Chapter 4 Female Reproductive Physiology
 The Menstrual Cycle
The Menstrual Cycle
Each menstrual cycle represents a complex interaction among the hypothalamus, pituitary gland, ovaries, and endometrium. Cyclic changes in gonadotropins (peptide hormones) and steroid hormones induce functional as well as morphologic changes in the ovary, resulting in follicular maturation, ovulation, and corpus luteum formation. Similar changes at the level of the endometrium allow for successful implantation of the developing embryo or a physiologic shedding of the menstrual endometrium when an early pregnancy does not occur.
 Hypothalamic-Pituitary Axis
Hypothalamic-Pituitary Axis
PITUITARY GLAND
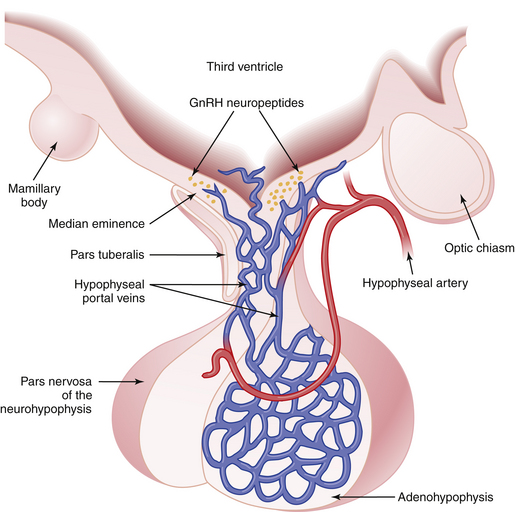
The pituitary gland lies below the hypothalamus at the base of the brain within a bony cavity (sella turcica) and is separated from the cranial cavity by a condensation of dura mater overlying the sella turcica (diaphragma sellae). The pituitary gland is divided into two major portions (Figure 4-1). The neurohypophysis, which consists of the posterior lobe (pars nervosa), the neural stalk (infundibulum), and the median eminence, is derived from neural tissue and is in direct continuity with the hypothalamus and central nervous system. The adenohypophysis, which consists of the pars distalis (anterior lobe), pars intermedia (intermediate lobe), and pars tuberalis—which surrounds the neural stalk—is derived from ectoderm.
Prolactin is secreted by lactotrophs. Unlike the case with other peptide hormones produced by the adenohypophysis, pituitary release of prolactin is under tonic inhibition by the hypothalamus. The half-life for circulating prolactin is about 20 to 30 minutes. In addition to its lactogenic effect, prolactin may directly or indirectly influence hypothalamic, pituitary, and ovarian functions in relation to the ovulatory cycle, particularly in the pathologic state of chronic hyperprolactinemia (see Chapter 32).
GONADOTROPIN SECRETORY PATTERNS
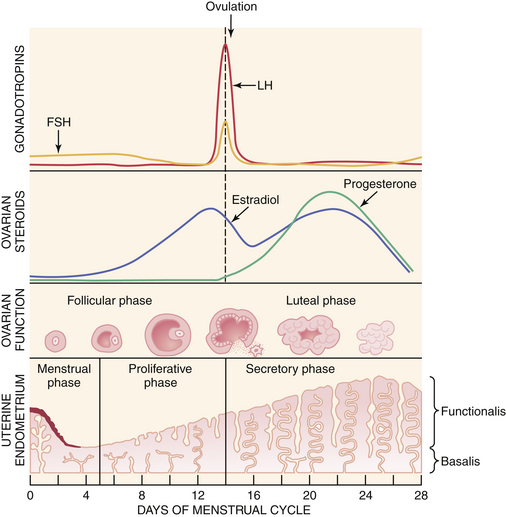
A normal ovulatory cycle can be divided into a follicular and a luteal phase (Figure 4-2). The follicular phase begins with the onset of menses and culminates in the preovulatory surge of LH. The luteal phase begins with the onset of the preovulatory LH surge and ends with the first day of menses.
