CHAPTER 100 Failed Disc Replacement
Despite a more than 20-year history of total disc replacements worldwide, detailed analyses of results and complications from comprehensive, prospective randomized studies have only become available through the U.S. investigational device exemption (IDE) trials.1–5 Box 100–1 is a comprehensive list of reported failures to date organized by mode/type of failure. Note that Box 100–1 does not include the myriad of approach-related complications including dysphagia, esophageal perforation, vocal cord paralysis, carotid artery injuries, retrograde ejaculation, ureteral injury, or large vessel injury. These complications are not unique to total disc replacements. They are inherent to anterior spinal surgery in general and have been reviewed elsewhere.6–8 The discussion that follows will review selected failures and focus on the prevention, evaluation, and management of these problems.
Failures of Lumbar Disc Replacement
Prevention of Failures
Benjamin Franklin’s profound words, “an ounce of prevention is worth a pound of cure,” cannot be overstated when discussing failures of the lumbar TDR. With current TDR designs, the lumbar spine is approached anteriorly for numerous reasons. These include (1) minimal morbidity allowing for short recovery times; (2) unobstructed visualization of the disc space, allowing for total discectomy and accurate implant sizing; (3) the absence of the need to enter or retract posterior neural structures in the spinal canal; and (4) the familiarity of territory for many spine and vascular surgeons. Accordingly, the approach-related risks are both predictable and relatively low.1–10 In contrast, revision approaches to the anterior lumbar spine have about a six times higher risk for major bleeding or thromboembolic complications due to adhesion formation, which prevents accurate identification of the great vessels.10 The anterior lumbar spine therefore remains a relatively facile approach as an index procedure but is fraught with potential complications in any revision situation. In our experience, many failures of lumbar disc replacement could have been avoided because they can be traced to surgeon-specific factors (as opposed to patient-specific factors) such as incomplete discectomy, improper device insertion, or inappropriate indications.
Total Discectomy
Rauschning has demonstrated that the bone at the periphery of the vertebral body endplate is strongest and all TDR implants have been designed to be seated along this peripheral rim of bone.11 Performing a complete or radical discectomy therefore becomes paramount for accurate total disc sizing and endplate placement. Implants that are too anterior or do not achieve good “rim fit” are more prone to dislodge or subside. A fundamental impediment to complete discectomy is poor visualization of the disc space. Regan and colleagues12 have demonstrated the importance of the “learning curve” for optimal outcomes for CHARITÉ TDR. Access surgeons and spinal surgeons who are uncomfortable or unfamiliar with the anterior approach to the lumbar spine will be less likely to retract the great vessels far enough to permit complete exposure of the lateral aspect of the disc. In contrast to lumbar interbody fusion, a relatively larger window into the intervertebral disc space is required to properly perform total disc replacement. We routinely use handheld Hohmann retractors, which pierce the vertebral bodies above and below the disc. Large Steinmann pins can serve this purpose as well. This technique of retraction prevents the iliac vessels and vena cava from gradually creeping into the surgical field, thus narrowing the exposure of the intervertebral disc. If one prefers to use fixed table–based retractor systems, then it is wise to continuously assess and reassess the field of exposure to ensure that adequate visibility is maintained to assist complete disc space preparation.
Sizing, Lordosis, and Placement
Maintaining lumbar lordosis is intuitive in terms of spinal balance in the sagittal profile; however, with lumbar disc replacement, especially L5-S1, maintaining and matching the patient’s lordosis is imperative to reduce shear and achieve good endplate contact, thereby reducing the risk of anterior dislodgement or dislocation. If the disc space lordosis measures 15 degrees, for example, but the prosthetic lordosis only reaches a maximum of 10 degrees, one or both of the prosthetic endplates will not be in complete contact with the host vertebral endplate. Assuming the prosthesis can match the 15 degrees of lordosis, it is even more favorable to have a mechanism by which the articulating portion of the prosthesis can assume a more horizontal (parallel to the floor when standing) position. One way to achieve this is to “build up” the caudal prosthetic endplate with a 10-degree or more lordotic endplate. By “horizontalizing” the disc space, the magnitude of the shear forces at the articulating surfaces is reduced but will still be significant at the caudal bone-implant interface. A note of caution might be in order for patients who have an especially steep sacral inclination of the S1 endplate. In conjunction with these concerns, L5-S1 presents with additional and theoretical risks for postoperative spondylolisthesis. The subject of facet orientation introduces the idea that if the facets are more in the sagittal plane rather than the coronal plane, there is a predisposition of future destabilization over time and increase in facet stress in flexion and extension. This has not been directly studied due to the limitation of cadaveric specimens with their respective facet orientation. Additionally, as Rousseau and colleagues showed in a comparison of constrained and semiconstrained disc arthroplasties with intact specimens, facet forces vary during flexion-extension and lateral bending. He found that one implant tended to unload the facets in extension and to a lesser degree in lateral bending, which was attributed to the implant’s instantaneous axis of rotation. The other tended to increase in these areas. Also, the flexion forces on the facets were not statistically different than for the intact specimen with a potted sacral inclination of 60 degrees.13
In the first U.S. IDE to reach completion, McAfee and colleagues3 demonstrated that inaccurate positioning of the lumbar TDR was statistically correlated with reduced patient outcome.
