-98.
114. Tremellen K., et al. A randomised control trial examining the effect of an antioxidant (Menevit) on pregnancy outcome during IVF-ICSI treatment [see comment]. Aust N Z J Obstet Gynaecol. 2007;47(3):216-221.
115. Safarinejad M., Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. 2009;181(2):741-751.
116. Scott R., et al. The effect of oral selenium supplementation on human sperm motility. Br J Urol. 1998;82(1):76-80.
117. Vézina D., et al. Selenium-vitamin E supplementation in infertile men. Effects on semen parameters and micronutrient levels and distribution. Biol Trace Elem Res. 1996;53(1–3):65-83.
118. Hawkes W., et al. Selenium supplementation does not affect testicular selenium status or semen quality in North American men. J Androl. 2009;30(5):525-533.
119. Iwanier K., Zachara B. Selenium supplementation enhances the element concentration in blood and seminal fluid but does not change the spermatozoal quality characteristics in subfertile men. J Androl. 1995;16(5):441-447.
120. Lenzi A., et al. Use of carnitine therapy in selected cases of male factor infertility: a double-blind crossover trial. Fertil Steril. 2003;79(2):292-300.
121. Costa M., et al. L-carnitine in idiopathic asthenozoospermia: a multicenter study. Italian Study Group on Carnitine and Male Infertility. Andrologia. 1994;26(3):155-159.
122. Vitali G., et al. Carnitine supplementation in human idiopathic asthenospermia: clinical results. Drugs Exp Clin Res. 1995;21(4):157-159.
123. Cavallini G., et al. Cinnoxicam and L-carnitine/acetyl-L-carnitine treatment for idiopathic and varicocele-associated oligoasthenospermia. J Androl. 2004;25(5):761-770.
124. Garolla A., et al. Oral carnitine supplementation increases sperm motility in asthenozoospermic men with normal sperm phospholipid hydroperoxide glutathione peroxidase levels. Fertil Steril. 2005;83(2):355-361.
125. Hansen M., et al. Assisted reproductive technologies and the risk of birth defects—a systematic review. Hum Reprod. 2005;20(2):328-338.
126. Chambers G.M., et al. Assisted reproductive technology treatment costs of a live birth: an age-stratified cost-outcome study of treatment in Australia. Med J Aust. 2006;184(4):155-158.
127. Verhaak C.M., et al. Women’s emotional adjustment to IVF: a systematic review of 25 years of research. Hum Reprod update. 2007;13(1):27-36.
128. Rajkhowa M., et al. Reasons for discontinuation of IVF treatment: a questionnaire study. Hum Reprod. 2006;21(2):358-363.
129. Stankiewicz M., et al. The use of complementary medicine and therapies by patients attending a reproductive medicine unit in South Australia: a prospective survey. Aust N Z J Obstet Gynaecol. 2007;47(2):145-149.
130. Cheong Y., et al. Acupuncture and assisted conception. Cochrane Database Syst Rev. 8(4), 2008. CD006920
131. Battaglia C., et al. Adjuvant L-arginine treatment for in-vitro fertilization in poor responder patients. Hum Reprod. 1999;14(7):1690-1697.
132. Food and Nutrition Board. Institute of Medicine. In Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids (macronutrients). Washington, DC: National Academy Press; 2002.
133. Moore M.C. Nutritional assessment and care. Missouri: Elsevier Mosby; 2005.
134. Miles L., Foxen R. New guidelines on caffeine in pregnancy. Nutrition Bulletin. 2009;34(2):203.
135. Turner R.E. Nutrition during pregnancy. In: Shils M.E., et al, editors. Modern nutrition in health and disease. Philadelphia: Lippincott Williams & Wilkins; 2006:771-783.
136. Food and Nutrition Board. Institute of medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press; 2001.
137. Casanueva E., et al. Weekly iron as a safe alternative to daily supplementation for nonanemic pregnant women. Arch Med Res. 2006;37(5):674-682.
138. Holst L., et al. The use and the user of herbal remedies during pregnancy. J Altern Complement Med. 2009;15(7):1-6.
139. Adams J., et al. Women’s use of complementary and alternative medicine during pregnancy: a critical review of the literature. Birth. 2009;36(3):237-245.