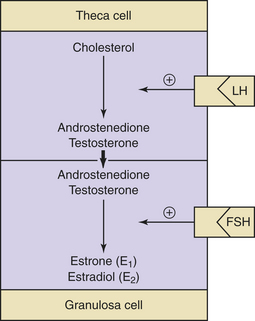
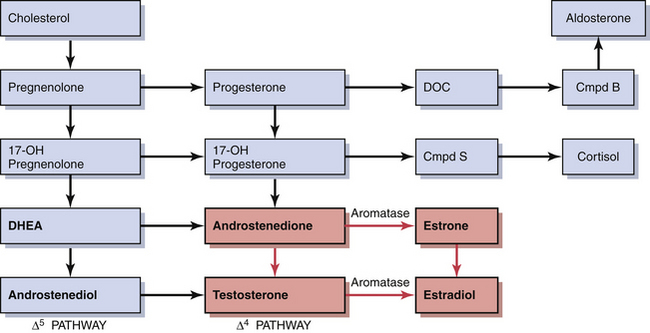
Decreasing levels of estradiol and progesterone from the regressing corpus luteum of the preceding cycle initiate an increase in FSH by a negative feedback mechanism, which stimulates follicular growth and estradiol secretion. A major characteristic of follicular growth and estradiol secretion is explained by the two-gonadotropin (LH and FSH), two-cell (theca cell and granulosa cell) theory of ovarian follicular development. According to this theory, there are separate cellular functions in the ovarian follicle wherein LH stimulates the theca cells to produce androgens (androstenedione and testosterone) and FSH then stimulates the granulosa cells to convert these androgens into estrogens (androstenedione to estrone and testosterone to estradiol), as depicted in Figure 4-3. Initially, at lower levels of estradiol, there is a negative feedback effect on the ready-release form of LH from the pool of gonadotropins in the pituitary gonadotrophs. As estradiol levels rise later in the follicular phase, there is a positive feedback on the release of storage gonadotropins, resulting in the LH surge and ovulation. The latter occurs 36 to 44 hours after the onset of this midcycle LH surge. With pharmacologic doses of progestins contained in contraceptive pills, there is a profound negative feedback effect on gonadotropin-releasing hormone (GnRH) so that none of the gonadotropin pool (ready-release or storage) is released. Hence, ovulation is (generally) blocked (see Chapter 26).
During the luteal phase, both LH and FSH are significantly suppressed through the negative feedback effect of elevated circulating estradiol and progesterone. This inhibition persists until progesterone and estradiol levels decline near the end of the luteal phase as a result of corpus luteal regression, should pregnancy fail to occur. The net effect is a slight rise in serum FSH, which initiates new follicular growth for the next cycle. The duration of the corpus luteum’s functional regression is such that menstruation generally occurs 14 days after the LH surge in the absence of pregnancy.
HYPOTHALAMUS
GnRH is a decapeptide that is synthesized primarily in the arcuate nucleus. It is responsible for the synthesis and release of both LH and FSH. Because it usually causes the release of more LH than FSH, it is less commonly called LH-releasing hormone (LH-RH) or LH-releasing factor (LRF). Both FSH and LH appear to be present in two different forms within the pituitary gonadotrophs. One is a releasable form and the other a storage form. GnRH reaches the anterior pituitary through the hypophyseal portal vessels and stimulates the synthesis of both FSH and LH, which are stored within gonadotrophs. Subsequently, GnRH activates and transforms these molecules into releasable forms. GnRH can also induce immediate release of both LH and FSH into the circulation. Some recent research that found receptors for GnRH in other tissues including the ovary suggests that GnRH may have a direct effect on ovarian function as well.
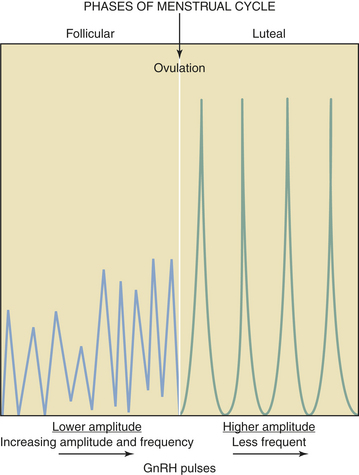
GnRH is secreted in a pulsatile fashion throughout the menstrual cycle as depicted in Figure 4-4. The frequency of GnRH release, as assessed indirectly by measurement of LH pulses, varies from about every 90 minutes in the early follicular phase to every 60 to 70 minutes in the immediate preovulatory period. During the luteal phase, pulse frequency decreases while pulse amplitude increases. A considerable variation among individuals has been identified.
