Chapter 37 Fasting
 Introduction
Introduction
Humans, unlike chimpanzees, have the ability to survive on water for extended periods of time.1 The primary adaptation is the ability to use ketones from fat metabolism as an alternate fuel for the central nervous system.2 The beauty of this adaptation is that during fasting the body prioritizes fat catabolism, the most concentrated caloric energy resource (9 vs 4 kcal for carbohydrates and protein), and thus safeguards catabolism of essential structures (nerves, muscles, organs, etc.) until stored adipose tissue is severely depleted—after fasting several weeks to months, depending on fat stores as well as metabolic, stress, and activity level. However, once this threshold is crossed in unattended fasting, starvation ensues (the body uses essential tissue for fuel, relying on protein as a major fuel source), resulting in death due to organ failure.3
 History
History
Throughout history, people of various cultures and religions have recognized the value of fasting. Numerous references appear in the Bible, Koran, pagan writings, and writings of the ancient Greeks.4–6 One of the earliest doctors to use therapeutic fasting in the United States was Isaac Jennings (1788 to 1874). In 1822, Jennings discarded the use of drugs and, through the influence of Presbyterian preacher Sylvester Graham (1794 to 1851), began advocating fasting and other aspects of hygienic treatment (vegetarian diet, pure water, sunshine, clean air, exercise, emotional poise, and rest). This treatment later came to be known as “natural hygiene.”5–9 Other doctors who followed in the hygienic tradition were James C. Jackson (1811 to 1895), Russell T. Trall (1812 to 1877), William A. Alcott (1798 to 1859), Mary Grove Nichols (1810 to 1884), Thomas L. Nichols (1815 to 1901), Edward H. Dewey (1837 to 1904), George H. Taylor (1821 to 1896), Harriet Austin (1826 to 1891), Charles E. Page (1840 to 1925), Emmett Densmore (1837 to 1911), Helen Densmore (? to 1904), Susanna W. Dodds (1830 to 1915), Felix Oswald (1845 to 1906), Robert Walter (1841 to 1921), John H. Tilden (1851 to 1940), and George S. Weger (1874 to 1935). Most of these physicians graduated as medical doctors (MDs) from eclectic medical schools and published various works on lifestyle, diet, and fasting.7–14
The hygienic lineage continued into the mid-1900s, mainly due to Herbert M. Shelton, DC, ND (1895 to 1985), who developed a stricter protocol for fasting (water only; no enemas, exercise, or treatments; and complete rest). Shelton began his study of fasting in 1911 by reading the popular writers of his day: Sinclair, Carrington, Hazzard, Haskell, Purinton, Tilden, and MacFadden. He studied under the fasting authorities of his time at MacFadden’s College (Chicago, Ill.), Crane’s Sanatorium (Elmhurst, Ill.), and Crandall’s Health School (York, Penn.).7,12 (Among the earliest fasting institutions of this time were Lindlahr’s Nature Cure Sanatoriums, including the Jungborn—the last operated by Benedict Lust, the founder of naturopathy in the United States, the MacFadden’s Healthatorium, and the Tilden’s Health School.)8 In 1928, he founded a fasting institution and health school that provided services for more than 40 years.7
In 1949, Dr, Shelton along with William Esser, ND, DC; Christopher Gian-Cursio, ND, DC; and Gerald Benesh, ND, DC, formed the American Natural Hygiene Society, now called the National Health Association,7,14 a lay organization dedicated to preserving the tenets of hygiene. In 1978, a professional branch was formed (International Association of Hygienic Physicians [IAHP]) to study and promote therapeutic fasting. Today, the IAHP organizes clinical training and examination, leading to certification in therapeutic fasting.15
 Physiology
Physiology
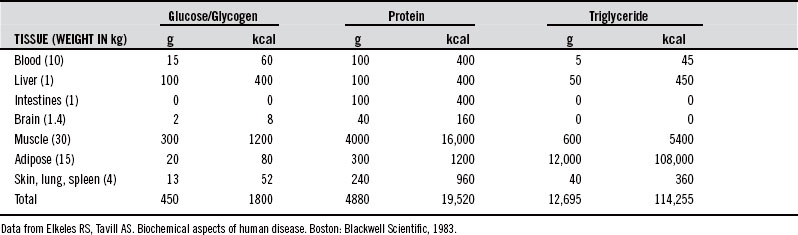
It has been suggested that humans, like other species, have evolved special biochemical pathways to subsist for long periods without food during periods of food scarcity (climate, injury, illness).16 While fasting, the body primarily uses fat stores from adipose tissue for energy while recycling nonessential tissue for maintenance of pivotal systems. This streamlining utilizes nonessential protein sources, including digestive and glycolytic enzymes, muscle contractile fibers, and other connective tissue. Research has determined that an average 70-kg man has the fat stores to maintain basic caloric requirements for 2 to 3 months of fasting17–21 (Tables 37-1 and 37-2). However, as this threshold approaches, the body can no longer effectively or efficiently mobilize fat stores for fuel, and significant protein catabolism again becomes necessary for energy production.17
| ENERGY SOURCE | RESERVE* |
|---|---|
| Glucose | 1 h |
| Digestion | 4-8 h |
| Glycogen | 12 h |
| Amino acids | 48 h |
| Protein | 3 wks (if protein were the only fuel used for gluconeogenesis) 24 wks (obligatory loss only) |
| Triglycerides | 8 wks |
* These estimates are based on 100% utilization of each fuel.
Data from Shils ME. Modern nutrition in health and disease, 9th ed. Philadelphia: Lea & Febiger, 1998; White A, Handler P, Smith EL. Principles of biochemistry, 6th ed. New York: McGraw-Hill, 1978; Montgomery R, Dryer RL, Conway TW, Spector AA. Biochemistry: a case-oriented approach, 6th ed. St Louis: CV Mosby, 1996; Nutrition reviews’ present knowledge in nutrition, 5th ed. Washington, DC: Nutrition Foundation, 1984:439-453.