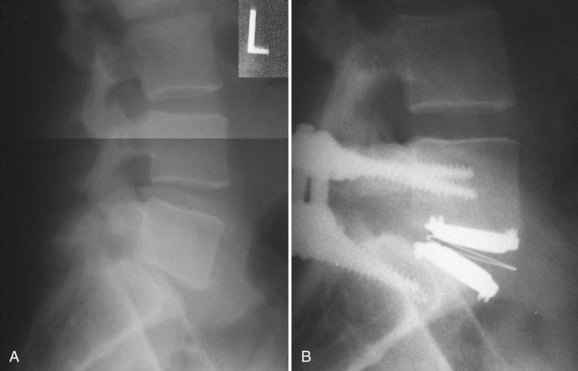
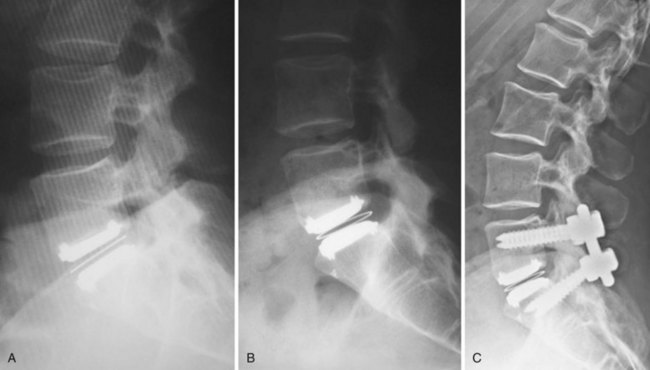
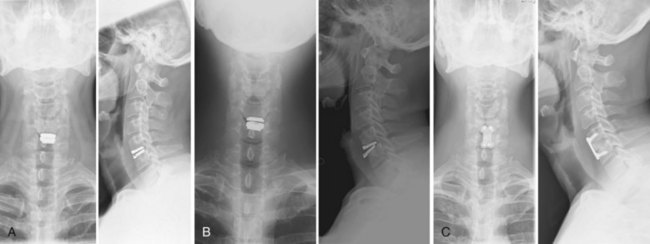
Appropriate patient selection may be the most important factor in reducing the risk of failures. Strict adherence to the inclusion and exclusion criteria from the U.S. IDE trials such as patients with osteoporosis, metabolic bone disease, history of chronic steroid use, or age older than 60 years should reduce the risk of TDR endplate subsidence. Failure to recognize an isthmic spondylolisthesis is another known reason for failure (Fig. 100–1). In the U.S. CHARITÉ trial there were five unrecognized pars defects for fractures that became apparent at the 6-week follow-up visit.10 In four of the five cases the defect was appreciable after retrospective scrutiny of the preoperative flexion and extension images was performed. One such case demonstrated migration of the device requiring posterior pedicle screw instrumentation and fusion. In the fifth case, bilateral pedicle fractures occurred in the postoperative setting and this case was revised to an anterior interbody fusion with posterior pedicle screw-rod instrumentation and fusion. While patients with a degenerative spondylolisthesis of less than 3 mm are not excluded from U.S. IDE trials, we prefer not to perform TDR on patients with more than 2 degrees of intervertebral translation. Computed tomography (CT) is the study of choice to evaluate the presence of an isthmic spondylolisthesis when the diagnosis is in question on preoperative flexion-extension radiographs or magnetic resonance imaging (MRI). CT scans will also reveal the degree of facet arthrosis better than MRI, which might also exclude certain candidates from lumbar TDR.
Failures (see Box 100–1)
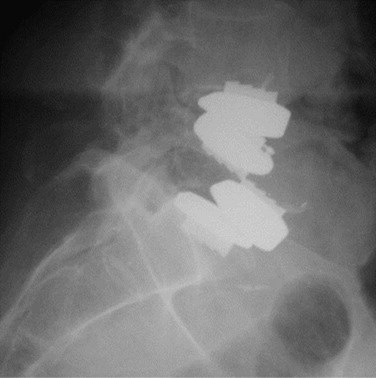
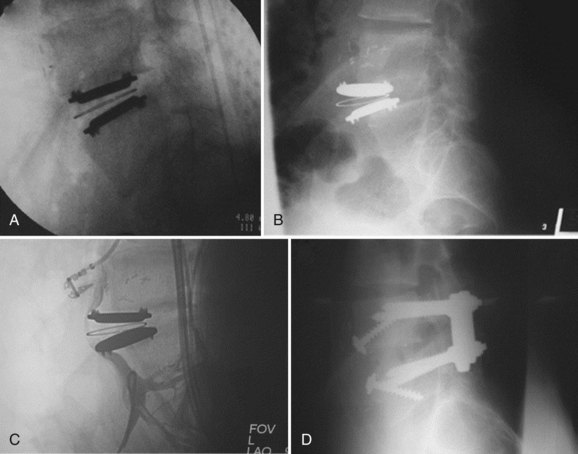
Implant failures such as polyethylene core fractures, core dislocations, or implant breakage have been uncommon occurrences thus far. Our knowledge of them derives from case reports or small case series.14–17 Instances of excessive polyethylene wear have been quite rare and have uniformly occurred in patients implanted before 1997. David reported a case of polyethylene failure due to oxidation 9.5 years following implantation.14 Similarly, as reported in the largest series of TDR failures reported to date, there was one case of detectable polyethylene core wear noted 12 years postoperatively.17 Since 1997, an industry-wide enhancement to the sterilization process of polyethylene, whereby irradiation occurs in an inert gas such as nitrogen rather than air, has resulted in dramatically improved wear characteristics due to a reduction in the incidence of oxidation. This corrective preventative action of avoiding polymerization and reducing the UHMWPE wear rate was born out of the total joint implant experience on partially cross-linked UHMWPE. In contrast to the previously mentioned cases, analysis of a retrieved polyethylene core from a revision surgery performed for bone-implant loosening 1.6 years postoperatively in a patient implanted post-1997 demonstrated low levels of oxidation with mechanical properties that were not substantially degraded.18 At the time of this writing, there are only two published cases of anterior dislocation of the polyethylene inlay of a ProDisc artificial disc replacement.16 A case of posterior core dislocation presented to our clinic (Fig. 100–2). There are no reported cases of metal endplate fatigue failures or deformation for modern prosthetic designs.
