Chapter 11 FDG-PET/CT and the Evaluation of Breast Cancer
Positron emission tomography (PET) scanning has gained widespread acceptance for the diagnosis, staging, and management of a variety of malignancies, including breast cancer. This has heralded an exciting new era of molecular imaging research, of which the use of 2-deoxy-2-(18F)fluoro-D-glucose (fluorodeoxyglucose, or FDG) as the primary PET tracer is only the beginning. The fundamental strength of PET over conventional imaging is the ability to convey functional information that even the most exquisitely detailed anatomic image cannot provide. The standard PET radiotracer in current clinical use, FDG is a glucose analog that is taken up by cells in proportion to their rate of glucose metabolism. The increased glycolytic rate and glucose avidity of malignant cells in comparison to normal tissue is the basis of the ability of FDG-PET imaging to accurately differentiate cancer from benign tissue regardless of morphology (Gambhir, 2002). The level or intensity of FDG uptake on PET is semi-quantified and reported as the standardized uptake value (SUV).
PET/CT Scanning Principles
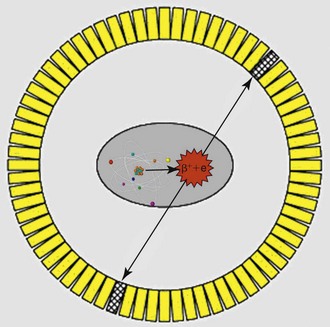
The radiotracers employed in PET imaging, such as 18FDG, always emit pairs of photons that are 180 degrees apart as they decay. This is the key difference from the conventional radiotracers used in nuclear medicine, which only emit single photons in any direction at each decay event. Accordingly, PET scanners are designed as a ring of multiple pairs of photon detectors arranged 180 degrees apart with the patient lying in the middle of this ring. These pairs of photon detectors, which are made of either bismuth germanium oxide (BGO), gadolinium orthosilicate (GSO), lutetium orthosilicate (LSO) crystals, or lutetium yttrium orthosilicate (LYSO) (Box 11-1) are electronically linked such that they accept only pairs of photons that arrive at both detectors at exactly the same time point and reject scattered photons that arrive at incongruent time points (Fig. 11-1). This design allows for superior photon sensitivity and spatial resolution compared to conventional nuclear medicine scanners, which rely on the use of lead-based collimators that act as filters to reduce scattered photons that hit the camera face at tangential angles.
PET/CT scanners are integrated units with the individual PET and computed tomography (CT) components nearly identical to their stand-alone counterparts. Integrated PET/CT has the added advantage of using the CT data for attenuation correction and anatomic localization; additionally, more sophisticated PET/CT fusion software can be utilized for interpretation. CT-based attenuation correction, however, may introduce artifacts on PET when imaging high-density materials, such as bowel contrast and metallic prostheses. By comparison, stand-alone PET units do not utilize CT-based attenuation correction, but rely on a transmission scan generated by a built-in germanium-68 rod source within the scanner. The field of view of a modern PET/CT scanner is approximately 16 to 18 cm wide (see Box 11-1). The bed is advanced into the scanner in 6 to 7 increments so the patient can be scanned from the base of the skull to the midthigh. An additional 6 to 7 increments are required for scanning the extremities. Typically, each increment, termed a bed position, requires 3 to 7 minutes of acquisition time before moving on to the next section of the body. In contrast to conventional cross-sectional imaging, scanning starts at the pelvis and moves cranially to minimize the amount of urine accumulation in the bladder (Box 11-2).
Box 11-2 Imaging Protocol Summary for FDG-PET/CT
Patient preparation before and after FDG PET/CT scanning is critical for high-quality scanning (see Box 11-2). High levels of either endogenous or injected insulin cause significant uptake of FDG in muscle and greatly decrease the sensitivity of the scan. For this reason, diabetic patients who have utilized regular insulin within 4 hours of FDG injection usually require rescheduling, as do patients who have eaten a meal within 8 hours. A less significant and somewhat controversial factor is that high levels of circulating glucose may potentially compete with FDG at tumor sites and, thereby, decrease sensitivity of the scan as well.
Accurate interpretation requires knowledge of the physiology of FDG as well as the limitations of PET scanning (Box 11-3). While greater SUV levels suggest a greater likelihood of malignancy, it is not reliable when used alone. Equally important is learning to differentiate focal from non-focal lesions through experience (there is no clinically used quantitative parameter for focality), with focal lesions more likely to be malignant than benign.
