Chapter 90 Fatty Acid Metabolism
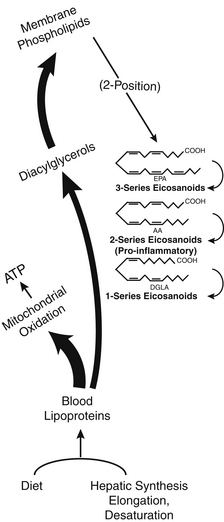
The essential hormone function is mediated by some special fatty acids that affect energy flow, cell division, immune responses, and many other body controls. These critical fatty acids are used to make powerful tissue-specific compounds called eicosanoids. Figure 90-1 illustrates the various roles of fatty acids. This chapter discusses more about these functions after some basic fatty acid relationships are explained.
 Fatty Acid Structure and Metabolism
Fatty Acid Structure and Metabolism
About 30 structural isomers and homologs of fatty acids occur in human tissues. They are named in various ways, including those with Latin prefixes such as penta– and hexa– for the number of carbon atoms. These are the chemical names approved by the International Union of Pure and Applied Chemists. Many also have common names. Because common names are in widest use among clinicians, they are used here. Table 90-1 provides a lexicon of fatty acid terms.
| TERM OR ABBREVIATION | DEFINITION OR EXPLANATION |
|---|---|
| AA | Arachidonic acid |
| ALA | Alpha-linolenic acid |
| Alpha-linolenic acid | Delta 9,12,15 octadecatrienoic acid; 18:3 ω-3 |
| Arachidonic acid | Delta 5,8,11,14 eicosatetraenoic acid; 20:4 ω-6 |
| Beta-oxidation | The mitochondrial metabolic pathway whereby fatty acids are converted into acetyl CoA, which enters the citric acid cycle to ultimately yield adenosine triphosphate. The carbon atoms of the fatty acid are oxidized to carbon dioxide. |
| Carnitine | The molecule to which fatty acids are attached in the process of their enzyme-mediated entry into the mitochondrial matrix. This enzyme is inhibited by malonyl CoA formed during fatty acid synthesis from carbohydrates. |
| Cerebronic acid | The 2-hydroxy derivative of lignoceric acid (24:0); found in glycosphingolipids in the brain |
| Cervonic acid | Another name for docosahexaenoic acid |
| cis | Geometrical isomer in which two groups are on the same side of a double bond |
| Clupanodonic acid | Another name for δ-7,10,13,16,19-docosapentaenoic acid (DPA3), an ω-3 series, long-chain, highly unsaturated fatty acid; found in fish oils and phospholipids in the brain |
| Delta | Used to describe the position of double bonds relative to the carboxyl end of a fatty acid |
| Desaturase | Tightly bound to the endoplasmic reticulum membrane, this enzyme, in association with cytochrome b5 and cytochrome b5 reductase, uses reduced nicotinamide adenine dinucleotide and oxygen to introduce double bonds into fatty acids. There are at least four separate desaturases, named according to the position of inserted double bonds. Delta-9-desaturase, δ-6-desaturase, and δ-5(4) desaturase act on fatty acetyl CoA thioesters, always inserting double bonds between the thioester bond (carboxylate group) and the double bond closest to it, leaving a three-carbon gap. Activities of these enzymes fluctuate according to dietary fat intake to maintain optimal fluidity state of the membrane lipids. Their concentrations decrease in starvation and increase greatly on refeeding carbohydrates. They are suppressed when dietary unsaturated fatty acid intake (including trans isomers) is high. |
| DGLA | Dihomogammalinolenic acid |
| Dihomogammalinolenic acid | Delta 8,11,4 eicosatrienoic acid; 20:3 ω-6 |
| DHA | Delta 4,7,10,13,16,19 docosahexaenoic acid; 22:6; an ω-3 series long-chain, highly unsaturated fatty acid; found in fish oils and the phospholipids in the brain (also known as cervonic acid) |
| Dienoic | Contains two double bonds |