140. Adams J., et al. Complementary and alternative medicine and pregnancy: the attitudes and referral practices of obstetricians and other maternity care providers. Int J Gynecol Obstet. 2009. In press
141. Bone K. Safety considerations during pregnancy and lactation. In: Mills S., Bone K., editors. The essential guide to herbal safety. St Louis: Churchill Livingstone, 2005.
142. Scientific Committee of the British Herbal Medical Association. British herbal pharmacopoeia, 1st edn. Bournemouth: British Herbal Medicine Association; 1983.
143. Blumenthal M., et al. Herbal medicine, editor. expanded commission E monographs (English translation). Austin: Integrative Medicine Communications, 2000.
144. European Scientific Cooperative on Phytotherapy. ESCOP Monographs. Stuttgart: Thieme; 2003.
145. McFarlin B., et al. A national survey of herbal preparation use by nurse-midwives for labor stimulation: review of the literature and recommendations for practice. J Nurse-Midwifery. 1999;44(3):205-216.
146. Bamford D., et al. Raspberry leaf tea: a new aspect to an old problem. Br J Pharmacol. 1970;40(1):161-162.
147. Simpson M., et al. Raspberry leaf in pregnancy: its safety and efficacy in labor. J Midwifery Womens Health. 2001;46(2):51-59.
148. Parsons M., et al. Raspberry leaf and its effect on labour: safety and efficacy. J Aust Coll Midwives. 1999;12(3):20-25.
149. Parsons M., et al. Raspberry leaf and its effect on labour: safety and efficacy. Aust Coll Midwives Inc J. 1999;12(3):20-25.
150. Holst L., et al. Raspberry leaf—Should it be recommended to pregnant women? Complement Ther Clin Prac. 2009. In press
151. Felter H.W., Lloyd J.U. King’s American Dispensary, 18th edn. Portland: Eclectic Medical Publications; 1905. reprinted 1983
152. Chadwick C.M. Embolus of the basilar artery. Br Med J. 1886;1(1313):391.
153. Wilson J.H. Viburnum prunifolium, or black haw, in abortion and miscarriage. Br Med J. 1886;1(1318):640.
154. Napier A.D.L. Viburnum prunifolium in abortion. Br Med J. 1886;1(1315):489.
155. Campbell W.M.F. Note on Viburnum prunifolium in abortion. Br Med J. 1886;1(1313):391.
156. Bone K. A clinical guide to blending liquid herbs. Missouri: Churchill Livingstone; 2003.
157. Veleva Z., et al. High and low BMI increase the risk of miscarriage after IVF/ICSI and FET. Hum Reprod. 2008;23(4):878-884.
158. Barclay L. Bariatric surgery before pregnancy may improve pregnancy outcomes in obese women CME. JAMA. 2008;300:2286-2296.
159. Gadsby R., et al. A prospective study of nausea and vomiting during pregnancy. Br J Gen Pract. 43, 1993. 245
160. Gadsby P. Some functions of prostaglandin e2 in early pregnancy. Some reasons why prostaglandin e2 can be associated with nausea and vomiting in pregnancy. 2000;50:4.
161. Jewell D., Young G. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. (4):2003;. CD000145
162. Borrelli F., et al. Effectiveness and safety of ginger in the treatment of pregnancy-induced nausea and vomiting. Obstet Gynecol. 2005;105:849-856.
163. Ozgoli G., et al. Effects of ginger capsules on pregnancy, nausea, and vomiting. J Altern Complement Med. 2009;15(3):243-246.
164. Pongrojpaw D., et al. A randomized comparison of ginger and dimenhydrinate in the treatment of nausea and vomiting in pregnancy. J Med Assoc Thai. 2007;90(9):1703-1709.
165. Danish Veterinary and Food Administration. [The Danish veterinary and food administration warn against food supplements containing ginger for pregnant women] [in Danish], 2008.
166. Chaiyakunapruk N., et al. The efficacy of ginger for the prevention of postoperative nausea and vomiting: a meta-analysis. Am J Obstet Gynecol. 2006;194:95-99.