A multitude of common physiologic variants can also occur. These include marked fat activity, particularly in the head and neck (termed brown fat uptake), skeletal muscle uptake caused by physical activity after the injection of FDG, and increased areolar and ovarian activity, which is dependent on lactation and hormonal cycles. Even with abnormal FDG uptake beyond these physiologic variants, a host of benign processes may cause abnormal findings on PET scanning. These include infection and inflammation, certain benign or adenomatous lesions (including fibroadenomas occasionally), granulomatous disease, as well as other inflammatory conditions (Box 11-4).
Current efforts to improve PET resolution and sensitivity include the development of time-of-flight calculation techniques that account for the slight difference in time at which the pairs of photons arrive at the detectors to further localize the origin of positron decay within the patient (Surti et al., 2006). On the horizon, the first integrated PET/MRI scanners are being developed at several institutions (Pichler et al., 2006).
Clinical Utility of FDG-PET and PET/CT for Breast Cancer
Initial Diagnosis
Although noninvasive breast cancer has been previously shown to be poorly imaged by FDG-PET), the majority of FDG-PET studies in the literature have been performed on patients with invasive breast cancer. Significant variations among studies are noted when surveying across several studies; earlier small studies in selected patient groups reported 100% sensitivity and accuracy, which is in contrast to the findings of larger series conducted by Avril and colleagues and Schirrmeister and colleagues, which showed FDG-PET sensitivity ranging from 84% to 93%. The overall specificity of FDG-PET is relatively high, but false positives do occur in patients with some benign inflammatory lesions and fibroadenoma (Jones et al., 1999; Palmedo et al., 1997).
The two major contributing factors that explain the varied statistical results between studies are histopathology and size of the lesion. Invasive breast cancer includes multiple histologic types, including infiltrating ductal, infiltrating lobular, and combined infiltrating ductal carcinoma. Infiltrating ductal carcinoma has a higher level of FDG uptake and therefore is detected at a significantly higher sensitivity than infiltrating lobular breast cancer (Avril et al., 2000; Crippa et al., 1998) (see Box 11-3). This difference in FDG uptake between the two histologic types suggests that tumor aggressiveness is not the sole determinant of FDG uptake but that the mechanism of the variable FDG uptake by breast cancer cells is likely modulated by multiple factors including glucose transport-1 (GLUT-1) expression, hexokinase I activity, tumor microvessel density, amount of necrosis, number of lymphocytes, tumor cell density, and mitotic activity index (Bos et al., 2002).
Not surprisingly, several studies show that breast tumor size significantly affects FDG-PET scan results. Early studies (Adler et al., 1993; Wahl et al., 1993) of lesions larger than 1 cm show that FDG-PET can detect such tumors with both a sensitivity and specificity in the range of 96% to 100%. However, a more recent series by Yutani and colleagues showed that FDG activity was very low or nondetectable in patients with tumor sizes ranging from 0.4 to 1.5 cm. These small lesions are at the limit of the resolution of modern PET systems, which is approximately 6 mm (the newest PET/CT systems possibly have a spatial resolution of 4 mm), and lesions in this size range and smaller will be below the threshold of detectability.
In short, the results of FDG-PET for the initial detection and diagnosis of primary breast cancer vary, largely due to the heterogeneity of the disease and tumor size (Boxes 11-4 to 11-6). Although some nuclear medicine physicians expected that FDG-PET would serve as a “metabolic biopsy” as a means of screening, this is not yet the case for breast cancer. Improvements in spatial resolution and scanner sensitivity, as well as the advent of dual-modality PET/CT scanning, may lead to FDG-PET being more useful for breast cancer diagnosis (Fueger et al., 2005). FDG-PET may also play an important adjunctive role in selected patients with dense breasts, in whom mammography has a much poorer sensitivity (Vranjesevic et al., 2003).
Initial Staging
It appears that PET is even less sensitive in detecting metastases identified by SLN biopsy than those identified by ALND (Guller et al., 2002). This is presumably because SLN biopsy, with its more detailed pathologic examination of a small number of nodes, is more likely to detect micrometastatic disease that cannot be identified with FDG-PET (Wahl et al., 2004). PET in its current format is not yet sensitive enough to replace SLN biopsy, but its high specificity may be useful in determining the extent of local and systemic disease (see Box 11-3).