| EFA | Essential fatty acid |
| Eicosanoid | A product of the specific, enzyme-directed oxidation of polyunsaturated fatty acids containing 20 (eicosa)carbons. This term encompasses the prostaglandins, thromboxanes, and leukotrienes. |
| Eicosapentaenoic acid | Delta 5,8,11,14,17 eicosapentaenoic acid; 20:5 ω-3; one of the most abundant fatty acids in fish oils |
| Elongase | An enzyme that adds 2-carbon units (acetate) to the carboxyl end of an existing saturated or unsaturated fatty acid. Mitochondrial form uses acetyl CoA, whereas endoplasmic form has malonyl CoA as substrate. |
| EPA | Eicosapentaenoic acid |
| Gamma linolenic acid | Delta 6,9,12 octadecatrienoic acid; 18:3 ω-6 |
| GLA | Gamma-linolenic acid |
| HUFA | Highly unsaturated fatty acid; generally having five or six double bonds |
| LA | Linoleic acid |
| Lauric acid | Tetradecanoic acid; C12:O; first isolated and identified from the laurel plant |
| LCP | Long-chain polyunsaturated fatty acid |
| Linoleic acid | Delta 9,12 octadecadienoic acid; 18:2 ω-6 |
| Meads acid | Delta 5,8,11 eicosatrienoic acid; 20:3 ω-9. This compound is not normally produced in appreciable amounts due to the preferential loading of the desaturase enzymes with the more strongly binding essential fatty acids and their metabolic products. It accumulates, however, in essential fatty acid deficiency, making it a marker for this condition. |
| Monoenoic | Containing one double bond |
| MUFA | Monounsaturated fatty acid |
| Omega | Used to describe the position of double bonds relative to the methyl end of a fatty acid |
| P + M/S ratio | Polyunsaturated + monounsaturated to saturated fatty acid ratio |
| P/S ratio | Polyunsaturated-to-saturated fatty acid ratio |
| Phospholipase A2 | An enzyme that catalyzes the hydrolysis of the fatty acid ester from position 2 of phosphoglycerides. Dependent on calcium, this enzyme is responsive to intracellular calcium, calmodulin, etc. It releases primarily polyenoic fatty acids (arachidonic acid, etc.) from membranes for eicosanoid synthesis. |
| PUFA | Polyunsaturated fatty acid |
| Phosphatide | Diacylglycerol phosphate, to which various groups may be attached through phosphoester linkage; the principal components of cell membranes |
| Polyenoic | Containing two or more double bonds |
| Saturated fatty acid | A fatty acid in which all of the carbon atoms except for the carboxyl carbon are fully hydrogenated as −CH2− (and −CH3) |
| Stearidonic acid | Delta 6,9,12,15 octadecatetraenoic acid; 18:4 ω-3; a product of elongation of ALA and a precursor to EPA. A good source is black currant seed oil. |
| Timnodonic acid | Another name for EPA or 20:4 ω-6 |
| Trans | Geometrical isomer in which two groups are on opposite sides of a double bond |
| Unsaturated fatty acid | A fatty acid in which two or more adjacent pairs of carbon atoms are lacking hydrogen atoms, having instead an additional carbon–carbon bond or double bond |
| δ | The Greek letter delta |
| ω | The Greek letter omega |
CoA, coenzyme A.
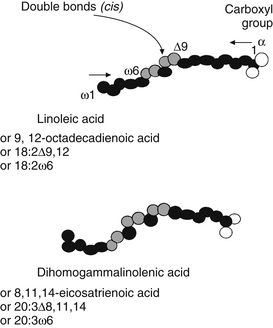
Three major families of UFAs are found in human tissues: the ω-9, ω-6, and ω-3 UFAs (or n-9, n-6, and n-3). The ω-6 and ω-3 PUFAs are defined by the position of the double bond closest to the terminal methyl group of the fatty acid molecule. In the ω-6 family, the first double bond occurs between the sixth and seventh carbons from the methyl group end of the molecule, whereas in the ω-3 family, the first double bond occurs between the third and fourth carbons (Figure 90-2 provides naming conventions).