During feeding, the conversion of fatty acids to acetyl coenzyme-A (CoA) is regulated by the availability of L-glycerol 3-phosphate (derived from glucose through the glycolytic pathway). As the concentration of acetyl CoA rises, it is resynthesized into triglycerides, with L-glycerol 3-phosphate serving as the accepter to which three acyl CoA groups are attached (through esterification). Conversely, during fasting, there is inadequate glucose to provide the needed glycerol for triglyceride synthesis, resulting in acetyl CoA levels in excess of the oxidative capacity of the Krebs cycle. The excess is then shunted into the synthesis of ketone bodies.22
Research using respiratory quotient and urinary nitrogen studies has repeatedly shown that triglycerides are the major fuel during fasting.17–23 Inadequate blood glucose in fasting prompts hydrolysis (lipolysis) of triglycerides within adipocytes, allowing fatty acids and glycerol to leave the cell. The fatty acids are transported in a physical complex with albumin to the liver, muscle, and other tissues. Fatty acid oxidation results in large quantities of ketones secreted into the blood stream, usually noted on urinalysis by day three.24 These ketone bodies (acetoacetic acid, acetone, and β-hydroxybutyric acid) are utilized by the heart and, in fasting, by the brain for energy production.2 Because the ketone bodies are acids, their entry into the plasma results in a rise in hydrogen ions. This change is buffered by the conversion of bicarbonate into carbonic acid and then to carbon dioxide, which is exhaled. In extended fasts, the buffering capacity is surpassed and the plasma pH decreases, leading to mild metabolic acidosis with a compensatory increase in respiratory rate with noted electrolyte imbalance.22
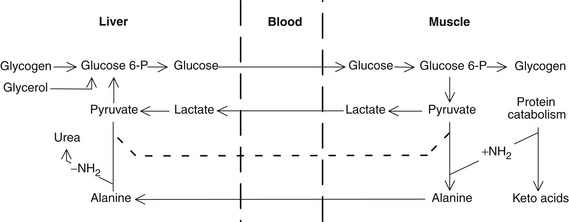
The initial physiologic response to fasting is the liver’s increased release of glucose to maintain adequate blood levels as undigested calories are exhausted in 4 to 8 hours. After only 12 hours, the liver’s glycogen stores become exhausted, and blood glucose is maintained by gluconeogenesis from triglyceride glycerol in fat reserves as well as from glucogenic amino acids and primarily from lactate (Cori) and alanine cycle from muscles.25,26 Interestingly, muscles contain more glycogen than the liver, but lack the enzyme required to convert glycogen to glucose (D-glucose-6-phosphatase). Through the Cori cycle, stored energy is shuttled as lactate to the liver and then used by body systems as glucose, where as in the feeding state, it would commonly be shuttled back to the muscles19 (Figure 37-1). As the fast proceeds, the kidneys become progressively more important in the maintenance of blood glucose levels, and eventually, the renal cortex synthesizes more glucose from amino acids than does the liver.16 Note that glucose is also recycled by the breakdown of blood cells in the liver.19–21
Under normal feeding conditions, the energy requirement of the mature brain is met almost entirely by glucose. Because the glycogen content of the brain is very low (0.1%), there is essentially no brain glucose reserve. Although the brain converts to oxidation of β-hydroxybutyrate after 4 to 7 days, there is still an obligatory need for approximately 80 g/day of glucose for the brain, red cells, muscles, and other tissues (400 to 600 kcal/day of glucose).24,27 Approximately 16 g of glucose is synthesized from triglyceride glycerol, with the rest of the glucose requirement (and the other metabolic processes requiring amino acids, such as enzyme turnover) being met by the catabolism of 18 to 24 g/day of protein.
All amino acids are glucogenic (with the exception of leucine, which appears to be a regulator of protein turnover in muscle),28 but alanine plays a prominent role analogous to lactate in the Cori cycle.25,26 The alanine cycle provides the mechanism for the recycling of a fixed supply of glucose and the effective transportation to the liver of amino acid nitrogen derived from muscle breakdown. Because muscle, unlike the liver, is incapable of synthesizing urea, most of the amino nitrogen from protein breakdown is transferred to pyruvate to form alanine. The alanine enters the blood and is taken up by the liver. The amino groups are removed to form urea, and the resulting pyruvate is converted to glucose. The newly synthesized glucose is secreted into the blood, taken up by the muscle, and catabolized to pyruvate to reseed the alanine cycle.22
Specific physical changes during fasting include decreases in body weight, pulse,3,18,29 and blood pressure (BP),3,18,30,31 and a drop in the basal metabolic rate by about 1% per day until stabilizing at about 75% of normal.31 Other cardiac adaptations noted on an electrocardiogram present as sinus bradycardia, decreased QRS complex and T-wave amplitude, elongation of the QT interval, and shifts to the right of the QRS and T-wave axes. These changes return to normal with return to food,3,29,31,32 similar to those animals that have prescribed adaptive mechanisms and hibernation cycles.
 Research
Research
Research into fasting has been reported since 1880, with the earliest record of therapeutic fasting in the medical literature appearing in 1910. The earliest research was primarily observational, as physiologic and metabolic changes were recorded while an individual fasted—Tanner (40 days in 1880),33 Jacques (30 days in 1887 and 40 days in 1888),34 Penny (30 days in 1905),35 and Levanzin (31 days in 1912).18
In 1923, the classic Fasting and Undernutrition provided in-depth analysis of animal and human physiologic changes and reactions during fasting by Morgulis at the University of Nebraska.30 In 1950, Ancel Keys3 at the University of Minnesota compiled two volumes entitled The Biology of Human Starvation describing the detailed observations of 32 volunteers who fasted for up to 8 months with comparisons to food deprivation observations made during the Second World War. Perhaps the most important observation was that fasting did not cause vitamin or mineral deficiencies. Related starvation research in developing countries noted that those who fasted completely lived longer than those on protein-deficient diets.16
Since these groundbreaking works, published clinical studies on therapeutic fasting have demonstrated benefit in almost every organ system. The following is a partial list of diseases and conditions that are beneficially influenced by fasting: chemical poisoning, cardiovascular disease and hypertension, diabetes, epilepsy, obesity, pancreatitis, and immune/inflammatory conditions (all expanded upon), as well as asthma, lumbago, depression and psychosomatic diseases, neurogenic bladder, irritable bowel syndrome, dysorexia nervosa (impaired or deranged appetite),36 neurosis and schizophrenia,37 parasites,38 duodenal ulcers,39 uterine fibroids,40 varicose ulcers,41 thrombophlebitis,42 eczema,3,43 and psoriasis.3,44,45
Appendicitis
A case report, published in 2011, of a patient advised of mainstream treatment for appendicitis refused surgery to try medically supervised water-only fasting. Pre-fasting ultrasound confirmed inflammatory dilation of the appendix, which was found to be relieved post-fasting by negative clinical and ultrasound findings with no return of symptoms at 2-year follow-up.46
Chemical Poisoning
Another encouraging finding for the use of fasting was published in the American Journal of Industrial Medicine in 1984. This study involved patients who had ingested rice oil contaminated with polychlorinated biphenyls. All patients reported improvement in symptoms, and some experienced “dramatic” relief, after undergoing 7- to 10-day fasts.47 This research supported past studies conducted by Inamura with polychlorinated biphenyls poisoned patients and suggested a detoxification effect of fasting.