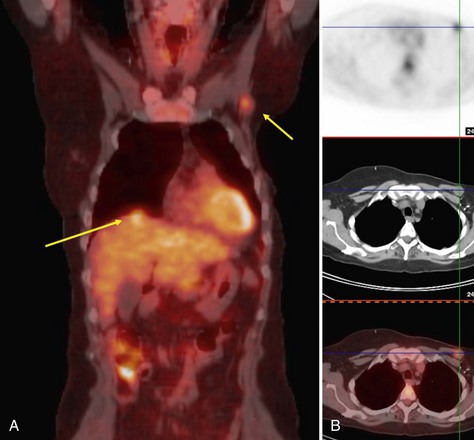
In contrast to the mixed results of FDG-PET in axillary lymph node staging, many studies have consistently demonstrated that FDG-PET is superior to CT in the detection of nodal metastases in internal mammary or mediastinal lymph nodes (Jones et al., 1999; Bellon et al., 2004; Eubank et al., 2001). In studies comparing PET to CT staging directly, the overall sensitivity, specificity, and accuracy in detection of mediastinal and internal mammary nodal metastases by PET was 85%, 90%, and 88%, respectively, versus 54%, 85%, and 73%, respectively, by CT. These data are promising for FDG-PET to play a role in staging internal mammary and mediastinal lymph node involvement, which is an important prognostic factor in patient management (Fig. 11-2).
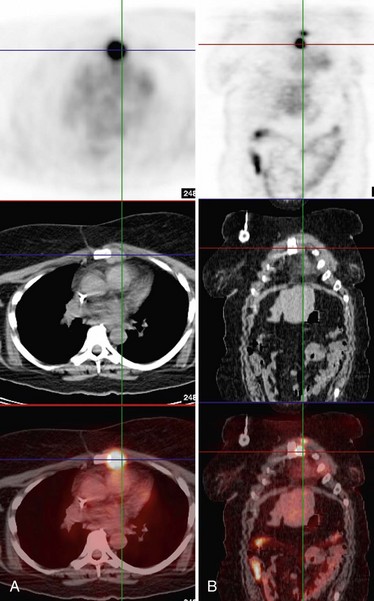
FDG-PET has also proved effective in detecting distant lesions and provides staging information even at the time of initial diagnosis. Several investigators, including Dose and colleagues, Lonneux and colleagues, and Moon and colleagues, have shown that PET is relatively sensitive (84% to 93%) and has a good negative predictive value (>90%) in the evaluation of distant metastases. Whole-body FDG-PET is able to detect metastases involving liver, lymph nodes, bone, lung, and bone marrow (Fig. 11-3). Specificity and positive predictive values are not quite as high, in the range of 55% to 86% and 82%, respectively, largely due to false-positive findings caused by muscle uptake, inflammation, blood pool activity, and bowel uptake (Moon et al., 1998). In respect to bone metastases, in which technetium-99m methyldisphosphonate (99mTc-MDP) bone scanning has been the established standard, recent studies by Ohta and colleagues and by Yang and colleagues independently showed that FDG-PET identified bone metastases with similar sensitivity and higher accuracy relative to 99mTc-MDP bone scanning. Further, a report by Garcia and colleagues and a preliminary study at our center suggests that FDG-PET and particularly dual-modality PET/CT may in fact be superior to bone scanning in the evaluation of lytic bone metastases (Taira et al., 2007).
Treatment Monitoring, Tumor Recurrence, and Restaging
FDG-PET is a metabolic imaging modality that has high sensitivity in the detection of therapy-induced glucose metabolic rate changes, which may not be evident in anatomic images, particularly early after treatment. This concept has naturally led to the evaluation of PET for treatment monitoring. Many groups of investigators (Avril and Weber, 2005; Gennari et al., 2000; Jansson et al., 1995; Schelling et al., 2000; Smith et al., 2000) have reported that FDG-PET can reliably differentiate responding and nonresponding breast cancers as early as after the first cycle of chemotherapy. For example, Schelling and colleagues reported that in a group of 22 patients, all responders were correctly identified, with sensitivity of 100% and specificity of 85%, using a decrease in FDG intensity (i.e., SUV) of greater than 55% compared to baseline. An additional phenomenon has been reported in estrogen receptor-positive tumors, in which metastases may actually show increased FDG intensity as a predictive response to successful anti-estrogen treatment, described as metabolic flare. This transient increase in FDG activity after hormone therapy initiation may be the result of an initial stimulation of tumor growth by estrogen-like agonist effects induced by increased levels of the hormone (Mortimer et al., 2001). The metabolic flare typically occurs 7 to 10 days after treatment initiation and may be the earliest and most accurate predictor of hormonal therapy response. It should be noted that all of these studies involve relatively small numbers of patients. Clearly more patients need to be evaluated, although there is at least preliminary evidence that FDG-PET may be used for early therapy evaluation of patients with locally advanced or metastatic breast cancer.
