22 Facial nerve
Facial Nerve
The facial nerve arises from the branchial (special visceral) efferent cell column caudal to the motor nucleus of the trigeminal nerve (Figure 17.2). The facial nucleus occupies the lateral region of the tegmentum in the caudal part of the pons (Figures 17.15, 22.1). Before emerging from the brainstem, it loops, as the internal genu, around the abducens nucleus, creating the facial colliculus in the floor of the fourth ventricle.
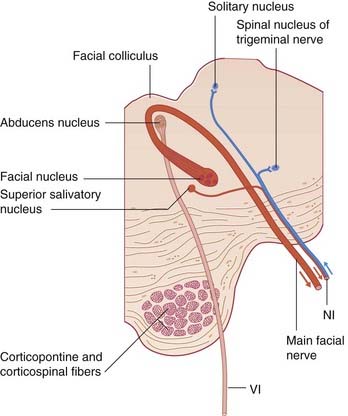
Figure 22.1 Transverse section of the pons, showing the facial nerve and the nervus intermedius (NI).
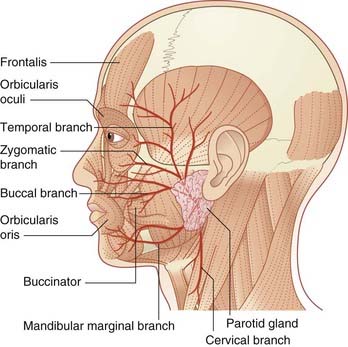
The nerve emerges at the lower border of the pons together with the nervus intermedius. Both nerves cross the subarachnoid space in company with the vestibulocochlear nerve, to the internal acoustic meatus. Above the vestibule of the labyrinth, it enters a 7-shaped bony canal having a backward bend at the external genu of the facial nerve. Prior to escaping the canal at the stylomastoid foramen, it supplies the stapedius muscle. Upon escape, it supplies the posterior belly of the occipitofrontalis, the stylohyoid, and the posterior belly of the digastric. It then turns forward within the substance of the parotid gland while breaking up into the five named branches to the muscles of facial expression (Figure 22.2).
Supranuclear connections
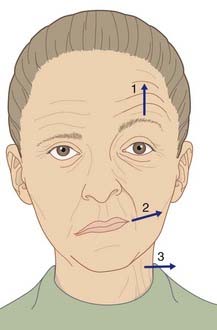
All of the cells of the motor nucleus receive a corticonuclear supply from the ‘face’ area of the contralateral motor cortex. In addition, those to the muscles of the upper face (occi-pitofrontalis and orbicularis oculi) receive an equal supply from the ipsilateral motor cortex. The bilateral supply for the upper facial muscles is reflected in their habitual paired activities in wrinkling the forehead, blinking, and squeezing the eyes closed. The muscles around the mouth, on the other hand, are often activated unilaterally for some expressive purpose. The partial bilateral supply to the facial muscles helps to distinguish a supranuclear from a nuclear or infranuclear lesion of the nerve (Clinical Panel 22.1).
Clinical Panel 22.1 Lesions of the facial nerve
Nuclear lesions
The main motor nucleus may be involved in thrombosis of one of the pontine branches of the basilar artery. As might be anticipated from the relationships depicted in Figure 22.1, the usual result of such a lesion is an alternating (crossed) hemiplegia: complete paralysis of the facial and/or abducens nerve on one side combined with motor weakness of the limbs on the opposite side owing to concomitant involvement of the corticospinal tract.
Infranuclear lesions
Four out of five patients recover completely within a few weeks because the nerve has only suffered a conduction block (neuropraxia). In the remainder, the nerve undergoes Wallerian degeneration (Ch. 9); recovery takes about 3 months and is often incomplete. During regeneration, some preganglionic fibers of the nervus intermedius may enter the greater petrosal nerve instead of the chorda tympani, with the result that the lacrimal gland becomes active at mealtimes (so-called ‘crocodile tears’).
Other causes of infranuclear palsy include a patch of demyelination within the pons in the course of multiple sclerosis (Figure CP 22.1.1), tumors in the cerebellopontine angle (Clinical Panel 22.2), middle ear disease, and tumors of the parotid gland. Herpes zoster oticus is a rare but well-recognized viral infection of the geniculate ganglion. Severe pain in one ear precedes a vesicular rash in and around the external acoustic meatus. Swelling of the geniculate ganglion may result in a complete facial palsy (Ramsay Hunt syndrome).