Cardiovascular Disease
Studies of the effects of fasting on patients with heart disease began in the early 1960s. Duncan et al44 noted improvements in hypertension and chronic cardiac disease. Others also found fasting to be beneficial in heart disease: Gresham,48 Lawlor,42 Imamura,47 and Vessby.49 Improvements noted included reductions in serum triglyceride values, BP, atheromas, and total cholesterol levels; increased ratio of high-density lipoprotein cholesterol to total cholesterol; and alleviation of congestive heart failure.3,24,48,50–53
In the June 2001 issue of the Journal of Manipulative and Physiological Therapeutics, Goldhamer et al54 reported on a study involving medically supervised water-only fasting in the treatment of hypertension. In this evaluation of 174 consecutive patients with high BP, all patients were able to achieve BP sufficient to eliminate the need for medication, and more than 90% became normotensive. In patients with Stage III hypertension (systolic BP greater than 180 mm Hg) the average reduction in systolic BP exceeded 60 points. This is the largest effect ever published in the scientific literature. Nine months later, Goldhamer et al55 reported on a study involving 68 consecutive patients with borderline high BP. The average ending BP in these subjects was 99 mm Hg systolic/67 mm Hg diastolic. In a letter to the editor published in Journal of Alternative and Complementary Medicine in December 2002, Goldhamer56 described initial results in 30 patients with high BP participating in a residential health education program that included the supervision of water-only fasting for an average of 14 days. BP, weight, and cost of treatment and medications were compared for the year before and the year after fasting. Preliminary data demonstrated sustained clinical improvement in terms of BP reduction and weight reduction and an average reduction in combined medical and drug costs of almost $2700 per year per subject.
Diabetes
Guelpa recorded the benefits of fasting in type 2 diabetes and gout as well as in inflammation and after surgery.57 The treatment of diabetes with fasting was further explored by Allen in 1915. He noted that rest and fasting usually stopped glycosuria, and he also observed improvements in gangrene and carbuncles.58 In 1950, Keys also noted improvement in diabetic patients.3 Over the last 25 years, type 2 diabetics have successfully fasted, with subsequent reduction or elimination of required medications through successful long-term follow-up, given appropriate lifestyle maintenance post-fasting.59
Epilepsy
The treatment of seizures through fasting began in the early 1900s in France by Guelpa and Marie.60 In 1924, Hoeffel and Moriarty61 described fasting’s beneficial effects in epilepsy. In 1928, concurring with other researchers, Lennox62 found that the induction of ketosis via fasting decreased the duration, severity, and number of seizures.
Immune and Inflammatory Disorders
The beneficial effect of fasting on certain autoimmune diseases was reported in Lancet in 1958. The researchers found that fasting shortened the early stages of acute glomerulonephritis (reduced glomerular filtration rate, high BP, and edema), thus improving prognosis. They concluded that “all patients with acute glomerulonephritis should fast.”63 Other autoimmune diseases that have responded to fasting are rosacea, systemic lupus erythematosus, chronic urticaria, and colitis.40,64,65
The subject of arthritis and fasting has received substantial attention in the scientific literature, with most of the research coming from Scandinavia. Scientists documented the anti-inflammatory effects of fasting with observations of decreases in the erythrocyte sedimentation rate (ESR), arthralgia, pain, stiffness, and need for medication.43,66–72 Consistent with those findings, a 1984 U.S. study of 43 patients with definite or classic rheumatoid arthritis found significant improvements in grip strength, pain, swelling of proximal interphalangeal joints, ESR, and functional activity after a fast of 7 days.69
A strong link between arthritis and food intolerance has been revealed through fasting (see also Chapter 205). The diminution in symptoms of rheumatoid arthritis during fasting may be due to the decrease in gut permeability that accompanies fasting,68 which would reduce the absorption of antigenic molecules into the blood from the gastrointestinal tract. In a 1984 study in Bulletin on Rheumatic Diseases, Panush73 proposed the following theories: (1) nutritional modification might alter immune responsiveness and thereby affect manifestations of rheumatic diseases, and (2) that rheumatic disease may be a manifestation of a food allergy or hypersensitivity.
Fasting, in conjunction with food challenging, is now being used as a diagnostic test to determine food intolerances. Patients fast for a minimum of 4 days, and then individual foods are given to determine whether a reaction occurs. This method correlates well with skin prick and radioallergosorbent testing. A letter in a 1984 Lancet issue states, “When food avoidances prevent headaches, [irritable bowel syndrome], arthralgia and depression, it is more effective and less costly than traditional treatment and the observation also throws light on the etiology of the disorder.”74
Obesity
Fasting for obesity has probably received more attention in the scientific literature than any other aspect. The earliest studies were conducted by Folin and Denis,50 who in 1915, advocated short fasts as a safe and effective way to lose weight. Bloom,75 Duncan et al,44,45 Drenick et al,51,76 and Thompson et al52 published numerous works on the use of short and long fasts in obesity. Perhaps the most famous study on obesity appeared in the Postgraduate Medical Journal of 1973, which reported the experience of a 27-year-old man who fasted without complications for 382 days and lost 276 pounds.53
In general, initial weight loss during fasting is approximately 0.30% of body weight per day, with a gradual decrease to 0.10% per day after 30 days. The initial weight lost is primarily that of water, glycogen, and salt. For every pound lost, the body loses approximately 140 g of protein and 250 g of fat.24
Although fasting is very effective for weight reduction, fasting alone, without counseling and other lifestyle modifications, does not ensure long-term maintenance of the lower body weight. This fact is well documented in a study of 121 obese patients who were monitored for 7.3 years after fasts that averaged 2 months. After 2 to 3 years, 50% of patients returned to their pre-fast weights, and by the end of the study, 90% weighed the same as before their fasts.77
Pancreatitis
In a 1984 randomized clinical trial (n = 88), fasting was determined to be the treatment of choice for patients with acute pancreatitis. The researchers suggested that “fasting alone be initially used as the simpler and more economical therapy.” They found that “neither nasogastric suction nor cimetidine offer any advantage over fasting alone in the treatment of mild to moderate acute pancreatitis of any etiology.”78
 Application
Application
Therapeutic fasting is best conducted under supervision at an inpatient facility. Such facilities will exercise care in terminating a fast and supervising post-fast recuperation, monitor laboratory values in light of fasting physiology, review imaging as necessary, and demonstrate prudence when using adjunctive therapies during fasting. Several facilities now exist in the United States, Canada, England, and Australia, and these centers follow the standards of care and principles of ethics established by the IAHP.15
It is important to recognize that therapeutic fasting can be viewed to have three stages or phases. Stage I fasting (also called early or turbulent fasting) lasts up to 7 days, as the body adapts to fasting physiology with the common symptoms of excessive cellular waste or slowed elimination—malaise, headaches, and muscle aches—that are generally transitory, but can be disconcerting. Only the most genetically capable detoxifiers or the least toxic individuals glide comfortably through this stage. After passing through this period, it is more “the mind” rather than “the body” that continually obsesses on eating. Stage II, or balanced fasting, is the most significant fasting stage, as remodeling of tissues promotes healing. It can last for weeks to months. Patients often experience one or more “healing crisis” (where chronic conditions/symptoms become acute) and/or go through less significant detoxification reactions. Stage III fasting is often called depletion fasting (or simply “starvation”) as the body can no longer effectively or efficiently mobilize fat stores. Although many old texts refer to “fasting to completion” (i.e., exhaustion of nutrient reserves), this practice is now uncommon and not usually necessary,79 as serial fasting after appropriate refeeding appears to be a safer alternative.