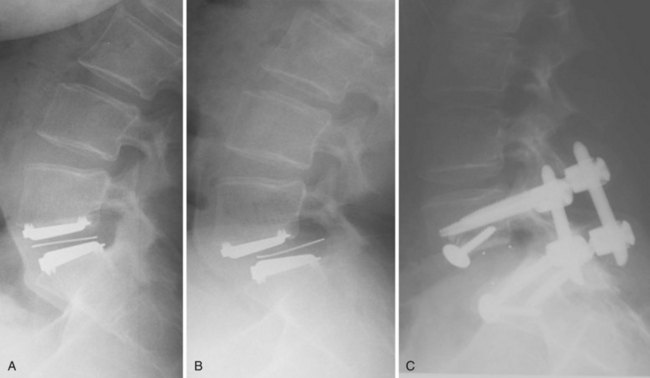
Bone-implant failures account for the greatest number of failures of lumbar disc replacement, and the mode of failure depends on the type of device. Sagittal vertebral body fractures can occur with keeled devices because the keel slot creates a stress riser in the bone (Fig. 100–3).19 Two-level implantations in which keel slots are introduced into the superior and inferior aspects of the intercalated vertebra are at highest risk for this complication. To reduce this risk, the MAVERICK keel has been reduced from 11 mm to 7 mm.20 In contrast, implants with smaller “anchors” at the bone-implant interface such as the CHARITÉ, in which six 3-mm teeth engage the vertebral endplate, exhibit a greater tendency to migrate or dislodge if improperly placed. In the CHARITÉ U.S. IDE study, there were 15 of 347 implantations that required removal and none of these had been inserted in the “ideal” position.3 Regardless of the design, TDR placement anterior to the center of rotation, especially in a hyperlordotic segment, will tend to migrate or dislocate anteriorly due to excessive shear.21 As previously mentioned, spondylolysis and spondylolisthesis present a biomechanically less stable environment for TDR and explain some of the early failures by migration (Fig. 100–4). Damage to the vertebral endplate during insertion, placement of a TDR in an osteoporotic spine, inaccurate positioning, and insertion of an implant that is too small are all factors that can contribute to TDR subsidence. In van Ooij and colleagues’17 report of 27 complications of the CHARITÉ device, there were 16 cases of subsidence. The cause, prevalence, or incidence of these failures could not be determined because the study was retrospective without mention of the total number of cases. Strict adherence to indications, surgeon education, and modern insertion instrumentation systems should theoretically reduce the incidence of this complication.
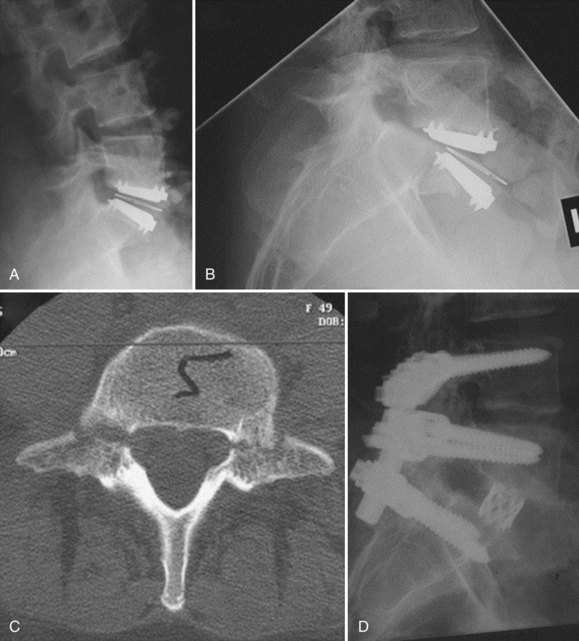
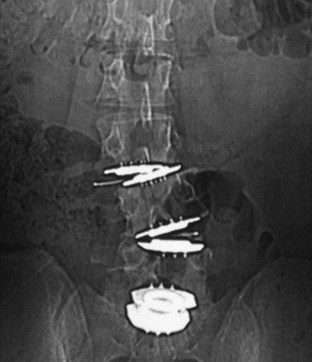
Iatrogenic deformity has a greater tendency to occur in multilevel implantations (Fig. 100–5). Preoperative scoliosis is a relative contraindication to TDR; however, subtle coronal and sagittal-plane deformities may not be taken into account. TDR insertions in these scenarios will likely exacerbate rather than reduce any deformity because stabilizing structures such as the anterior and posterior longitudinal ligament, as well as the majority of the annulus, are removed during the implantation. Even in the well-aligned spine, incomplete discectomy and “off axis” TDR placement at one level can create an “off-axis” situation at the next level (Fig. 100–6). Thus when more than one vertebral level is to be implanted, device placement errors will tend to be compounded at sequential levels of insertion, resulting in a “Z deformity.” Kyphosis is not prevalent following TDR unless the implant is inserted backwards (i.e., a lordotic device inserted in kyphosis) or severe subsidence of the implant into the posterior half of the vertebral body.
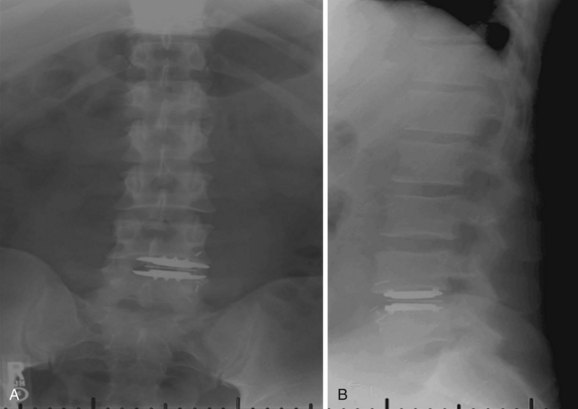
FIGURE 100–6 A and B, CHARITÉ disc replacement placed at an outside facility with off-axis placement.
Host response to particulate debris generated from virtually any articulating biomaterial is characterized by macrophage recruitment and proinflammatory cytokine release. The culmination of this cascade is matrix metalloprotease activation, which leads to bone resorption and osteolysis. Osteolysis is a well-documented complication of total joint replacement22,23; however, most investigators agree that it will not be prevalent following TDR. One reason for the potentially reduced risk in the spine is the fact that the intervertebral disc lacks a surrounding synovial space, the key source for macrophages and other inflammatory cells. The second reason is the relatively reduced range of motion and hence reduced volumetric wear in TDR compared with typical diarthrodial joint replacement. With more than 20 years of global experience and more than 10,000 TDR implantations worldwide, there are only anecdotal reports of osteolysis, and to our knowledge only one has been documented histologically.10 Heterotopic ossification (HO) surrounding lumbar TDR implants has been classified previously by McAfee and colleagues24 in preparation for the CHARITÉ U.S. IDE trial. In this trial, the incidence of HO was 4.3%; however, the presence of HO did not have any impact on flexion-extension range of motion or clinical outcome.25 We know of no other lumbar TDR trial in which the presence of HO has been systematically evaluated; however, isolated cases of periannular ossifications have been reported. To be fair, however, the imaging characteristics of the lumbar devices, which are typically cobalt-chrome or stainless steel alloys, do not allow for identification of all but the larger amounts of periannular bone formation.