The hypothalamus produces PIF, which exerts chronic inhibition of prolactin release from the lactotrophs. A number of pharmacologic agents (e.g., chlorpromazine) that affect dopaminergic mechanisms influence prolactin release. Dopamine itself is secreted by hypothalamic neurons into the hypophyseal portal vessels and inhibits prolactin release directly within the adenohypophysis. Based on these observations, it has been proposed that hypothalamic dopamine may be the major PIF. In addition to the regulation of prolactin release by PIF, the hypothalamus may also produce prolactin-releasing factors (PRFs) that can elicit large and rapid increases in prolactin release under certain conditions, such as breast stimulation during nursing. All PIFs and PRFs have not been clearly characterized biochemically as of 2008. TRH serves to stimulate prolactin release as well. This phenomenon may explain the association between primary hypothyroidism (with secondary TRH elevation) and hyperprolactinemia. The precursor protein for GnRH, called GnRH-associated peptide (GAP), has been identified to be both a potent inhibitor of prolactin secretion and an enhancer of gonadotropin release. These findings suggest that this GnRH-associated peptide may also be a physiologic PIF and could explain the inverse relationship between gonadotropin and prolactin secretions seen in many reproductive states.
 Ovarian Cycle
Ovarian Cycle
ESTROGENS
During early follicular development, circulating estradiol levels are relatively low. About 1 week before ovulation, levels begin to increase, at first slowly, then rapidly. The conversion of testosterone to estradiol in the granulosa cell of the follicle occurs through an enzymatic process called aromatization and is depicted in Figure 4-3. The levels generally reach a maximum 1 day before the midcycle LH peak. After this peak and before ovulation, there is a marked and precipitous fall. During the luteal phase, estradiol rises to a maximum 5 to 7 days after ovulation and returns to baseline shortly before menstruation. Estrone secretion by the ovary is considerably less than secretion of estradiol but follows a similar pattern. Estrone is largely derived from the conversion of androstenedione through the action of the enzyme aromatase (Figure 4-5).
SERUM-BINDING PROTEINS
Circulating estrogens and androgens are mostly bound to specific sex hormone–binding globulins (SHBG) or to serum albumin. The remaining fraction of sex hormones is unbound (free), and this is the biologically active fraction. It is unclear whether steroids bound to serum proteins (e.g., albumin) are accessible for tissue uptake and utilization. The synthesis of SHBG in the liver is increased by estrogens and thyroid hormones but decreased by testosterone.
FOLLICULAR DEVELOPMENT
During early stages of folliculogenesis, LH receptors are present only on the theca interna layer. LH stimulation induces steroidogenesis and increases the synthesis of androgens by thecal cells. In nondominant follicles, high local androgen levels may enhance follicular atresia. However, in the follicle destined to reach ovulation, FSH induces aromatase enzyme and its receptor formation within the granulosa cells. As a result, androgens produced in the theca interna of the dominant follicle diffuse into the granulosa cells and are aromatized into estrogens. FSH also enhances the induction of LH receptors on the granulosa cells of the follicle that is destined to ovulate. These are essential for the appropriate response to the LH surge, leading to the final stages of maturation, ovulation, and the luteal phase production of progesterone. Thus, the presence of greater numbers of FSH receptors and granulosa cells and increased induction of aromatase enzyme and its receptors may differentiate between the follicle of the initial cohort that will develop normally and those that will undergo atresia.
LUTEINIZATION AND CORPUS LUTEUM FUNCTION
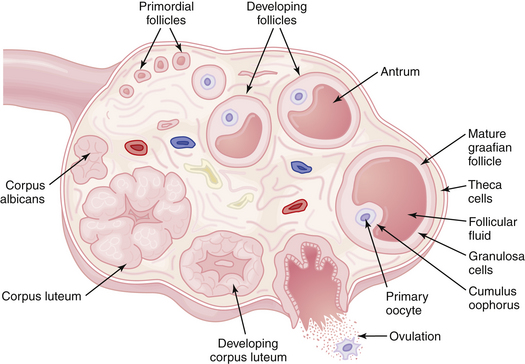
After ovulation and under the influence of LH, the granulosa cells of the ruptured follicle undergo luteinization. These luteinized granulosa cells, plus the surrounding theca cells, capillaries, and connective tissue, form the corpus luteum, which produces copious amounts of progesterone and some estradiol. The normal functional life span of the corpus luteum is about 9 to 10 days. After this time it regresses, and unless pregnancy occurs, menstruation ensues, and the corpus luteum is gradually replaced by an avascular scar called a corpus albicans. The events occurring in the ovary during a complete cycle are shown in Figure 4-6.
 Histophysiology of the Endometrium
Histophysiology of the Endometrium
MENSTRUAL PHASE
Because it is the only portion of the cycle that is visible externally, the first day of menstruation is taken as day 1 of the menstrual cycle. The first 4 to 5 days of the cycle are defined as the menstrual phase. During this phase, there is disruption and disintegration of the endometrial glands and stroma, leukocyte infiltration, and red blood cell extravasation. In addition to this sloughing of the functionalis, there is a compression of the basalis due to the loss of ground substances. Despite these degenerative changes, early evidence of renewed tissue growth is usually present at this time within the basalis of the endometrium.