When we fast, we recreate the natural occurrence of our ancestors when a “late spring” meant depleted food reserves followed by unintentional fasting while waiting for the snow to melt and the new shoots to rise for sustenance. Today, voluntary and attended therapeutic fasting provides the opportunity to mobilize or peel back the layers of our structural and functional adaptations within and between cells that are no longer required or beneficial. Therefore, like an onion that may have a superficial bruise, during a fast, the body “peels the onion” by deconstructing the buttressing (“the disease”) back to a healthy vital core or foundation that health can be built upon. Thus, a health promoting diet is pivotal to growth and maintenance of healing, and further, even with shorter annual fasts, allows less symptomatology and the ability to retain vitality throughout the year.59
Unfortunately, determining optimal fast length is difficult to predict and includes many factors, including size of reserves, individual metabolism, financial limitations, work schedule, severity of disease, age, and sex. This decision is based on all factors, especially the patient’s mental state. Experience shows that extending a fast beyond a healing crisis (or noteworthy detoxification reaction) provides significant clinical benefit where stopping midcourse suppresses healing until the same symptomatology appears, albeit earlier, in the course of a subsequent fast. Overall, “[the] doctor will look for good practical recovery where patient is symptom free and signs of regeneration are present.”79
General Principles
The use of a low salt, vegan, high fiber, low fat, low protein, and low sugar diet before and after fasting has been found beneficial and avoids complications of fasting. This diet also promotes pre-fast bowel movements and subsequent post-fast bowel movements that are sooner to evolve, easier to pass, and less problematic. (To commence fasting, bowel movement frequency must be at least daily.) Using broth or fruit and other juices are examples of restricted diets. They do not initiate fasting metabolic processes since they contain carbohydrates, protein, and/or fat. Nonetheless, restricted diets are often useful before and after fasting and for patients in whom a healing crisis develops during a fast or when a fast is contraindicated.80 In most cases, a fast is superior to the restricted diet because (1) hunger almost totally disappears,3,44 (2) ketosis occurs more quickly and efficiently,3,44 (3) famine edema does not occur,3 (4) sodium diuresis is more pronounced,75 (5) weight loss is more dramatic and is from fat rather than protein stores, (6) healing time is shorter, and (7) patient strength may be greater.81
Exercise while fasting is discouraged. Fuel conservation is necessary to allow maximal healing and the avoidance of unnecessary gluconeogenesis.81,82 The body utilizes certain muscle proteins early in a fast, thus initiating the natural restriction of activity. Short walks or light stretching is permissible, but intense exercise inhibits repair and elimination. In serious chronic disease, an excess of activity has been suspected as cause of death during fasting.83 Even moderate activity can double caloric utilization.84
Pure water (distilled, filtered, or reverse osmosis) is recommended.81,82 Researchers of fasting obese individuals often recommend 3 L/day,24 and 64 to 96 oz/day is commonly adequate, although upwards of 160 oz is commonly ingested without affecting serum sodium status. Although increased water intake will allow for less detoxification reactions during days of greater mobilization, excess consumption will cause electrolyte depressions that are clinically significant and require refeeding. Physiologically, the body is able to modulate “available water” through reduced obligatory water excretion (owing to lower excretion of urea, the major osmotic solute) and by access to released water from catabolized fat.16
Laboratory tests such as a complete blood count and serum chemistry screen are usually performed weekly and others are performed as necessary. Regular urine tests are performed and vital signs are checked daily.77,85
Upon refeeding there is a sudden shift from a low insulin, fat-burning metabolism to a high insulin, glucose-based state. As the plasma insulin rises, potassium, phosphate, and magnesium are driven intracellularly and sodium extracellularly, acting to expand and dilute the circulating volume. Sodium restriction during refeeding therefore should be emphasized to not precipitate dilution, edema, or acute heart failure.86
 Laboratory Values
Laboratory Values
Most laboratory values for body fluids during fasting do not follow specific patterns, but are unique to the individual and the disease process.32,52 Assessment of a fasting patient’s progress is based not on a sign or symptom, but on the total clinical picture. Although specific predictions of laboratory values during fasting are not possible, some general observations have been made.
Urinalyses may be difficult to interpret during fasting, because the body discards considerable waste via the kidneys.77 It is not uncommon to see various types of casts, red blood cells, white blood cells (WBC), bilirubin (+1 to +2), protein (trace, +2), and ketones (+4), and, if liver disease is present, urobilinogen elevation. Trace leukocytes and blood are common incidental findings, particularly in women. Specific gravity is commonly elevated (possibly to 1.035), a finding that may reflect inadequate hydration.85
Complete blood counts usually show no significant change.77 Low hemoglobin and hematocrit values have been observed,42,85,87 requiring rule out of hemolysis or hemorrhage,88 whereas elevations in hematocrit, hemoglobin, and red blood cell count usually indicate reduced hydration.77,85,89 WBC counts are usually unchanged or decrease slightly with fasting; however, infection may cause an increase. Further, WBCs may increase, particularly if levels are low before fasting.88
All of the electrolytes have reductions over the long term as the body’s mobilized stores are lost, but it is significant to note the ability to redistribute stores even with only distilled water during extended fasts. Serum electrolyte levels are not good indicators of tissue stores, but they are considered the most important blood values during fasting,88 because they usually do not change significantly during fasting unless there is a concern that needs management.