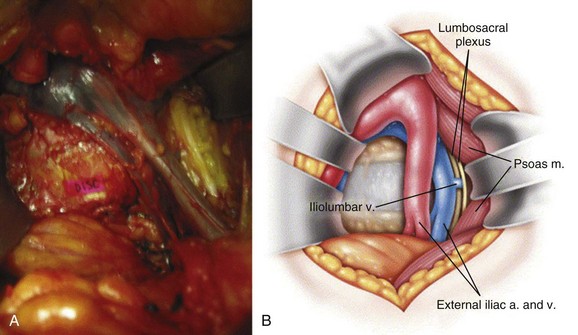
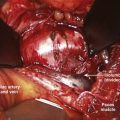
Neurologic injury has not been shown to be more prevalent in cases of lumbar TDR when compared with fusion controls in prospective randomized evaluation.26 Most cases of revision surgery in the CHARITÉ U.S. IDE trial involved reoperation with laminectomy and foraminotomy for new neurologic symptoms in the lower extremities. After any anterior discectomy and instrumentation, patients should be asked if they have any “new” pain in their legs that was not present preoperatively and a thorough neurologic examination should be performed in the postanesthesia care unit. If there are any new neurologic complaints or physical examination findings (i.e., motor or sensory loss), a CT scan with myelographic contrast should be performed to assess positioning of the implant and ensure that a hematoma, bone fragment, or disc material is not present in the spinal canal. MRI is not particularly helpful postimplantation due to metal artifact obscuring the regions of greatest interest. By rapidly diagnosing the cause for new neurologic symptoms with appropriate imaging, early revision such as implant repositioning is possible. Neurologic injury without radiographic abnormalities is a complication that typically presents with left-sided leg pain in the L4 or L5 dermatome. First reported by Thalgott and colleagues, this complication has been thought by some to be due to excessive retraction of the lumbar plexus during the retroperitoneal exposure.27 The lumbar plexus is at highest risk when exposing the L4-5 disc space. To mobilize the left common iliac vein to the patient’s right side, the iliolumbar vein(s) needs to be identified and ligated as it courses dorsally between the lumbar nerve roots located under the psoas muscle (Fig. 100–7). Alternatively, such symptoms have been thought to be sympathetic mediated and result from the vascular exposure because similar symptoms may be seen following nonspinal vascular dissections. In either case, whatever the etiology, the dysesthetic pain pattern typically resolves after 6 to 8 weeks; however, corticosteroid selective nerve root blocks may help minimize the symptoms. Notwithstanding, the burden of proof remains to demonstrate that the acquired symptoms are not due to new nerve root compression, which may be remedied with further surgery.
Supraphysiologic motion is a phenomenon that is not a prevalent mode of failure for TDR but is likely underdiagnosed. It is defined as motion exceeding the natural motion arc in six degrees of freedom from the native motion in a specific patient’s functional spinal unit. This motion results in subclinical instability that the body senses and in turn attempts to combat. This can present with postoperative muscle spasms and unexplained and chronic postoperative pain. It is a diagnosis of exclusion. The TDR is typically in excellent position without signs of dislodgement, dislocation, or subsidence. The hallmark is significant hypertrophy of the facet joints (Fig. 100–8). Because the workup algorithm follows closely to ruling out spondylolysis, a CT scan can be helpful in characterizing this hypertrophy. To determine surgical candidacy following exhaustive conservative management, which typically includes long-acting narcotics, local facet blocks are the major objective tool to assess pain relief. Care must be taken to counsel the patient to give feedback following the injections to pay close attention to the mere hours of relief that may ensue corresponding to the half-life of bupivacaine hydrochloride (Marcaine). If the patient notices a significant decrease in pain, a stabilizing procedure in the form of a posterolateral fusion with instrumentation can be discussed as an option of treatment. There is presently no way of predicting which patients will be affected by this phenomenon. Some surgeons have pointed to facet orientation as a predisposition. Especially at L5-S1, more sagittally oriented facets in conjunction with sacral inclination can predispose to increased facet forces and this mode of failure. When diagnosed properly with facet blocks, patients have had resolution of pain and spasms and were likewise weaned off of narcotics completely.
Revision Strategies
Revision total disc replacement is a potentially life-threatening procedure and should be reserved for indications that justify the increased risk (Box 100–2).
BOX 100–2 Contraindications of Cervical Disc Replacement
Timing
Revision anterior exposure within 2 weeks of TDR incurs relatively little additional morbidity because adhesion formation is minimal. For this reason, surgeons should have a low threshold for revising implants that are clearly malpositioned or exhibit early migration within this 2-week time frame. If the prosthesis can be repositioned or revised to another TDR, without damage to host bone, it seems reasonable to do so. Beyond this period of time, a revision strategy must be individualized to the particular clinical situation. A posterior instrumented fusion with or without a decompressive laminectomy is currently the most effective salvage procedure. Preoperative planning is critical to a successful anterior revision. The authors analyze the cause for failure to establish individual goals for the revision. Corrections such as polyethylene replacement, size, or position changes are relatively easy to anticipate. Appropriate patient counseling regarding the increased risks, potential for changes, or need to abort the original plan for safety considerations is also critical. Reviewing the initial operative reports, clinic notes, and original indications can be particularly enlightening if a revision to a TDR is contemplated. If a patient failed to meet indications for a TDR at the index procedure, it is unlikely that he or she will meet indications at the revision. Patients with significant host bone loss, deformity, or instability, along with any of the indications in Box 100–2, should be revised to an interbody arthrodesis.
TDR revision approaches depend on the reason for failure and the original surgical approach; however, the currently available options include posterior decompression, posterior decompression and instrumented fusion, anterior TDR removal and fusion, or anterior TDR removal and reinsertion. For patients meeting one of the criteria in Box 100–2, anterior TDR removal and fusion is the safest salvage strategy because gaining the exposure necessary to implant a new disc replacement is rarely possible due to adhesion formation (Fig. 100–9). Anterior interbody fusion devices typically require less exposure and can be inserted obliquely across the disc space. This can be extremely handy when the only accessible area to the disc is to one side. Revision to a TDR may be considered in the early (<2-week) postoperative period when exposure is excellent and an easily correctable problem such as inaccurate placement to anterior has been identified.