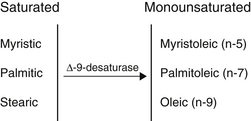
The geometry of desaturase enzymes will not allow insertion farther than nine carbons (δ-9) from the carboxyl group. Thus, linoleic acid (LA; δ-9, 12) cannot be synthesized in humans. Fatty acids of various chain lengths can act as desaturase substrates, as well as those that already possess double bonds at other positions. Figure 90-3 shows the products of the δ-9 desaturase acting on three saturated fatty acids.
The activity of the desaturase enzymes is critical for maintaining the ratio of saturated and unsaturated components in cell membranes. Tumor tissue and virus-transformed cells have a higher content of UFAs, especially oleic acid, which increases relative to the amount of stearic acid. Such shifts increase the metabolic rates of many lipid-dependent enzymes and are associated with a higher capacity for cell division.1 Individuals with recurrent tumors may already have too much oleic acid relative to stearic acid, and encouraging a diet high in olive oil or other highly UFAs may be contraindicated.
In the tissues of plants and higher animals, the insertion of double bonds always results in the cis geometry. The phrase “partially hydrogenated vegetable oil” on food labels indicates that a natural oil has been chemically modified in a process that converts some of the cis UFAs to the trans form. Oleic acid is changed into its trans isomer, elaidic acid, which is the most abundant trans fatty acid in most hydrogenated oils. In human metabolism, trans unsaturated fat behaves similar to saturated fat, meaning that their accumulation tends to increase the risk of heart disease. Adverse effects of trans fatty acids on high-density lipoprotein or low-density lipoprotein cholesterol are well documented in medical research literature.2–4
Interference with eicosanoid production is also suspected, and research in the area of health risks of trans fatty acids is accelerating. Trans fatty acid levels in cell membranes are determined by dietary intake of hydrogenated oils.5 A person eating a doughnut for breakfast (3.19 g of trans fatty acids), a small order of French fries with lunch (3.43 g), two teaspoons of margarine on bread with dinner (1.24 g), and two cookies for a snack (1.72 g) would ingest a total of 9.58 g of trans fatty acids, enough to negate the serum cholesterol-lowering effects of a decrease in saturated fat of 10% of total energy intake.6
Dietary PUFAs are largely composed of the ω-6 family (e.g., linolenic acid [LA]), which is abundant in vegetable seed oils, and the ω-3 family (e.g., α-linolenic acid [ALA]), which is high in vegetable leaves, flaxseeds, walnuts, canola, and modest in soybean oil.7 Table 90-2 lists other members of these classes. When the diet supplies adequate LA and ALA, they may be used to form the other members in their classes by desaturation and elongation reactions.
TABLE 90-2 Polyunsaturated Fatty Acid Families
| FAMILY | CONFIGURATION | NAME |
|---|---|---|
| Omega-6 | 18:2 | Linoleic acid (LA) |
| 18:3 | Gamma linolenic acid (GLA) | |
| 20:3 | Dihomogammalinolenic acid (DGLA) | |
| 20:4 | Arachidonic acid (AA) | |
| Omega-3 | 18:3 | Alpha-linolenic acid (ALA) |
| 20:5 | Eicosapentaenoic acid (EPA) | |
| 22:5 | Docosapentaenoic acid (DPA-3) | |
| 22:6 | Docosahexaenoic acid (DHA) |
The δ-6 desaturase enzyme requires a zinc ion for activity. For this reason, supplementing a patient with sources of LA and ALA does little good if zinc deficiency is present. The changes in body growth, organ weights, and lipid concentrations in plasma and liver produced by zinc deficiency are reversed by the addition of γ-linoleic acid (GLA)-rich primrose oil, but not LA-rich safflower oil, showing the role of zinc in the δ-6 desaturase enzyme.8 The enzyme is also inhibited by high concentrations of saturated fatty acids, monounsaturated acids, and trans fatty acids, all of which compete for the enzyme binding site and yield products that are of less significance to the cell.