The total body store of sodium is 83 to 97 g (of which 65% is exchangeable), and that of potassium is 115 to 131 g (of which 98% is exchangeable). The typical daily dietary intake of sodium is 3 to 7 g, and of potassium, 3 to 5 g.90 During early fasting, the body loses 150 to 250 mEq (3.5 to 5.8 g) of sodium and 40 to 45 mEq (1.6 to 1.8 g) of potassium a day; later, these values drop to 1 to 15 mEq (0.02 to 0.35 g) and 10 to 15 mEq (0.4 to 0.6 g), respectively. Serum potassium usually decreases (but may become elevated) and values less than 3 mEq/L or above 6 mEq/L often require breaking of the fast. The electrolytes calcium and chloride are usually stable, but levels often diminish, especially if vomiting is present.88
Liver enzyme values may increase considerably if liver disease is present and may rise even if liver disease is not present. Triglyceride, cholesterol, and uric acid levels usually rise during fasting,85,91,92 indicating mobilization of tissue stores. Post-fast values often show a decrease from pre-fast values,49,61,91 but lipid panels will not show a new baseline value until 4 to 6 weeks post-fast. The serum protein value usually declines with fasting. Pancreatic lipase and amylase values also usually drop with fasting.88
A rise in blood urea nitrogen (BUN) value may occur, but some authorities have observed a decrease.3,62,42 The serum creatinine value may be elevated49,85 or may remain stable,93 and depression requires prompt retesting or fast termination. Closely monitor creatinine for elevations, particularly in those with renal compromise. Blood glucose values drop in most patients,43,58,61,62 possibly below 30 mg/dL. If the blood glucose value is low before fasting, it may rise after fasting. ESR and C-reactive protein usually drop after fasting, although they may rise during the fast.68,70,85
Hormonal changes during fasting typically consist of decreases in insulin16,20,23,32,94 and thyroid hormone levels.29,32,95 Increased levels of growth hormone,29,32,95 cortisol,89 glucagon,16,20 plasma norepinephrine,96 serum melatonin,96 and certain prostaglandins (in animals) usually occur.97 In contrast, a decrease in growth hormone is usually found in obese individuals.32 In one study conducted in 10 postmenopausal women who underwent short-term fasts, no significant changes in adrenal hormones, androgens, serum and urinary estrogens, plasma epinephrine, or dopamine were recorded.96
After a 44 day fast, the following changes were noted in less commonly ordered bloodwork—elevations: cortisol, somatostatin, insulin-like growth factor–binding protein 1; very low levels: leptin, ghrelin, insulin-like growth factor 1; normal values: peptide YY, agoutirelated peptide, α-melanocortin-stimulating hormone, neuropeptide Y, and pro-opiomelanocortin.98
Adjunctive Care
Dietary Supplements
Loss of minerals or vitamins is usually not a concern, and deficiencies during fasting are rare. Problems such as nausea and indigestion were reported when mineral and vitamin supplements were taken during fasting.76,93 For example, it is well known that nicotinic acid supplementation inhibits the release of free fatty acids from adipose tissue.24 In one patient in whom vitamin deficiency was reported in the medical literature, the actual fasting protocol was not described; in addition, the patient’s physical activity was not restricted and oral medication for intercurrent illness was maintained during fasting.76 Vitamin and mineral excretion becomes very low after 10 days.
Enemas
Enemas are not usually necessary and do not generally offer any added benefit during fasting.66 Some authorities have found that enemas also cause discomfort.81 To help prevent constipation, pre-fast meals of only fresh fruit and vegetables for at least 2 days will assist in establishing proper elimination before the fast is initiated. Lack of bowel movement 3 to 5 days post-fast (particularly on longer fasts) may point to the need for stewed prunes with meals until resolution or an enema becomes necessary.
Intravenous Therapy
Intravenous administration requires much care and is best avoided entirely, except for emergent conditions. Saline should be avoided due to plasma expansion and edema, which has precipitated acute heart failure. Glucose, in contrast, should be accompanied by vitamin B1 and B6 co-administration to avoid acute thiamine deficiency and lactic acidosis.99
 Contraindications
Contraindications
Contraindications to fasting are few, and each case must be judged individually, because no two cases are alike. For example, an inexperienced practitioner may assume that emaciated patients should not fast, but about this issue Shelton81 had the following to say: “Extreme emaciation: In such cases a long fast is impossible. A short fast of 1-3 days may be found beneficial, or a series of such short fasts with longer periods of proper feeding intervening may be found advisable.”
Contraindications to fasting include severe anemia, porphyria, and serious malnutrition. Individuals with a rare fatty acid deficiency of the enzyme medium-chain acyl-CoA dehydrogenase should also avoid fasting.40
The fasting of children and pregnant women is controversial. Although a short fast is appropriate for the sick child who does not want to eat, fasting in a pregnant woman may be strongly contraindicated; ketosis in pregnant diabetic women is known to be associated with fetal damage. Although this association is commonly recognized, the fact that this information has come only from research of diabetic pregnant women is not as widely known.100 There appear to be no studies of the effects of nondiabetic ketosis on fetal development. Doctors (e.g., Shelton, Benesh, Sidwha, and Burton) with considerable experience of fasting pregnant women during all three trimesters have found no adverse effects with fasts of a few days to 2 to 3 weeks. Although the fasting of pregnant women appears from clinical observation to be safe, definitive pronouncement cannot be made until careful research is performed (such as a controlled retrospective analysis of existing cases).
Fasts for children and pregnant women should be shorter and should be meticulously supervised by an experienced doctor. In The Science and Fine Art of Fasting, Shelton81 wrote, “Few infants require more than 2-3 days of fasting. … I have never hesitated to permit a sick infant to fast and I have yet to see one harmed by it.” Regarding pregnancy he said, “The author would object to a long fast in chronic ‘disease’ during this period. There can, however, be no objection to a short fast.”81
It is well recognized that fasting during lactation is not generally advised, because milk flow is halted by fasting and is difficult to resume.81 Although fasting is considered inappropriate in renal insufficiency,24 we have seen patients with 65% renal function return to normal as a result of fasting and dietary management.
With regard to fasting contraindications in general, Burton79 stated:
Supervised fasting as a therapeutic procedure is generally safe and effective. The incidence of death at fasting institutions is low, a fact that is promising because many of the patients have serious chronic diseases and have exhausted other therapeutic options. Of the hundreds of cases of fasting described in the scientific literature, only seven cases of death were reported before 1985.36,83,101–104 In all cases, the patients had serious chronic disease before fasting, and in five of the seven cases, drugs were given to the patients while fasting; in the other two, no description of protocol was provided.