Given the aforementioned technical challenges, the most common salvage procedure for failed TDR is posterior instrumented fusion with pedicle screws. This technique essentially locks the prosthesis from any further movement and allows for bone grafting in the posterolateral intertransverse region. Cunningham and colleagues28 found that pedicle screws alone combined with a lumbar disc replacement were not found to be statistically different biomechanically from pedicle screws and a femoral ring allograft. Posterior hemilaminectomy or laminotomy without fusion is an alternative for focal disc or bone displacements into the spinal canal; however, extreme care must be taken to avoid destruction of stabilizing structures like the facet joints.
Conclusions
Recently in the literature, anterior cervical discectomy and fusion (ACDF) and cervical arthroplasty were found to be slightly superior to the results of total hip and total knee arthroplasty with respect to patient perceived outcomes.29 Considering that total joint arthroplasty has provided the standard by which most orthopedic procedures are compared, this contradicts previous stereotypes of mediocre outcomes in spine surgery. In addition, the complication rate of the anterior approach to the cervical spine is much lower than other orthopedic procedures, especially infection.30
Since the introduction of the Bryan Total Cervical Disc Prosthesis in 2000, many other discs have entered the market as viable options and are in widespread use today. There are two FDA-approved implants presently on the market with 19 others under study. Although ACDF continues to be the gold standard for treatment of cervical radiculopathy and myelopathy, cervical disc arthroplasty has proven to match the goals of ACDF including decompression of the spinal canal, disc height and alignment restoration, and cessation of neurologic compression. Because of this parallelism in goals, complications are similar to a degree—namely, approach-related complications, recurrence of symptoms, instrumentation malposition, fixation related, alignment related, or stability related. Conversely, with the introduction of a motion segment rather than fusion, there is a divergence of complications. Problems identified with ACDF include the incidence of adjacent segment disease, the need for perioperative immobilization, bone graft site morbidity (if used), and pseudoarthrosis. With arthroplasty, the obvious reasons are due to motion including supraphysiologic motion or hypermobility, traumatic instability, heterotopic ossification, and dynamic neural compression.31 The primary reason to choose cervical disc arthroplasty over fusion in the properly selected patient is maintenance of motion and avoidance of adjacent segment disease, which has been studied and reported, but long-term studies are pending.32
Prevention of Failures
Complications in cervical disc replacements are far from numerous. With most of our knowledge gleaned from prospective randomized IDE studies for new implants, long-term outcomes are lacking. Success of arthroplasty is truly related to the proper patient selection. A close second is technique of placement. The importance of the latter is confirmed by most arthroplasty implant companies requiring formal attendance to implant methodology and intraoperative placement training. The complication of infection and others related to the approach to the anterior cervical spine is not discussed in this manuscript. In U.S. IDE studies of the Bryan (Medtronic Sofamor Danek, Memphis, Tenn.); Prestige (Medtronic Sofamor Danek); and Prodisc-C (Synthes, Paoli, Pa.) cervical disc replacements, investigators have reported no surgical site infections in either the control (fusion) or investigational (disc replacement) groups.33–35 All three of these studies revealed that the most common reason for removal of cervical disc arthroplasty was for recurrent or new radiculopathy related to incomplete decompression.
The discussion of complications in any surgery is followed closely by a review of revision techniques for these diagnoses. Unlike anterior lumbar procedure revisions, cervical revisions have much less risk through the same incision.36 As would be thought, any revision approach increases the rate of complications. In the anterior cervical spine, this rate is already low for the index procedure and thus subsequent revision has been found to be 10% including infection.37 In our experience, many of the failures of anterior cervical disc replacement can be linked to iatrogenic factors like improper implant placement, insertion or sizing, and inappropriate indications.
Patient Selection
Proper patient selection cannot be overstated. A decision to operate can be just as detrimental to outcome as performing poor technique during device insertion. Absolute contraindications include present or previous trauma, morbid obesity, hypermobility, endocrine/autoimmune or metabolic bone abnormalities or related medications known to be detrimental to bone formation, severe facet arthrosis, metal allergy, DISH/ankylosing spondylitis, absence of motion, ossification of the posterior longitudinal ligament (OPLL), osteoporosis diagnosed by DEXA (T ≤ −2.5), and active malignancy (see Box 100–2). Additionally, as mentioned previously, this procedure is not intended for axial neck pain. The presence of radiculopathy and/or myelopathy must exist.
Verification of Midline, Sizing/Lordosis, and Placement
The ultimate goal of any arthroplasty prosthesis is to reproduce exactly the motion of the native joint. The functional unit of the spine is no different. This linkage with the posterior ligamentous complex and facet joints posteriorly and disc space anteriorly provide for a complex kinematic loading system that includes coupled motion. Motion is typically a combination of two planes. It has been documented that the center of rotation is not a fixed point but rather follows an elliptical path in the sagittal plane.38 These facts stress the importance of placing the implant in the midline of the endplates. Verification of midline is not a common practice when placing interbody fusion devices or grafts except when trying to make an x-ray appearance symmetric. Frankly, this can be an added step in ACDF surgery that is time consuming and unnecessary. Additionally, a device placed too far off of the midline can cause problems with the exiting nerve root or vertebral artery. This could introduce overdistraction on one side, causing a traction phenomenon, and stenosis on the other, away from the prosthesis, causing recurrent or new radicular symptoms. To add insult to injury, this could be a dynamic phenomenon because of the preservation of motion.
When sizing the implant, your goal is to use the largest footprint available. This occasionally requires slight squaring of the uncus-to-uncus distance when your initial trial has too much room on the lateral borders of the prosthesis and appears undersized. Sagittal plane sizing is not much of a problem due to judicious use of intraoperative fluoroscopy. The goal is to arrive at a placement of the device’s posterior edge that corresponds to the posterior edge of the vertebral body. This placement correlates with the proper placement of the center of rotation, which allows for coupled motion to recreate normal physiologic motion.38 As more cervical discs are placed and more reports are published, subsidence has become more of a theoretical issue. Lin and colleagues39 showed in a finite element study that implants with a keel or flanges may increase the propensity to subsidence due to high-contact stresses that could induce resorption of bone. This study also showed the importance of avoiding an undersized implant on the basis of increased stress concentrations per unit area. Lordosis preservation is important as well for maintenance of normal kinematics of the cervical spine. Implants come with differing degrees of lordosis to match the patient’s anatomy. Studies have shown that single-level segmental kyphosis can cause accelerated adjacent segment degeneration in fusion,40 but others have shown that it does not affect it. Pickett and colleagues41 reported 12 cases of preoperative lordosis with postoperative disc arthroplasty kyphosis but no long-term follow-up or short-term complications from this. The remainder of the cervical spine compensated for this single kyphotic segment and maintained an overall lordotic alignment.