PROLIFERATIVE PHASE
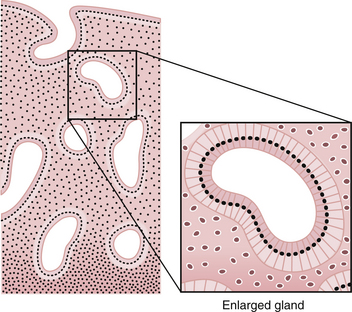
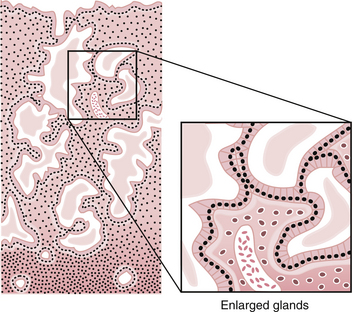
During this phase of the cycle, the large increase in estrogen secretion causes marked cellular proliferation of the epithelial lining, the endometrial glands, and the connective tissue of the stroma (Figure 4-7). Numerous mitoses are present in these tissues, and there is an increase in the length of the spiral arteries, which traverse almost the entire thickness of the endometrium. By the end of the proliferative phase, cellular proliferation and endometrial growth have reached a maximum, the spiral arteries are elongated and convoluted, and the endometrial glands are straight, with narrow lumens containing some glycogen.
SECRETORY PHASE
Following ovulation, progesterone secretion by the corpus luteum stimulates the glandular cells to secrete glycogen, mucus, and other substances. The glands become tortuous and the lumens are dilated and filled with these substances. The stroma becomes edematous. Mitoses are rare. The spiral arteries continue to extend into the superficial layer of the endometrium and become convoluted (Figure 4-8).
If pregnancy does not occur by day 23, the corpusluteum begins to regress, secretion of progesterone and estradiol declines, and the endometrium undergoes involution. About 1 day before the onset of menstruation, marked constriction of the spiral arterioles takes place, causing ischemia of the endometrium followed by leukocyte infiltration and red blood cell extravasation. It is thought that these events occur secondary to prostaglandin production by the endometrium. The resulting necrosis causes menstruation or sloughing of the endometrium. Thus, menstruation, which clinically marks the beginning of the menstrual cycle, is actually the terminal event of a physiologic process that enables the uterus to be prepared to receive another conceptus.
 Spermatogenesis, Sperm Capacitation, and Fertilization
Spermatogenesis, Sperm Capacitation, and Fertilization
Capacitation is the physiologic change that sperm must undergo in the female reproductive tract before fertilization. Human sperm can also undergo capacitation after a short incubation in defined culture media without residence in the female reproductive tract, which allows for in vitro fertilization (see Chapter 34).
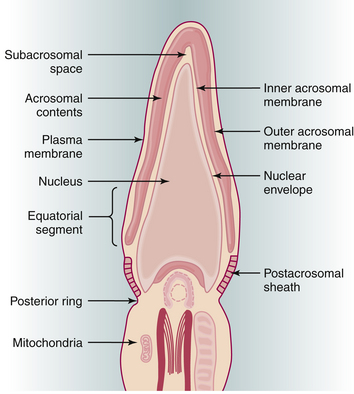
The acrosome reaction is one of the principal components of capacitation. The acrosome, a modified lysosome, lies over the sperm head as a kind of “chemical drill-bit” designed to enable the sperm to burrow its way into the oocyte (Figure 4-9). The overlying plasma membrane becomes unstable and eventually breaks down, releasing hyaluronidase, a neuraminidase, and corona-dispersing enzyme. Acrosin, bound to the remaining inner acrosomal membrane, may play a role in the final penetration of the zona pellucida. The latter contains species-specific receptors for the plasma membrane. After traversing the zona, the postacrosomal region of the sperm head fuses with the oocyte membrane, and the sperm nucleus is incorporated into the ooplasm. This process triggers release of the contents of the cortical granules that lie at the periphery of the oocyte. This cortical reaction results in changes in the oocyte membrane and zona pellucida that prevent the entrance of further sperm into the oocyte.