An emerging application of PET/CT may be in radiation treatment planning. Fused PET and CT images provide radiation oncologists with two pieces of critical information in a single study: the volume and extent of viable tumor and its exact location. Initial studies in patients with varied tumor types have confirmed that using PET/CT both in pretreatment planning and in follow-up evaluations has a significant impact on radiotherapy management in up to 56% of patients (Ciernik et al., 2003; Giraud et al., 2001). Certainly, evaluation of PET/CT for radiation treatment planning is still in the nascent stages, lacking rigorous randomized trials but nevertheless showing early promise.
Key Elements
Patients require adequate fasting to optimize scan quality.
Histologic subtype and lesion size greatly effect sensitivity for malignant breast lesions.
Many benign breast lesions may have FDG uptake that mimic malignancy.
PET/CT is not appropriate for staging axillary nodal disease.
PET/CT is effective for evaluation of distant sites of disease, response to therapy, and surveillance, particularly in high-risk patients based on family history, histologic features, and/or extent of disease at initial staging.
PET/CT is nearly as sensitive as Tc-99m MDP for identification of osteoblastic metastases and likely superior for identification of osteolytic metastases.
Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL. Evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2-fluoro-D-glucose PET. Radiology. 1993;187:743-750.
Adler LP, Faulhaber PF, Schnur KC, et al. Axillary lymph node metastases: screening with [F-18]2-deoxy-2-fluoro-D-glucose (FDG) PET. Radiology. 1997;203:323-327.
Avril N, Rose CA, Schelling M, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495-3502.
Avril NE, Weber WA. Monitoring response to treatment in patients utilizing PET. Radiol Clin North Am. 2005;43:189-204.
Bellon JR, Livingston RB, Eubank WB, et al. Evaluation of the internal mammary lymph nodes by FDG-PET in locally advanced breast cancer (LABC). Am J Clin Oncol. 2004;27:407-410.
Bos R, van Der Hoeven JJ, van Der Wall E, et al. Biologic correlates of 18fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20:379-387.
Centers for Medicare and Medicaid Services. NCA tracking sheet for positron emission tomography (FDG) for solid tumors (CAG-00181R). available at www.cms.gov/mcd/viewtrackingsheet.asp?from2=viewtrackingsheet.asp&id=218&, April 3, 2009.
Ciernik IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Intl J Radiat Oncol Biol Physics. 2003;57:853-863.
Crippa F, Agresti R, Seregni E, et al. Prospective evaluation of fluorine-18-FDG PET in presurgical staging of the axilla in breast cancer. J Nucl Med. 1998;39:4-8.
Dose J, Bleckmann C, Bachmann S, et al. Comparison of fluorodeoxyglucose positron emission tomography and “conventional diagnostic procedures” for the detection of distant metastases in breast cancer patients. Nucl Med Comm. 2002;23:857-864.
Eubank WB, Mankoff DA, Takasugi J, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001;19:3516-3523.
Fueger BJ, Weber WA, Quon A, et al. Performance of 2-deoxy-2-[F-18]fluoro-D-glucose positron emission tomography and integrated PET/CT in restaged breast cancer patients. Mol Imaging Biol. 2005;7:369-376.
Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nature Rev. 2002;2:683-693.
Garcia JR, Simo M, Perez G, et al. 99mTc-MDP bone scintigraphy and 18F-FDG positron emission tomography in lung and prostate cancer patients: different affinity between lytic and sclerotic bone metastases. Eur J Nucl Med Molecul Imaging. 2003;30:1714.
Gennari A, Donati S, Salvadori B, et al. Role of 2-[18F]-fluorodeoxyglucose (FDG) positron emission tomography (PET) in the early assessment of response to chemotherapy in metastatic breast cancer patients. Clin Breast Cancer. 2000;1:156-163.