Failures
The focus of this review is to point out implant-related failures of total disc arthroplasty. Complications that can occur commonly with ACDF procedures are not covered here because they are patient selection, neurologic recovery, as well as approach related. Because long-term outcome studies are lacking, there is a paucity of literature showing common methods of failure. Articles in the past have categorized the complications into time dependence with early and late.41,42 The most obvious failures are migration and loosening because they are found on routine follow-up imaging (Fig. 100–10). The predominance of these cases is asymptomatic. The exact cause is rarely known. Theoretical reasons include poor ingrowth, poor sizing, shear force of a whiplash injury, or endplate resorption coupled with subsidence. Most common out of these is likely poor sizing. Implants now have the importance of a press-fit size that is usually difficult to remove during the procedure. If implants are easily adjustable in the sagittal plane intraoperatively, they are likely undersized and have an increased chance of displacement in the future. Keels, teeth, and screw fixation of implants have all been improvements in this initial fit and subsequent risk of displacement. Cunningham and colleagues43 conducted a biomechanical pull-out strength test of multiple constructs that were compared with anterior plate and tricortical iliac crest. No correlation was noted with total surface area of the implant itself, but rather the presence of screws, which was strongest, as well as toothed ridges and keels, which were next in strength.
Vertebral body fracture is the most extreme case of loosening. Presently there is only one case report and many anecdotal reports of these in the cervical spine with multilevel adjacent implants. This complication is at most risk when a keel cut is required in the same vertebral body. Datta and colleagues44 presented a case with an intraoperative fracture of the intervening vertebral body in a two-level case. Postoperative CT showed a fracture in the superior-most vertebral body, as well as the middle vertebral body with two keel cuts. The authors cited dull chisels as the reason for fracture because there was at least 5 mm of space between the two keel cuts. This complication presumably gave birth to the side-cutting milling drill bit, which requires no mallet or force to produce a keel cut. The lumbar spine has a report of this complication by Shim and colleagues.19 Another type of fracture includes a posterior vertebral body fracture, perhaps referred to as a reverse tear drop because it is of the triangular piece of bone at the posterior edge of vertebral body. The mechanism is provided by too shallow keel cuts. In this situation, attempts are made at placing the implant in the optimal position under fluoroscopy, but the keel is bottomed out. Harder strikes of the mallet cause this fracture, which can displace fragments into the canal, causing compression. Shim and colleagues45 described this as an avulsion fracture, but this is a misnomer. This is a direct fracture of the vertebral body by the implant or use of keel chisel rather than a ligament pulling off the piece of bone. The use of side-cutting milling drill bits has decreased the risk of this complication, as well as finesse with implant placement. If the implant does not want to arrive at the optimum position, attempt to recut the keel.
Heterotopic ossification (HO) is another failure of cervical disc replacement. This complication, although not catastrophic, defeats the purpose of placing a motion-preserving device. The goal is to be able to predict who will develop it and perform an ACDF instead. Parkinson and colleagues46 presented the first case report on spontaneous fusion of cervical arthroplasty. A nonkeeled device was used. Lack of motion was noted immediately postoperatively on the basis of flexion-extension radiographs. The authors speculated that the cause was not directly known but because of the acute decrease in preoperative and postoperative motion that it was a device malfunction versus overdistraction and overstuffing the disc space. Mehren and colleagues found an incidence of 66% with any HO present following cervical disc arthroplasty in a prospective series of 77 keeled implants. Grade of HO was assessed at 1 year postoperatively on the basis of McAfee and colleagues’24 classification system described for the lumbar spine. Complete fusion (grade 4) was noted in 9%. Loss of motion and fusion is described as either 2 degrees or less of dynamic motion or change in the interspinous distance of 2 mm or less.48,49 Heller and colleagues50 found that not only did the ossification typically occur in the first 100 days of surgery but it was typically lateral near the uncovertebral joints. The authors recommended judicious use of nonsteroidal anti-inflammatories (NSAIDs) in high-risk patients. What is a high-risk patient? No evidence in the literature points to a specific set of variables that will predict HO. Perhaps in the older patient with mild global spondylosis, NSAIDs are indicated, but this introduces the potential for poor ingrowth into these implants, although this is theoretical. As the indication for disc replacement expands, implants are being placed in older, “borderline” patients who perhaps require an ACDF instead. More long-term studies that compare age with onset, as well as the use of a high-speed burr, need to be conducted.
Inflammation or hyaline cartilage formation after placement of a total cervical disc arthroplasty refers to a host response complication. The most obvious reason is inflammation from particulate debris. Wear characteristics of cervical disc replacements have been studied. Several studies have reported on the wear debris production and inflammatory effects of disc replacements. They all showed minimal debris and no inflammatory response after 40 million cycles in one.51,52 So although theoretical, wear debris has not been a problem to date in the literature. A 37-year-old woman treated with a disc replacement with a polyethylene articular component presented to our facility with increasing pain 9 months postoperatively. Her radiculopathy had subsided, but axial neck pain was increased from her preoperative state and required increasing amounts of narcotics. Her examination revealed lower extremity hyperreflexia and relative weakness 4/5 throughout the lower extremities. Imaging studies including a CT myelogram revealed no canal encroachment or myelomalacia. Infection indices were negative. The patient was convinced that the implant was causing the problems. After considerable counseling of the patient on risks of revision and encouragement to avoid another surgery, she wished to proceed with conversion to a fusion. During the case, neuromonitoring was used. Baseline EMGs revealed poor amplitudes throughout. Interestingly and surprisingly, at the moment the total disc replacement was removed, amplitudes increased instantaneously in all leads. Her postoperative course was characterized by improvement of her strength and reduced pain. In a similar case, Cavanaugh and colleagues53 reported a case characterized by radiographic evidence of a soft tissue mass 9 months after cervical disc placement with recurrence of radicular symptoms. Gross exploration revealed a thick layer of hyaline cartilage with histologic analysis revealing chronic inflammatory debris. Both cases display a possible metal hypersensitivity reaction rather than wear debris due to the short time course of the symptom onset.