The PUFAs impact health due to their conversion to compounds, collectively called eicosanoids, which possess extremely potent biological activities. Their functions of regulating blood vessel leaking, lipid accumulation, inflammation, and immune cell behavior are relevant to the initiation and progress of cardiovascular disease.9 These compounds, in turn, are used to amplify and balance signals to the organs, the blood clotting system, and the immune system.
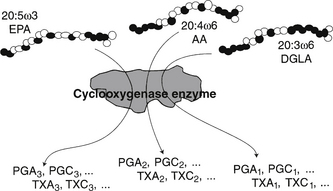
Production of eicosanoid hormones is shown in Figure 90-4. The parent compounds are dihomogammalinolenic (DGLA), arachidonic acid (AA), and EPA, and the pathway uses the same cyclooxygenase enzyme for all three series of products. The relative concentrations of the three fatty acids present in the phospholipids of the cell membranes where the hormones are produced determine which product will predominate, due to substrate competition for the active site, as depicted in Figure 90-4. In a similar manner, the lipoxygenase enzyme initiates the sequence of reactions leading to leukotriene formation. The 2-series that is derived from AA is by far the most proinflammatory. The effects of GLA and DGLA on rheumatoid arthritis and many other inflammatory diseases are mediated via this mechanism. Because dietary intake is a primary determinant of the fatty acids that enter this pathway, there is a direct link between the balance of specific fats in the diet and inflammatory responses and other local control processes. Long-term health maintenance is highly dependent on proper balance of dietary fatty acids.
It is no longer adequate simply to recommend the replacement of saturated fats with PUFAs. Balanced intake of n-3 and n-6 fatty acids, not just a high PUFA-to-saturated ratio, is required for optimal control of every tissue in the body.10 Chapter 14 discusses the interpretation of fatty acid testing and clinical interventions.
 Control of Fatty Acid Supply and Distribution
Control of Fatty Acid Supply and Distribution
Fatty acids are present in the diet in the form of triglycerides in solid fats or liquid oils and as phosphatides in cell membranes of whole foods. They are rarely present in nature as free fatty acids. Table 90-3 lists food sources of the more abundant fatty acids. Foods always contain complex mixtures of fatty acids with enrichment of any single fatty acid rarely exceeding 70%.
TABLE 90-3 Dietary Sources of Even-numbered Medium-chain and Long-chain Saturated Fatty Acids
| NO. OF CARBONS | COMMON NAME | SOURCE |
|---|---|---|
| 10 | Capric (C10:O) | Most plants and butter |
| 12 | Lauric (C12:O) | Palm kernel, coconut, laurels |
| 14 | Myristic (C14:O) | Nutmeg, palm kernel, coconut, myrtle |
| 16 | Palmitic (C16:O) | Common in all animal and plant fats |
| 18 | Stearic (C18:O) | Common in all animal and plant fats |
| 20 | Arachidic (C20:O) | Peanut (arachis) oil |
| 22 | Behenic (C22:O) | Seeds |
| 24 | Lignoceric (C24:O) | Cerebrosides, peanut oil |
Effect of Low-Fat Diets
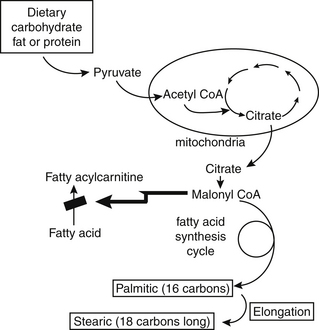
An individual on a low-fat, high-carbohydrate diet uses the fatty acid synthetic pathway extensively. As shown in Figure 90-5, this pathway requires citrate to leave the mitochondria to generate malonyl CoA. The resultant high concentrations of malonyl CoA inhibit the activity of the enzyme that is the gateway to fatty acid oxidation. For this reason, very low-fat diets do not result in weight loss nearly as quickly as one might expect. The burning of excess fat is actually inhibited by the high carbohydrate intake. In individuals with low body fat and good lean mass, high carbohydrate intake spares the use of fatty acids. In more sedentary individuals with lower rates of fatty acid oxidation, the medium-chain fatty acids that are otherwise so quickly used for energy may accumulate above normal levels in membranes.