There is no evidence in the scientific literature to suggest that fasting itself can be considered a cause of death. Death during fasting indicates that the remedial efforts of the body have been overpowered by the pathologic process. This situation occurs in serious disease, whether the patient is eating or fasting. In examining the fallacy of attributing the cause of death to fasting, Stewart and Fleming105 wrote, “Fasting short of emaciation is not hazardous; if death results, reasons other than those of the fast should be considered before concluding that all supervised fasts should be discouraged.”
 Side Effects
Side Effects
Side effects of fasting are rarely serious, but fasting may uncover disease and reveal weaknesses that were previously subclinical.44 Discomfort during fasting may be due to withdrawal from stimulants, hypoglycemia, acidosis, elimination of wastes, and enhancement of repair. Patients may experience headaches, insomnia, skin irritations, dizziness, nausea, coated tongue, body odor, aching limbs, palpitations, mucous discharge, and visual and hearing disturbances. Hair growth is usually arrested, and skin may become dry and scaly. Most signs and symptoms are usually brief as the body works to remove the disease.
• A sudden drop in BP (possibly due to peripheral circulatory collapse)
• Rapid, slow, feeble, or irregular pulse
Fasting elevates serum uric acid values and uric acid excretion, and if fluid intake is insufficient, gout or renal stones may be precipitated.24,106
A few studies have discussed the development of Wernicke encephalopathy during prolonged fasting, but because it rarely occurs during therapeutic fasting, it is difficult to determine whether the condition is related to methodology. It is important, however, to acknowledge the importance of utilizing B vitamins, especially thiamine, when any fast is broken with intravenous glucose.107,108 The decision to terminate the fast should be based on the complete clinical picture and not on an isolated sign or symptom.
 Conclusion
Conclusion
The vast potential of therapeutic fasting is only beginning to be realized, although results have been recorded since the early 1900s.109–114 Considerable empiric study has been accomplished, and fasting is generally a safe, economical, and effective therapy. Extensive research revealed pervasive and important effects, including the enhancement of immune system function.70,89,115–118 Unfortunately, a lot of prejudice exists. When we fast or assist another in fasting, perhaps the deepest human instinct is struck at depth—our need to eat, to survive, to exist. This often prevents otherwise intelligent and open-minded individuals from considering the facts regarding fasting and its unique benefits to human evolution and even health. There have been tragic deaths from fasting (almost exclusively inappropriately supervised), but fasting is not synonymous with starvation as our “instinctual self” would like to have us believe. Individual centers have successfully supervised the fasts of over 7000 people without fatalities over the last 27 years,59 and as more appreciate the “healing power of nature,” this therapy will become more mainstream.
1. Pollard K.S. What makes us human? Sci Am. 2009;300:44–49.
2. Wilson J.D., Braunwald E., Isselbacher K.J., et al. Harrison’s Principles of Internal Medicine, 12th ed., New York: McGraw-Hill, 1991.
3. Keys A., Brozek J., Henschel A., et al. The biology of human starvation, vols. 1 and 2. Minneapolis: University of Minnesota Press; 1950.
4. Arbesmann R. Fasting and prophecy in pagan and Christian antiquity. Traditio. 1951;7:1–71.
5. MacDermot V. The cult of the seer in the Ancient Middle East: a contribution to current research on hallucinations drawn from Coptic and other texts. Berkeley, CA: University of California Press; 1971.
6. Muhammad A. The religion of Islam: a comprehensive discussion of the sources, principles and practices of Islam. Lahore, India: The Ahmadiyya Anjuman Isha’at Islam; 1936.
7. Burns D. The greatest health discovery. Chicago: Natural Hygiene Press; 1972.
8. Shelton H.M. Some fasting history. Shelton’s Hygienic Review. 1964;XXV:291–293.
9. Shelton H.M. Rubies in the sand. San Antonio, TX: Shelton’s Health School; 1961.
10. Numbers R.L. Prophetess of health: a study of Ellen G White. New York: Harper & Row; 1976.
11. Weiss H.B., Kemble H.R. The great American water-cure craze: a history of hydropathy in the United States. NJ: The Past Times Press; 1967.
12. Shelton H.M. Natural hygiene: man’s pristine way of life. San Antonio, TX: Shelton’s Health School; 1968.
13. Shyrock R.H. Medicine in America: historical essays. Baltimore: Johns Hopkins Press; 1966.
14. American National Hygiene Society, 12816 Race Track Road, Tampa, FL, 33625.
15. Mark A. Huberman. IAHP Secretary/Treasurer Atty. Personal communication. 2005.
16. Young V.R., Scrimshaw N.S. The physiology of starvation. Sci Am. 1971;225:14–21.
17. Lehninger A. Biochemistry: the molecular basis of cell structure and function. New York: Worth Publishing; 1964. 841-845
18. Benedict F.G. A study of prolonged fasting. Publication #203. Washington, DC: Carnegie Institute; 1915.
19. Saudek C.D., Felig P. The metabolic events of starvation. Am J Med. 1976;60:117–126.
20. Cahill G.F., Jr., Owen O.E., Morgan A.P. The consumption of fuels during prolonged starvation. Adv Enzyme Regul. 1968;6:143–150.
21. Cahill G.F., Jr., Owen O.E. Starvation and survival. Trans Am Clin Climatol Assoc. 1968;79:13–20.
22. Montgomery R., Dryer R.L., Conway T.W., et al. Biochemistry: a case-oriented approach, 6th ed., St Louis: CV Mosby, 1996.
23. Felig P., Owen O.E., Morgan A.P., et al. Utilization of metabolic fuels in obese subjects. Am J Clin Nutr. 1968;21:1129–1133.
24. Shils M.E. Modern nutrition in health and disease, 9th ed., Philadelphia: Lea & Febiger, 1998.
25. Felig P., Pozefsky T., Marliss E., et al. Alanine: key role in gluconeogenesis. Science. 1970;167:1003–1004.
26. Mallette L.E., Exton J.H., Park C.R. Control of gluconeogenesis from amino acids in the perfused rat liver. J Biol Chem. 1969;244:5713–5723.