167. Sahakian V., et al. Vitamin B6 is effective therapy for nausea and vomiting of pregnancy: a randomized, double-blind placebo-controlled study. Obstet Gynecol. 1991;78(1):33-36.
168. Schuster K., et al. Morning sickness and vitamin B6 status of pregnant women. Hum Nutr Clin Nutr. 1985;39(1):75-79.
169. Vutyavanich T., et al. Pyridoxine for nausea and vomiting of pregnancy: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gyneco. 1995;173(3):881-884.
170. Wolf M., et al. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98:757-762.
171. Wergeland E., Strand K. Work place control and pregnancy health in a population-based sample of employed women in Norway. Scand J Work Environ Health. 1998;24:206-212.
172. Weissgerber T., et al. The role of regular physical activity in preeclampsia prevention. Med Sci Sports Exerc. 2003;36(12):2024-2031.
173. Roberts J., et al. Nutrient involvement in pre-eclampsia. J Nutr. 2003;133(Supp):S1684-S1692.
174. Dugoua J., et al. Safety and efficacy of cranberry (vaccinium macrocarpon) during pregnancy and lactation. Can J Clin Pharmacol. 2008;15(1):80-86.
175. Jepson R., et al. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. (1):2004. CD001321
176. Barrons R., Tassone D. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30(3):453-468.
177. Thorpe J., Anderson J. Supporting women in labour and birth. In: Pairman S., et al, editors. Midwifery: preparation for practice. Sydney: Elsevier Churchill Livingstone; 2006:393-415.
178. Saisto T., et al. Therapeutic group psychoeducation and relaxation in treating fear of childbirth. Acta Obstet Gynecol Scand. 2006;85(11):1315-1319.
179. Janssen P.A., et al. Outcomes of planned hospital birth attended by midwives compared with physicians in British Columbia. Obstet Gynecol Surv. 2007;62(11):701.
180. Tan W.M., et al. How do physicians and midwives manage the third stage of labor? Birth. 2008;35(3):220-229.
181. Morano S., et al. Outcomes of the first midwife-led birth centre in Italy: 5 years’ experience. Arch Gynecol Obstet. 2007;276(4):333-337.
182. Johnson K.C., Daviss B.A. Outcomes of planned home births with certified professional midwives: large prospective study in North America. BMJ. 2005;330(7505):1416.
183. Mottl-Santiago J., et al. A hospital-based doula program and childbirth outcomes in an urban, multicultural setting. Matern Child Health J. 2008;12(3):372-377.
184. Campbell D.A., et al. A randomized control trial of continuous support in labor by a lay doula. J Obstet Gynecol Neonatal Nurs. 2006;35(4):456-464.
185. Nommsen-Rivers L.A., et al. Doula care improves birth and breastfeeding outcomes for low-income, primiparous mothers. J Obstet Gynecol Neonatal Nurs. 2009;38(2):157-173.
186. Moses M.C., Potter R.H. Use of doulas for inmates in labor: continuous supportive care with positive outcomes. Corrections Today. 2008;70(3):4.
187. Thorogood C., Donaldson C. Disturbances in the rhythm of labour. In: Pairman S., et al, editors. Midwifery: preparation for practice. Sydney: Elsevier Churchill Livingstone; 2006:679-716.
188. Liston F.A., et al. Neonatal outcomes with caesarean delivery at term. Arch Dis Child Fetal Neonatal Ed. 2008;93(3):F176.
189. Pistiner M., et al. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122(2):274-279.
190. Thavagnanam S., et al. A meta-analysis of the association between caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629-633.
191. Beebe K.R., et al. The effects of childbirth self-efficacy and anxiety during pregnancy on prehospitalization labor. J Obstet Gynecol Neonatal Nurs. 2007;36(5):410-418.
192. Lee M.K., et al. Effects of SP6 acupressure on labor pain and length of delivery time in women during labor. J Altern Complement Med. 2004;10(6):959-965.
193. Kistin S.J., Newman A.D. Induction of labor with homeopathy: a case report. J Midwifery Women’s Health. 2007;52(3):303-307.