 Fatty Acids and Human Diseases
Fatty Acids and Human Diseases
Quantification of the approximately 30 fatty acids present in human tissues is now routinely available. Both deficiencies and excesses of individual fatty acids lead to metabolic problems. A brief discussion of a few highly relevant conditions affected by fatty acid intake is presented here (see Chapter 14 for additional discussion of specific fatty acid imbalances). Table 90-4 lists typical signs and symptoms of fatty acid abnormalities, and Table 90-5 lists some well-documented clinical applications of supplemental fatty acids.
TABLE 90-4 Signs and Symptoms Associated with Fatty Acid Abnormalities
| SIGNS AND SYMPTOMS | FATTY ACID ASSOCIATION | ACTION |
|---|---|---|
| Emaciation, weakness, disorientation | Caloric deprivation | Add sources of balanced ω-3 and ω-6 FAs |
| Reduced growth, renal dysplasia, reproductive deficiency, scaly skin deficiency | Classic essential fatty acid | Add good quality fats and oils |
| Eczema-like skin eruptions, loss of hair, liver degeneration, behavioral disturbances, kidney degeneration, increased thirst, frequent infections, poor wound healing, sterility (male) or miscarriage (female), arthralgia, cardiovascular disease, growth retardation | Linoleic acid insufficiency | Add corn or safflower oils |
| Growth retardation, weakness, impairment of vision, or learning disability, poor coordination, tingling in arms/legs, behavioral changes, mental disturbances, low metabolic rate, high blood pressure, immune dysfunction, acid insufficiency | Alpha- or γ-linolenic | Add flax, primrose, borage, black currant oils |
| Depression, anxiety, learning and behavioral disorders, age-related eye disorders | Long-chain PUFA-dependent | Add fish oils, avoid neuromembrane function hydrogenated oils |
| Cardiovascular disease risk | Prostanoid balance | Add ω-3 PUFAs |
| Cancer | Low stearic to oleic ratio | Use ω-6 PUFAs with caution |
| Rheumatoid arthritis | Low GLA and DGLA | Add primrose oil |
| Deficiency of vitamin B12 or carnitine, or both | Increased odd-numbered FAs | Add vitamin B12 or carnitine, or both |
| Myelinated nerve degeneration | Increased long-chain FAs | Add high-erucate canola or mustard oils |
| Fatty liver | Saturated and ω-9 | Restrict alcohol, add lecithin, accumulation in liver, increase methionine |
| Accelerated aging | High PUFA intake without increased antioxidants | Add vitamins E and C, Se, Mn, and Zn |
DGLA, dihomogammalinolenic acid; FAs, fatty acids; GLA, γ-linolenic acid; Mn, magnesium; PUFA, polyunsaturated fatty acid; Se, selenium; Zn, zinc.