27. Reinmuth O.M., Scheinberg P., Bourne B. Total cerebral blood flow and metabolism. Arch Neurol. 1965;12:49–66.
28. Buse M.G., Reid S.S. Leucine, a possible regulator of protein turnover in muscle. J Clin Invest. 1975;56:1250–1261.
29. Theorell T., Kjellberg J., Palmblad J. Electrocardiographic changes during total energy deprivation (fasting). Acta Med Scand. 1978;203:13–19.
30. Morgulis S. Fasting and undernutrition; a biological and sociological study of inanition. New York: EP Dutton & Company; 1923.
31. Consolazio C.F., Nelson R.A., Johnson H.L., et al. Metabolic aspects of acute starvation in normal humans: performance and cardiovascular evaluation. Am J Clin Nutr. 1967;20:684–693.
32. Kerndt P.R., Naughton J.L., Driscoll C.E., et al. Fasting: the history, pathophysiology and complications. West J Med. 1982;137:379–399.
33. Annon, Dr. Tanner’s fast. BMJ. 1880;2:171.
34. Paton D.N., Stockman R. Observations of the metabolism of man during starvation. Proc R Soc Edinburgh. 1888-89;16:121–131.
35. Penny F. Notes on a thirty day’s fast. BMJ. 1909;1:1414–1416.
36. Suzuki J., Yamauchi Y., Horikawa M., et al. Fasting therapy for psychosomatic disease with special reference to its indications and therapeutic mechanism. Tohoku J Exp Med. 1976;118:245–259.
37. Boehme D.H. Preplanned fasting in the treatment of mental disease: survey of the current Soviet literature. Schizophr Bull. 1977;3:288–296.
38. Milet V., Spencer M.J., Chapin M., et al. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Dig Dis Sci. 1983;28:335–339.
39. Johnston D.A., Wormsley K.G. The effects of fasting on 24-h gastric secretions of patients with duodenal ulcers resistant to ranitidine. Aliment Pharmacol Ther. 1989;3:471–479.
40. Fuhrman J. Fasting and eating for health. New York: St. Martin’s Press; 1995.
41. Spencer I.O. Death during therapeutic starvation for obesity. Lancet. 1968;1:1288–1290.
42. Lawlor T., Wells D.G. Metabolic hazards of fasting. Am J Clin Nutr. 1969;22:1142–1149.
43. Lithell H., Bruce A., Gustafsson I.B., et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397–403.
44. Duncan G.G., Jenson W.K., Cristofori F.C., et al. Intermittent fasts in the correction and control of intractable obesity. Am J Med Sci. 1963;245:515–520.
45. Duncan G.G., Duncan T.G., Schless G.L., et al. Contraindications and therapeutic results of fasting in obese patients. Ann N Y Acad Sci. 1965;131:632–636.
46. Gershfeld N., Goldhamer A., Sultana P. A case of non-pharmacological conservative management of suspected uncomplicated sub-acute appendicitis in an adult male. J Alt Comp Med. 2011;17:1–3.
47. Imamura M., Tung T. A trial of fasting cure for PCB poisoned patients in Taiwan. Am J Ind Med. 1984;137:147–153.
48. Gresham G.A. Is atheroma a reversible lesion? Atherosclerosis. 1976;23:379–391.
49. Vessby B., Boberg M., Karlstrom B., et al. Improved metabolic control after supplemented fasting in overweight type 2 diabetic patients. Acta Med Scand. 1984;216:67–74.
50. Folin O., Denis W. On starvation and obesity with special reference to acidosis. J Biol Chem. 1915;21:183–192.
51. Drenick E.J. Contraindications to long term fasting. JAMA. 1964;188:88.
52. Thompson T.J., Runcie J., Miller V. Treatment of obesity by total fast for up to 249 days. Lancet. 1966;2:992–996.
53. Stewart W.K., Fleming L.W. Features of a successful therapeutic fast of 382 days’ duration. Postgrad Med J. 1973;49:203–209.
54. Goldhamer A.C., Lisle D.J., Parpia B., et al. Medically supervised water-only fasting in the treatment of hypertension. J Manipulative Physiol Ther. 2001;24:335–339.
55. Goldhamer A., Lisle D., Sultana P., et al. Medically supervised water-only fasting in the treatment of borderline hypertension. J Altern Complement Med. 2002;8:643–650.
56. Goldhamer A. Initial cost of care results in medically supervised water-only fasting for treating high blood pressure and diabetes. J Altern Complement Med. 2002;8:696–697.
57. Guelpa G. Starvation and purgation in the relief of diabetes. BMJ. 1910;2:1050–1051.
58. Allen F.M. Prolonged fasting in diabetes. Am J Med Sci. 1915;150:480–485.
59. Goldhamer A. Personal communication on 1/18/2011.
60. Kerndt P.R., Naughton J.L., Driscoll C.E., et al. Fasting: the history, pathophysiology and complications. West J Med. 1982;137:379–399.
61. Hoeffel G., Moriarty M. The effects of fasting on the metabolism. Am J Dis Child. 1924;28:16–24.
62. Lennox W.G., Cobb S. Studies in epilepsy. Arch Neurol Psych. 1928;20:711–779.
63. Brod J., Pavkova L., Fencl V., et al. Influence of fasting on the immunological reactions and course of acute glomerulonephritis. Lancet. 1958;1:760–763.
64. Okamoto O., Murakami I., Itami S., et al. Fasting diet therapy for chronic urticaria: report of a case. J Dermatol. 1992;19:428–431.
65. Fuhrman J., Sarter B., Calabro D.J. Brief case reports of medically supervised, water-only fasting associated with remission of autoimmune disease. Altern Ther Health Med. 2002;8:110–111.
66. Skoldstam L., Larsson L., Lindstrom F.D. Effect of fasting and lactovegetarian diet on rheumatoid arthritis. Scand J Rheumatol. 1979;8:249–255.
67. Skoldstam L., Lindstrom F.D., Lindblom B. Impaired conA suppressor cell activity in patients with rheumatoid arthritis shows normalization during fasting. Scand J Rheumatol. 1983;12:369–373.
68. Sundqvist T., Lindstrom F., Magnusson K.E., et al. Influence of fasting on intestinal permeability and disease activity in patients with rheumatoid arthritis. Scand J Rheumatol. 1982;11:33–38.
69. Kroker G.F., Stroud R.M., Marshall R., et al. Fasting and rheumatoid arthritis: a multicenter study. Clin Ecol. 1984;2:137–144.
70. Uden A.M., Trang L., Venizelos N., et al. Neutrophil function and clinical performances after total fasting in patients with rheumatoid arthritis. Ann Rheum Dis. 1983;42:45–51.