194. Oberbaum M., et al. The effect of the homeopathic remedies Arnica montana and Bellis perennis on mild postpartum bleeding—a randomized, double-blind, placebo-controlled study—preliminary results. Complement Ther Med. 2005;13(2):87-90.
195. Davim R.M.B., et al. Effectiveness of non-pharmacological strategies in relieving labor pain. Rev Esc Enferm USP. 2009;43:438-445.
196. Baddock S., Dixon L. Physiological changes in labour and the postnatal period. In: Pairman S., et al, editors. Midwifery: preparation for practice. Sydney: Elsevier Churchill Livingstone; 2006:375-392.
197. Henderson A., Scobbie M. Supporting the breastfeeding mother. In: Pairman S., et al, editors. Midwifery: preparation for practice. Sydney: Elsevier Churchill Livingstone; 2006:507-523.
198. Gartner L., et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496-506.
199. Abascal K., Yarnell E. Botanical galactagogues. Altern Complement Ther. 2008;14(6):288-294.
200. Blumenthal M. British herbal pharmacopoeia. London: BHMA Publishing; 1996.
201. Gabay M. Galactogogues: medications that induce lactation. J Hum Lact. 2002;18:274-279.
202. Sharma S., et al. Randomized controlled trial of Asparagus racemosus (shatavari) as a lactogogue in lactational inadequacy. Indian Pediatr. 1996;33(8):675-677.
203. Osborn D., Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev. (3):2004;. CD003741
204. Allen L. Formulas and milks for infants and young children: making sense of it all. Mod Med. 1999;42(6):24-30.
205. Taylor S., et al. Intestinal permeability in preterm infants by feeding type: mother’s milk versus formula. Breastfeeding Med. 2009;4(1):11-15.
206. Uruakpa F.O., et al. Colostrum and its benefits: a review. Nutr Res. 2002;22(6):755-767.
207. Hunter L., et al. A selective review of maternal sleep characteristics in the postpartum period. J Obstet Gynecol Neonatal Nurs. 2009;38(1):60-68.
208. Ross L., et al. Sleep and perinatal mood disorders: a critical review. J Psychiatry Neurosci. 2005;30(4):247-256.
209. Fletcher R.J., et al. Addressing depression and anxiety among new fathers. Med J Aust. 2006;185(8):461-463.
210. Steiner M. Female-specific mood disorders. Clin Obstet Gynecol. 1992;35(3):599-611.
211. Omu A.E., et al. Indications of the mechanisms involved in improved sperm parameters by zinc therapy. Med Princ Pract. 2008;17(2):108-116.
212. Safarinejad M.R., Safarinejad S. Efficacy of selenium and/or N-acetyl-cysteine for improving semen parameters in infertile men: a double-blind, placebo controlled, randomized study. J Urol. 2009;181(2):741-751.
213. Ee C., et al. Acupuncture for pelvic and back pain in pregnancy: a systematic review. Am J Obstet Gynecol. 2008;198(3):254-259.
214. Ewies A., Olah K. Moxibustion in breech version—a descriptive review. Acupunct Med. 2002;20(1):26-29.
215. Cardini F., et al. A randomised controlled trial of moxibustion for breech presentation. BJOG. 2005;112(6):743-747.
216. Spinelli A., et al. Do antenatal classes benefit the mother and her baby? J Matern-Fetal Neonatal Med. 2003;13(2):94-101.
217. Fletcher R., et al. New fathers’ postbirth views of antenatal classes: Satisfaction, benefits, and knowledge of family services. J Perinat Educ. 2004;13(3):18-26.
218. Gerhar I., Wallis E. Individualized homeopathic therapy for male infertility. Homeopathy. 2002;91:133-134.
219. Smith C.A. Homoeopathy for induction of labour. Cochrane Database Syst Rev. (4):2003;. CD003399
220. Burns E., et al. Aromatherapy in childbirth: a pilot randomised controlled trial. BJOG. 2007;114(7):838-844.
221. Thakur R.S. Major medicinal plants of India. Lucknow: Central Institute of Medicinal and Aromatic Plants. 1989:361.