TABLE 90-5 Clinical Use of γ-linolenic Acid and α-linolenic Acid
| APPLICATION | ALPHA-LINOLENIC ACID | GAMMA-LINOLENIC ACID |
|---|---|---|
| Lower blood pressure, lower blood cholesterol, and lower risk of stroke and heart attack | X | XX |
| Normalize fat metabolism in diabetes and decrease the amount of insulin required by diabetics | X | X |
| Prevent liver damage due to alcoholism and decrease withdrawal symptoms after discontinuing the habitual use of alcohol | X | X |
| Provide adjunctive treatment for schizophrenics | X | XX |
| Cause weight loss by increasing metabolic rate and fat burn-off | X | XX |
| Relieve premenstrual breast pain (mastalgia) and premenstrual syndrome of bloating, irritability, depression, and aggressive behavior | XX | X |
| Prevent drying and atrophy of tear and salivary glands (Sjögren’s syndrome) | X | |
| Prevent arthritis in animals | X | X |
| Improve the condition of hair, nails, and skin | X | XX |
| Improve certain kinds of eczema | X | X |
| Slow down or stop deterioration in multiple sclerosis | X | X |
| Help treat diabetic neuropathy in type 2 diabetes | X | X |
| Kill cancer cells in tissue culture without harming normal cells | X | XX |
X, moderate response; XX, strong clinical response.
Developmental Disorders
The very-long-chain PUFA docosahexaenoic acid (DHA) has a special role in the formation of the developing nervous system.11,12 Children with attention deficit hyperactivity disorder have lower concentrations of key fatty acids in plasma and in red blood cells.13 Preterm infants deprived of EFAs during late pregnancy are likely to have failures of normal development, especially the visual system, unless ω-3 fatty acids are supplemented.14
Heart Disease
We are now seeing the emergence of adverse effects from the decades-long encouragement of intake of hydrogenated oils in the form of margarines and many other foods with lower, but hydrogenated fat content. By the late 1960s, processed vegetable fats had displaced animal fats in the diets of most people in industrialized countries due to lower costs and purported health benefits. Increased tissue levels of the trans fatty acids from hydrogenated oils is clearly a factor in the etiology of heart disease.15,16 Higher palmitic and lower ω-3 fatty acids in serum are correlated with higher incidence of coronary heart disease in middle-aged men at high risk for cardiovascular disease.17 Improvements in plasma fatty acids and vitamins E and C were the only factors found to be related to improvements in life expectancy and a 70% lowering of heart disease in a study population.18
Cancer
Because of the pivotal role in tumorigenesis of cell–cell recognition factors and the membrane-associated functions controlling that process, changes in the fatty acid composition of cell membranes signal tumor activity. Tumor tissue and virus-transformed cells have a higher content of UFAs, especially oleic acid, which increases relative to the amount of stearic acid. Such shifts increase the metabolic rates of many lipid-dependent enzymes and are associated with a higher capacity for cell division.19 Changes in dietary fatty acid intake cause alterations in immune response, including antitumor activity.20
Neurologic Disorders
Adrenal leukodystrophy and related conditions are associated with the accumulation in nerve sheath membranes of fatty acids more than 20 carbons long.21 This situation may be made worse by the lack of sufficient long-chain (C16-C20) PUFAs. The indicator for this situation is the sum of the 22-26 carbon length even-numbered fatty acids in erythrocytes or plasma.
The brain has a high content of lipid, and the fatty acid composition is unique in the high content of very-long-chain PUFAs, especially DHA. Strong evidence suggests a relationship between depleted DHA levels in postpartum depression and other forms of depression.22 These results led to acceleration of studies of the proper clinical application of essential fatty acid therapies in depressed patients.22–25
1. Wood C.B., Habib N.A., Apostolov K., et al. Reduction in the stearic to oleic acid ratio in human malignant liver neoplasms. Eur J Surg Oncol. 1985;11:347–348.
2. Mensink R.P., Zock P.L., Katan M.B., et al. Effect of dietary cis and trans fatty acids on serum lipoprotein[a] levels in humans. J Lipid Res. 1992;33:1493–1501.
3. Longnecker M. Do trans fatty acids in margarine and other foods increase the risk of coronary heart disease? Epidemiology. 1993;4:492–495.
4. Lichenstein A. Trans fatty acids, blood lipids, and cardiovascular risk. Where do we stand? Nutr Rev. 1993;51:340–343.