Dynamic radiculopathy may present another complication. Jan Goffin42 addresses this type of complication when referencing malposition of implants in his review of cervical disc arthroplasty complications. With an undersized implant that is not placed in the midline, preferential loading may cause a coronal plan abnormality that causes the contralateral foramen to decrease in size, effectively causing a bony stenosis of the foramen or possible impingement on the vertebral artery. Symptoms would include recurrence of radiculopathy if on the same side as the previous disc herniation or new contralateral radiculopathy. A reduction of symptoms may be seen with lateral bending toward the offset side of the implant. This requires revision.
Acknowledgments
The authors acknowledge Bryan Cunningham for his assistance in the preparation of this manuscript.
Permissions
The lumbar portion of this manuscript was used with the publisher’s permission in its entirety with additions and corrections. Reprinted from Tortolani PJ, McAfee PC, Saiedy S: Failures of lumbar disc replacement, Semin Spine Surg 18:78-86, 2006, with permission from Elsevier.54
Pearls
Pitfalls
1 Blumenthal SL, McAfee PC, Guyer RD, et al. A prospective, randomized, multi-center FDA IDE study of lumbar total disc replacement with the CHARITÉ Artificial Disc vs. lumbar fusion: Part I. Evaluation of clinical outcomes. Spine. 2005;30:1565-1575.
2 Gornet MF, Matthews HH, Burkus JK: MAVERICK total disc replacement: A review of 12-month data from five investigational centers. Proceedings of the NASS 20th Annual Meeting, Philadelphia, 2005.
3 McAfee PC, Cunningham BW, Holtsapple G, et al. A prospective, randomized, multi-center FDA IDE study of lumbar total disc replacement with the CHARITÉ Artificial Disc vs lumbar fusion: Part II. Evaluation of radiographic outcomes and correlation of surgical technique accuracy with clinical outcomes. Spine. 2005;30:576-583.
4 Zigler JE. Clinical results with ProDisc: European experience and US investigational device exemption study. Spine. 2003;28:S163-S166. (suppl)
5 Zigler JE, Burd T, Vialle EN, et al. Lumbar spine arthroplasty: early results using the ProDisc II: a prospective randomized trial of arthroplasty versus fusion. J Spinal Disord Tech. 2003;16:352-361.
6 Brau SA. Mini-open approach to the spine for anterior lumbar interbody fusion: Description of the procedure, results, and complications. Spine. 2002;2:216-223.
7 Brau SA, Delamarter RB, Schiffman ML, et al. Vascular injury during anterior lumbar surgery. Spine. 2004;4:409-412.
8 Hynes R, Wasselle J, Velez D: Complications of the lumbar anterior surgical approach for artificial disc replacement of the lumbar spine. Proceedings of the NASS 20th Annual Meeting, Philadelphia, 2005.
9 Tropiano P, Huang RC, Girardi FP, et al. Lumbar disc replacement: Seven to eleven year follow-up. J Bone Joint Surg Am. 2005;87:490-496.
10 McAfee PC, Geisler FH, Saiedy S, et al. Revisability of the CHARITÉ Artificial Disc Replacement: Analysis of 347 patients enrolled in the US IDE Study of the CHARITÉ Artificial Disc. Roundtables in Spine Surgery, Complications and Revision Strategies in Lumbar Spine Arthroplasty. 2005;1:73-96.
11 Rauschning W. Pathoanatomy of lumbar disc degeneration and stenosis. Acta Orthop Scand. 1993;251:3-12. (suppl)
12 Regan JJ, McAfee PC, Blumenthal SL, et al. Evaluation of the learning curve and the importance of surgical volume for lumbar total disc replacement: An analysis of the prospective, randomized, multicenter FDA IDE study of the CHARITÉ artificial disc, in Roundtables in Spine Surgery. Complications and Revision Strategies in Lumbar Spine Arthroplasty. 2005;1:97-108.
13 Rousseau M, Bradford D, et al. Disc arthroplasty design influences intervertebral kinematics and facet forces. Spine J. 2006;6:258-266.
14 David T. Revision of a CHARITÉ artificial disc 9.5 years in vivo to a new CHARITÉ artificial disc: Case report and explants analysis. Eur Spine J. 2005;14:507-511.
15 Mather P, Blackman M, Redla S, et al. Bilateral pedicle fractures following anterior dislocation for the polyethylene inlay of the Prodisc artificial disc replacement. Spine. 2005;30:E311-E314.
16 Mayer HM, Wiechert K, Korge A, et al. Minimally invasive total disc replacement: Surgical technique and preliminary clinical results. Eur Spine J. 2002;11:S124-S130. (suppl)
17 Van Ooij A, Oner FC, Verbout AJ. Complications of artificial disc replacement: A report of 27 patients with the SB CHARITÉ disc. J Spinal Disord Tech. 2003;16:369-383.
18 Kurtz SM, Peloza J, Siskey R, et al. Analysis of a retrieved polyethylene total disc replacement component. Spine J. 2005;5:344-350.
19 Shim CS, Lee S, Maeng DH, et al. Vertical split fracture of the vertebral body following total disc replacement using ProDisc: Report of two cases. J Spinal Disord Tech. 2005;18:465-469.
20 Matthews HH. Complications and revision considerations of total disc arthroplasty: MAVERICK metal-on-metal prosthesis. Roundtables in Spine Surgery, Complications and Revision Strategies in Lumbar Spine Arthroplasty. 2005;1:109-116.
21 Aunoble S, Donkersloot P, Le Huec JC. Dislocations with intervertebral disc prosthesis: Two case reports. Eur Spine J. 2004;13:464-467.
22 Han CD, Choe WS, Yoo JH. Effect of polyethylene wear on osteolysis in cementless primary total hip arthroplasty: Minimal 5-year follow-up study. J Arthroplasty. 1999;14:714-723.