71. Palmblad J., Hafstrom I., Ringertz B. Antirheumatic effects of fasting. Rheum Dis Clin North Am. 1991;17:351–362.
72. Kjeldsen-Kragh J., Mellbye O.J., Haugen M., et al. Changes in laboratory variables in rheumatoid arthritis patients during a trial of fasting and one-year vegetarian diet. Scand J Rheum. 1995;24:85–93.
73. Panush R.S. Controversial arthritis remedies. Bull Rheum Dis. 1984;34:1–10.
74. Gerrard J.W. Food intolerance. Lancet. 1984;2:413.
75. Bloom W.L. Fasting as an introduction to the treatment of obesity. Metabolism. 1959;8:214–220.
76. Drenick E.J., Swenseid M.E., Blahd W.H., et al. Prolonged starvation as a treatment for severe obesity. JAMA. 1964;187:100–105.
77. Scott DJ. Personal communication, 1986.
78. Navarro S., Ros E., Aused R., et al. Comparison of fasting, nasogastric suction and cimetidine in the treatment of acute pancreatitis. Digestion. 1984;30:224–230.
79. Burton A. Fasting too long. J Health Science. 1979;2:144–146.
80. Shelton H.M. Fasting for renewal of life. Chicago: Natural Hygiene Press; 1978.
81. Shelton H.M. The science and fine art of fasting. Chicago: Natural Hygiene Press; 1978.
82. Carrington H. Fasting for health and long life. Mokelumne Hill, CA: Health Research; 1963.
83. Kahan A. Death during therapeutic starvation. Lancet. 1968;1:1378–1379.
84. Cahill G.F. Famine symposium: physiology of acute starvation in man. Ecol Food Nutr. 1978;6:221–230.
85. Goldhamer A. Personal communication, 1986.
86. Korbonits M., Blaine D., Elia M., et al. Metabolic and hormonal changes during the refeeding period of prolonged fasting. Eur J Endocrinol. 2007;157:157–166.
87. Rooth G., Carlstrom S. Therapeutic fasting. Acta Med Scand. 1970;187:455–463.
88. Cinque R. Hematological changes during fasting. IAHP Newsletter. 1993;7:6–8.
89. Palmblad J., Cantell K., Holm G., et al. Acute energy deprivation in man. Effect on serum immunoglobulins, antibody response, complement factors 3 & 4, acute phase reactants and interferon producing capacity of blood lymphocytes. Clin Exp Immunol. 1977;30:50–55.
90. Nutrition reviews’ present knowledge in nutrition, 5th ed.. Washington, DC: Nutrition Foundation. 1984:439–453.
91. Valenta L.J., Elias A.N. Modified fasting in the treatment of obesity. Postgrad Med. 1986;79:263–267.
92. Ende N. Starvation studies with special reference to cholesterol. Am J Clin Nutr. 1962;11:270–280.
93. Rapoport G.L., From A., Hudson H. Metabolic studies in prolonged fasting: inorganic metabolism and kidney function. Metabolism. 1965;14:30–47.
94. Spark R.F., Arky R.A., Boulter P.R., et al. Renin aldosterone and glucagon in the natriuresis of fasting. N Engl J Med. 1975;292:1335–1340.
95. Harrison M.T., Harden R.M. The long-term value of fasting in the treatment of obesity. Lancet. 1966;2:1340–1342.
96. Beitins I.Z., Barkan A., Kiblanski A., et al. Hormonal responses to short term fasting in postmenopausal women. J Clin Endocrinol Metab. 1985;60:1120–1126.
97. Kim Y.C., Brodows R.G. Starvation stimulates pancreatic PGE content. Prostaglandins. 1983;25:365–371.
98. Korbonits M., Blaine D., Elia M., et al. Refeeding David Blaine–studies after a 44-day fast. N Engl J Med. 2005;353:2306–2307.
99. Centers for Disease Control and Prevention. Lactic acidosis traced to thiamine deficiency related to nationwide shortage of multivitamins for total parenteral nutrition–United States, 1997. JAMA. 1997;278:109–111.
100. Churchill J.A., Berendes H.W., Nemore J. Neuropsychological deficits in children of diabetic mothers. Am J Obset Gynecol. 1969;105:257–268.
101. Cubberley P.T., Polster S.A., Schulman C.L. Lactic acidosis and death after treatment of obesity by fasting. N Engl J Med. 1965;272:628–630.
102. Garnett E.S., Barnard D.L., Ford J., et al. Gross fragmentation of cardiac myofibrils after therapeutic starvation. Lancet. 1969;1:914–916.
103. Norbury F.B. Contraindication to long term fasting. JAMA. 1964;88:208.
104. Runcie J., Thompson T.J. Prolonged starvation-a dangerous procedure. BMJ. 1970;3:432–435.
105. Stewart W.K., Fleming L.W. Fragmentation of cardiac myofibrils after therapeutic starvation. Lancet. 1969;1:1154.
106. Drenick E.J. Hyperuricemia, acute gout, renal insufficiency and urate nephrolithiasis due to starvation. Arth Rheum. 1965;8:988–997.
107. Devathansen G. Wernicke’s encephalopathy in prolonged fasting. Lancet. 1982;2:1108–1109.
108. Falzi G., Ronchi E. Wernicke’s lethal encephalopathy in voluntary, total prolonged fasting. Forensic Sci Int. 1990;47:17–20.
109. Dewey E.H. The no-breakfast plan and the fasting-cure. New York: The Health Culture Co; 1900.
110. MacFadden B. Fasting for health: a complete guide on how, when and why to use the fasting cure. New York: MacFadden; 1923.
111. Hazzard L.B. Scientific fasting. New York: Grant Publications; 1927.
112. De Vries A. Therapeutic Fasting. Los Angeles: Chandler Book Co; 1963.
113. Shelton H.M. Fasting can save your life. Chicago: Natural Hygiene Press; 1964.
114. Oswald J.A., Shelton H.M. Fasting for the health of it. Pueblo, CO: Nationwide Press; 1983.
115. Wing E.J., Boehme S.M., Barczynski L.K. Effects of acute nutritional deprivation on immune function in mice I. Macrophages. Immunology. 1983;48:543–550.
116. Wing E.J., Stanko R.T., Winnkelstein A., et al. Fasting-enhanced immune effector mechanisms in obese subjects. Am J Med. 1983;75:91–96.
117. Friend P.S., Fernandes G., Good R.A., et al. Dietary restrictions early and late: effects on the nephropathy of NZB X NZW mouse. Lab Invest. 1978;38:629–632.
118. Miller J.D. Life extension. N Engl J Med. 1985;313:760–761.