5. Pettersen J., Opstvedt J. Fatty acid composition of lipids of the brain and other organs in suckling piglets. Lipids. 1992;27:761–769.
6. Litin L., Sacks F. Trans fatty acid content of common foods. N Engl J Med. 1993;329:1969–1970.
7. Gebauer S.K., Psota T.L., Harris W.S., et al. N-3 fatty acid dietary recommendations and food sources to achieve essentiality and cardiovascular benefits. Am J Clin Nutr. 2006 Jun;83(Suppl 6):S1526–S1535.
8. Huang Y.S., Cunnane S.C., Horrobin D.F., et al. Most biological effects of zinc deficiency corrected by gamma-linolenic acid, (18:3 omega 6) but not by linoleic acid (18:2 omega 6). Atherosclerosis. 1982;41:193–207.
9. Stamler J. Nutrition related risk factors for the atherosclerotic diseases—present status. Prog Biochem Pharmacol. 1983;19:245–308.
10. Horrobin D.F., ed. Clinical uses of essential fatty acids. Montreal: Eden Press, 1981.
11. Green P., Glozman S., Kamensky B., et al. Developmental changes in rat brain membrane lipids and fatty acids. The preferential prenatal accumulation of docosahexaenoic acid. J Lipid Res. 1999;40:960–966.
12. Farkas K., Noble R.C., Speake B.K. Developmental changes in the levels of molecular species of triacylglycerol that contain docosahexaenoic acid in adipose tissue of the chick embryo. Comp Biochem Physiol B Biochem Mol Biol. 1996;115:1–6.
13. Stevens L.J., Zentall S.S., Deck J.L., et al. Essential fatty acid metabolism in boys with attention-deficit hyperactivity disorder. Am J Clin Nutr. 1995;62:761–768.
14. Innis S.M. N-3 fatty acid requirements of the newborn. Lipids. 1992;27:879–887.
15. Ascherio A., Katan M.B., Zock P.L., et al. Trans fatty acids and coronary heart disease. N Engl J Med. 1999;340:1994–1998.
16. Nelson G.J. Dietary fat, trans fatty acids, and risk of coronary heart disease. Nutr Rev. 1998;56:250–252.
17. Simon J.A., Fong J., Bernert J.T., et al. Serum fatty acids and the risk of stroke. Stroke. 1995;26:778–782.
18. Renaud S., de Lorgeril M., Delaye J., et al. Cretan Mediterranean diet for prevention of coronary heart disease. Am J Clin Nutr. 1995;61:S360–S376.
19. Wood C.B., Habib N.A., Apostolov K., et al. Reduction in the stearic to oleic acid ratio in human malignant liver neoplasms. Eur J Surg Oncol. 1985;11:347–348.
20. Erikson K.L., Hubbard N.E., Chakrabarti R. Modulation of signal transduction in macrophages by dietary fatty acids. J Nutr. 1995;125:S1683–S1686.
21. van Geel B.M., Assies J., Haverkort E.B., et al. Progression of abnormalities in adrenomyeloneuropathy and neurologically asymptomatic X-linked adrenoleukodystrophy despite treatment with “Lorenzo’s oil.”. J Neurol Neurosurg Psychiatry. 1999;67:290–299.
22. Mischoulon D., Fava M. Docosahexanoic acid and omega-3 fatty acids in depression. Psychiatr Clin North Am. 2000;23:785–794.
23. Locke C.A., Stoll A.L. Omega-3 fatty acids in major depression. World Rev Nutr Diet. 2001;89:173–185.
24. Anonymous. Omega-3 fatty acids in the treatment of depression. Harv Ment Health Lett. 2001;18:4–5.
25. Stoll A.L., Damico K.E., Daly B.P., et al. Methodological considerations in clinical studies of omega 3 fatty acids in major depression and bipolar disorder. World Rev Nutr Diet. 2001;88:58–67.