23 Maloney WJ, Galante JO, Anderson M, et al. Fixation, polyethylene wear, and pelvic osteolysis in primary total hip replacement. Clin Orthop. 1999;369:157-164.
24 McAfee PC, Cunningham BW, Devine J. Classification of heterotopic ossification (HO) in artificial disc replacement. J Spinal Disord Tech. 2003;16:384-389.
25 Tortolani PJ, Cunningham BW, McAfee PC: The incidence of heterotopic ossification in total disc replacement: A prospective, randomized, FDA study in 276 patients. Proceedings of the SRS, 39th Annual Meeting, Buenos Aires, Argentina.
26 Geisler FH, Blumenthal SL, Guyer RD, et al. Neurological complications of lumbar disc replacement and comparison of clinical results with those related to lumbar arthrodesis in the literature: Results of a multicenter, prospective, randomized investigational device exemption study of the CHARITÉ intervertebral disc. From the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:143-154.
27 Thalgott JS, Giuffre JM, Klezl Z, et al. Anterior lumbar interbody fusion with titanium mesh cages, coralline hydroxyapetite, and demineralized bone matrix as part of a circumferential fusion. Spine. 2002;2:63-69.
28 Cunningham BW, Hu N, Beatson HJ, Serhan H, Sefter JC, McAee PC. Revision strategies for single- and two-level total disc arthroplasty procedures: a biomechanical prospective. Spine J. 2009;9:735-743.
29 Anderson PA, Puschak TJ, Sasso RC. Comparison of short-term SF-36 results between total joint arthroplasty and cervical spine decompression and fusion or arthroplasty. Spine. 2009;34(2):161-166.
30 Gloystein DM, Tortolani PJ, Stambough JL: Complications of spine surgery: Spinal infections. Orthopaedic Knowledge Online. Submitted and accepted, awaiting e-publication.
31 Pimenta L, Diaz R, McAfee PC, et al. Cervical Disc Replacement Revisions: Clinical and Biomechanical Considerations. Motion Preservation Surgery of the Spine: Advanced Techniques and Controversies Ch 35:287-96. Philadelphia: Elsevier. 2008.
32 Pickett G, Mitsis D, Sekhon L, et al. Effects of a cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus. 2004;17:E5.
33 Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275-286.
34 Sasso RC, Smucker JD, Hacker RJ, Heller JG. Clinical outcomes of BRYAN cervical disc arthroplasty: A prospective, randomized, controlled, multicenter trial with 24 month follow-up. J Spinal Disord Tech. 2007;20:481-491.
35 Porchet F, Metcalf NH. Clinical outcomes with the Prestige II cervical disc: Preliminary results from a prospective randomized clinical trial. Neurosurg Focus. 2004;17:E6.
36 Sekhon LH. Reversal of anterior cervical fusion with a cervical arthroplasty prosthesis. J Spinal Disord Tech. 2005;18:S125-8.
37 Gloystein DM, et al: Comprehensive analysis of 80 cases of failed cervical instrumentation: Comparison of revision to fusion versus arthroplasty–total of 142 operative levels. Proceedings from the Spine Arthroplasty Society Meeting, London, 2009.
38 Ishii T, Mukai Y, Hosono N, et al. Kinematics of the subaxial cervical spine in rotation: In vivo three-dimensional analysis. Spine. 2006;31:155-160.
39 Lin C, Kank H, Rouleau JP, Hollister SJ, Marca FL. Stress analysis of the interface between cervical vertebrae end plates and the Bryan, Prestige LP, Prodisc-C disc prostheses: An in vivo image-based finite element study. Spine. 2009;34:1554-1560.
40 Katsura A, Hukuda S, Imanaka T. Anterior cervical plate used in degenerative disease can maintain lordosis. J Spinal Discord. 1996;9:470-476.
41 Pickett GE, Sekhon L, Sears WR, Duggal N. Complications with cervical arthroplasty. J Neurosurg Spine. 2006;4:98-105.
42 Goffin J. Complications of cervical disc arthroplasty. Semin Spine Surg. 2006;18:87-98.
43 Cunningham BW, Hu N, Zorn CM, McAfee PC. Comparative fixation methods of cervical disc arthroplasty versus conventional methods of anterior cervical arthrodesis: Serration, teeth, keels, or screws? J Neurosurg Spine. 2010;12:214-220.
44 Datta JC, Janssen ME, Beckham R, Ponce C. Sagittal split fractures in multilevel cervical arthroplasty using keeled prosthesis. J Spinal Disord Tech. 2007;20:89-92.
45 Shim CS, Shin HD, Lee SH. Posterior avulsion fracture at an adjacent vertebral body during cervical disc replacement with ProDisc-C: A case report. J Spinal Disord Tech. 2007;20:568-572.
46 Parkinson JF, Sekhon L. Cervical arthroplasty complicated by delayed spontaneous fusion. J Neurosurg Spine. 2005;2:377-380.
47 Reference deleted in proofs.
48 Goffin J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, et al. Preliminary clinical experience with the Bryan Cervical Disc prosthesis. Neurosurg. 2002;51:840-847.
49 Cannada LK, Scherping SC, Yoo JU, Jones PK, Emery SE. Pseudoarthrosis of the cervical spine: A comparison of radiographic diagnostic measures. Spine. 2003;28:46-51.
50 Heller JG, Tortolani PJ, Park AE, et al. Computed tomography (CT) scan assessment of paravertebral bone after total cervical disc replacement: Prevalence, temporal relationships, and the effects of NSAIDs. Proc Cervical Spine Res Soc. 2003.
51 Anderson P, Sasso R, Rouleau J, et al. The Bryan cervical disc: wear properties and early clinical results. Spine J. 2004;4:303s-309s.
52 Hu N, Cunningham BW, McAfee PC, et al. Porous coated motion cervical disc replacement: A biomechanical, histomorphometric, and biologic wear analysis in a caprine model. Spine. 2006;31:1666-1673.
53 Cavanaugh DA, Nunley PD, Kerr EJ, Werner DJ, Jawahar A. Delayed hyper-reactivity to metal ions after cervical disc arthroplasty: A case report and literature review. Spine. 2009;34:E262-5.
54 Tortolani PJ, McAfee PC, Saiedy S. Failures of lumbar disc replacement. Semin Spine Surg. 2006;18:78-86.