The muscles of facial expression are more responsive to emotional states than any other muscle group. A limbic contribution to the supranuclear supply is to be expected, and indeed two have been identified. One is the nucleus accumbens at the base of the forebrain, identified in Figure 33.1. The nucleus accumbens is a ventral part of the basal ganglia, which in turn influence the motor cortex. That circuit is compromised in Parkinson’s disease, which is often characterized by a mask-like facies (Ch. 33). The other occupies the affective area of the cingulate gyrus (illustrated in Figure 34.10), an emotionally responsive region in the territory of the anterior cerebral artery. It is active during production of a spontaneous smile, and this is of clinical interest, as explained in Clinical Panel 22.1.
Nuclear connections
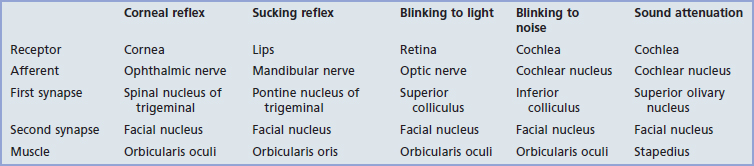
Five reflex arcs engaging the facial nucleus are listed in Table 22.1. Most important clinically is the corneal reflex.
Corneal reflex
The corneal reflex may be lost following a lesion of either the ophthalmic or facial nerve. A gradual compression of ophthalmic fibers in the sensory root of the trigeminal nerve may damage corneal neurons selectively. For this reason, the corneal reflex must be tested in patients under suspicion of an acoustic neuroma (Clinical Panel 22.2).
Clinical Panel 22.2 Syndromes of the cerebellopontine angle
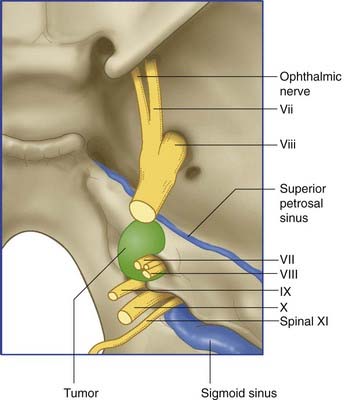
Several kinds of space-occupying lesions may compromise one or more of the nerves. The most frequent is an acoustic neuroma (Figure CP 22.2.1), a slow-growing, benign tumor of Schwann cells (neurolemmoma). The tumor originates on the vestibular nerve within the internal acoustic meatus, but the initial symptoms are more often cochlear than vestibular. An acoustic neuroma must be suspected in every middle-aged or elderly patient presenting with auditory or vestibular symptoms. Early diagnosis is important because of the difficulty of removing a large neuroma extending into the posterior cranial fossa; also because the cumulative motor and sensory disturbances may not show significant improvement after surgery.
The following is a fairly typical sequence of symptoms and signs in a case escaping early detection:
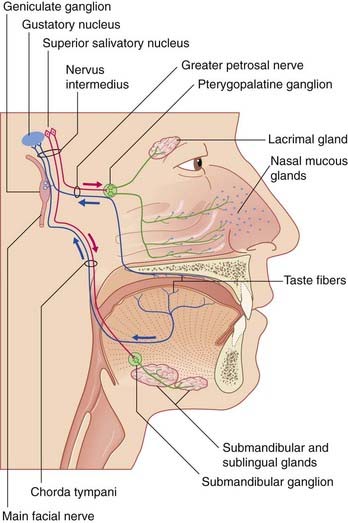
Nervus Intermedius
Nervus intermedius aligns with the facial nerve distal to the internal genu. It comprises two sets of parasympathetic and two sets of special sense fibers (Figure 22.3).
The special sense root of this nerve has unipolar cell bodies in the geniculate ganglion of the facial nerve. The peripheral processes of these ganglion cells supply taste buds in the palate via the great petrosal nerve, and taste buds in the anterior two-thirds of the tongue via the chorda tympani. The central processes enter the gustatory part of the solitary nucleus, which also receives fibers from the glossopharyngeal nerve (Ch. 18), as well as the vagus nerve (which carries taste fibers from the epiglottis). From here, second-order neurons project to the thalamus on the same side, for relay to the anterior parts of insula and cingulate cortex.
A few cells of the geniculate ganglion supply skin in and around the external acoustic meatus (Clinical Panel 22.1).
Fischer U, Hess CW, Rosler KM. Uncrossed corticonuclear projections in humans are abundant to facial muscles of the upper and lower face, but may differ between sexes. J Neurol. 2005;252:21-26.
Gilchrist JM. Seventh cranial neuropathy. Semin Neurol. 2009;29:5-13.
Lang J. Clinical anatomy of the cerebellopontine angle and internal acoustic meatus. Adv Oto-Rhino-Laryng. 1984;34:8-24.
Lemon CH, Katz DB. The neural basis of taste. BMC Neuroscience. 2007;8(S3):1-8.