Following penetration of the oocyte, the sperm nucleus decondenses to form the male pronucleus, which approaches and finally fuses with the female pronucleus at syngamy to form the zygote. Fertilization restores the diploid number of chromosomes and determines the sex of the zygote. In couples with infertility resulting from severe sperm abnormalities, fertilization and subsequent pregnancy can be successfully achieved after the injection of a single sperm, with or without its tail, into the cytoplasm of the oocyte (see Chapter 34).
 Implantation
Implantation
The fertilized ovum reaches the endometrial cavity about 3 days after ovulation.
Under the influence of progesterone, decidual changes occur in the endometrium of the pregnant uterus. The endometrial stromal cells enlarge and form polygonal or round decidual cells. The nuclei become round and vesicular, and the cytoplasm becomes clear, slightly basophilic, and surrounded by a translucent membrane. During pregnancy, the decidua thickens to a depth of 5 to 10 mm. The decidua basalis is the decidual layer directly beneath the site of implantation. Integrins, a class of proteins involved in cell-to-cell adherence, peak within the endometrium at the time of implantation and may play a significant role. Additional growth factors act in a synergistic fashion to enhance the implantation process. The decidua capsularis is the layer overlying the developing ovum and separating it from the rest of the uterine cavity. The decidua vera (parietalis) is the remaining lining of the uterine cavity (Figure 4-10). The space between the decidua capsularis and decidua vera is obliterated by the 4th month with fusion of the capsularis and vera.
 Placenta
Placenta
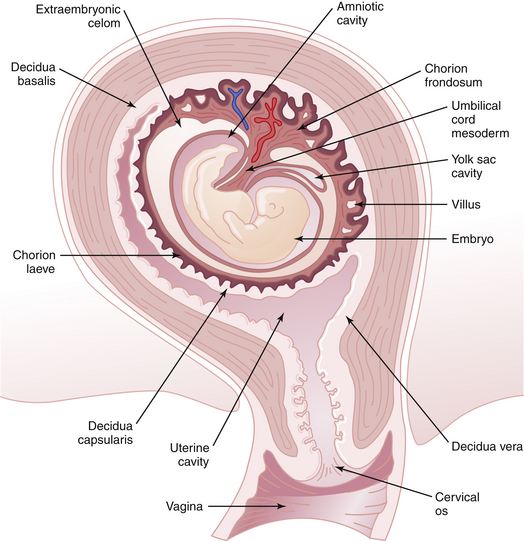
By 3 weeks, the relationship of the chorion to the decidua is evident. The greater part of the chorion, denuded of villi, is designated the chorion laeve (smooth chorion). Until near the end of the 3rd month, the chorion laeve remains separated from the amnion by the extraembryonic celomic cavity. Thereafter, amnion and chorion are in intimate contact. The villi adjacent to the decidua basalis enlarge and branch (chorion frondosum) and progressively assume the form of the fully developed human placenta (Figure 4-11). By 16 to 20 weeks, the chorion laeve contacts and fuses with the decidua vera, thus obliterating most of the uterine cavity.
Adashi E. The ovarian cycle. In Yen S.S.C., Jaffe R.B., editors: Reproductive Endocrinology, 4th ed., Philadelphia: WB Saunders, 1997.
Olive D.L., Palter S.F. Reproductive physiology. Berek and Novak’s Gynecology, 14th ed.. Philadelphia: Lippincott Williams & Wilkins; 2007.
Speroff L., Glass R.H., Kase N.G. Clinical Gynecologic Endocrinology and Fertility, 6th ed. Baltimore: Williams & Wilkins; 1999.
Strauss J., Gurpide E. The endometrium: Regulation and dysfunction. In Yen S.S.C., Jaffe R.B., editors: Reproductive Endocrinology, 4th ed., Philadelphia: WB Saunders, 1997.
Yen S.S.C. The human menstrual cycle: Neuroendocrine regulation. In Yen S.S.C., Jaffe R.B., editors: Reproductive Endocrinology, 4th ed., Philadelphia: WB Saunders, 1997.

 Cleavage, Morula, Blastocyst
Cleavage, Morula, Blastocyst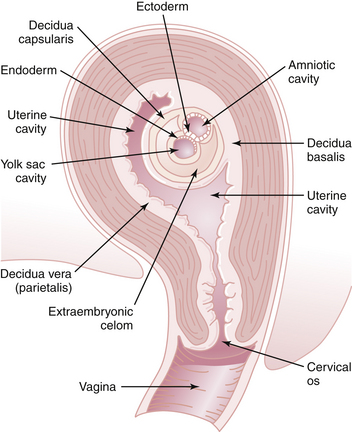
 Amniotic Fluid
Amniotic Fluid