Giraud P, Grahek D, Montravers F, et al. CT and 18F-deoxyglucose (FDG) image fusion for optimization of conformal radiotherapy of lung cancers. Intl J Radiat Oncol Biol Physics. 2001;49:1249-1257.
Guller U, Nitzsche EU, Schirp U, et al. Selective axillary surgery in breast cancer patients based on positron emission tomography with 18F-fluoro-2-deoxy-D-glucose: not yet!. Breast Cancer Res Treat. 2002;71:171-173.
Jansson T, Westlin JE, Ahlstrom H, et al. Positron emission tomography studies in patients with locally advanced and/or metastatic breast cancer: a method for early therapy evaluation? J Clin Oncol. Jun 1995;13:1470-1477.
Jones A, Bernstein V, Davis N, et al. Pilot feasibility study to assess the utility of PET scanning in the preoperative evaluation of internal mammary nodes in breast cancer patients presenting with medial hemisphere tumors. Clin Positron Imaging. 1999;2:331.
Lonneux M, Borbath II, Berliere M, et al. The place of whole-body PET FDG for the diagnosis of distant recurrence of breast cancer. Clin Positron Imaging. 2000;3:45-49.
Moon DH, Maddahi J, Silverman DH, et al. Accuracy of whole-body fluorine-18-FDG PET for the detection of recurrent or metastatic breast carcinoma. J Nucl Med. 1998;39:431-435.
Mortimer JE, Dehdashti F, Siegel BA, et al. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797-2803.
Ohta M, Tokuda Y, Suzuki Y, et al. Whole body PET for the evaluation of bony metastases in patients with breast cancer: comparison with 99mTc-MDP bone scintigraphy. Nucl Med Comm. 2001;22:875-879.
Palmedo H, Bender H, Grunwald F, et al. Comparison of fluorine-18 fluorodeoxyglucose positron emission tomography and technetium-99m methoxyisobutylisonitrile scintimammography in the detection of breast tumours. Eur J Nucl Med. 1997;24:1138-1145.
Pecking AP, Mechelany-Corone C, Bertrand-Kermorgant F, et al. Detection of occult disease in breast cancer using fluorodeoxyglucose camera-based positron emission tomography. Clin Breast Cancer. 2001;2:229-234.
Pichler BJ, Judenhofer MS, Catana C, et al. Performance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. J Nucl Med. 2006;47:639-647.
Schelling M, Avril N, Nahrig J, et al. Positron emission tomography using [(18)F]fluorodeoxyglucose for monitoring primary chemotherapy in breast cancer. J Clin Oncol. 2000;18:1689-1695.
Schirrmeister H, Kuhn T, Guhlmann A, et al. Fluorine18 2-deoxy-2-fluoro-D-glucose PET in the preoperative staging of breast cancer: comparison with the standard staging procedures. Eur J Nucl Med. 2001;28:351-358.
Smith IC, Welch AE, Hutcheon AW, et al. Positron emission tomography using [18F]-fluorodeoxy-D-glucose to predict the pathologic response of breast cancer to primary chemotherapy. J Clin Oncol. 2000;18:1676-1688.
Surti S, Karp JS, Popescu LM, et al. Investigation of time-of-flight benefit for fully 3-D PET. IEEE Trans Medl Imaging. 2006;25:529-538.
Taira AV, Herfkens RJ, Gambhir SS, Quon A. Detection of bone metastases: assessment of integrated FDG PET/CT imaging. Radiology. 2007;243:204-211.
Vranjesevic D, Schiepers C, Silverman DH, et al. Relationship between 18F-FDG uptake and breast density in women with normal breast tissue. J Nucl Med. 2003;44:1238-1242.
Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22:277-285.
Wahl RL, Zasadny K, Helvie M, et al. Metabolic monitoring of breast cancer chemohormonotherapy using positron emission tomography: initial evaluation. J Clin Oncol. 1993;11:2101-2111.
Yang SN, Liang JA, Lin FJ, et al. Comparing whole body (18)F-2-deoxyglucose positron emission tomography and technetium-99m methylene diphosphonate bone scan to detect bone metastases in patients with breast cancer. J Cancer Res Clin Oncol. 2002;128:325-328.
Yutani K, Shiba E, Kusuoka H, et al. Comparison of FDG-PET with MIBI-SPECT in the detection of breast cancer and axillary lymph node metastasis. J Computer-assist Tomogr. 2000;24:274-280.