CHAPTER 22 Eyelid and Facial Laser Skin Resurfacing
Skin rejuvenation has long been a goal of medical practitioners. Time-honored remedies have included a wide range of chemical and mechanical agents, but the results were often much ado about little improvement, which might also be an apt description for many modern efforts! Perhaps the major difference between current practitioners and their predecessors is an understanding, albeit an imperfect one, of the treatment goals and of the methods to achieve them. The epidermis is tightly bound to the papillary dermis with an intervening basement membrane. The papillary dermis is about equal thickness to the epidermis and it blends into the much thicker reticular dermis. In a simplified understanding of skin anatomy, the appearance of the skin is largely a product of the regularity, or lack thereof, of the external layers of the skin.
Of special interest for facial skin anatomy is the presence of generous numbers of appendages which include hair follicles, sebaceous glands, and sweat glands. These structures arise within the deeper reticular dermis and subdermis. They each are connected to the skin surface by duct like structures which are lined with epithelium. During ablative resurfacing, the surface epithelium is completely removed. The epithelium from these glands provides the reservoir from which much of the re-epithelialization occurs. In contrast, the neck and especially the anterior chest wall have a paucity of these structures. This skin is a poor choice for resurfacing because the healing process is slow and prone to scarring. The goal of resurfacing is to induce significant improvement in the surface appearance of the skin. Alteration of the collagen in the papillary and upper reticular dermis is the method to achieve the goal. Before the advent of lasers, those changes were produced by a variety of topically applied chemical agents or by mechanical appliances such as dermabraiders. The expectation for both modalities was reformation of the papillary and upper reticular dermis with new, tightly cross linked, collagen bundles followed by re-epithelialization. By the late 1980s and early 1990s, carbon dioxide (CO2) laser vaporization was added to these modalities. Erbium laser vaporization was available by the mid 1990s. Both the CO2 and the erbium lasers ablate or remove the epidermis and the papillary and upper reticular dermis with precision and predictability. Their successful use requires an understanding of lasers, skin anatomy, and laser tissue interaction. Common to all ablative modalities including chemical peels, dermabrasion and laser ablation is production of a partial thickness wound in the skin which requires a ‘down time’ to heal. By the late 1990s another group of lasers, light sources and radiofrequency devices were introduced with the goal of inducing change in the collagen of the papillary and reticular dermis without damaging the epidermis. This group can collectively be grouped together as non-ablative devices. The number of wavelengths in this group and the variable parameters used to induce collagen neogenesis offer mute testimony to the fact that this technology is still developmental.
Laser physics
If the beam is released in a pulse with the intent to conduct it to the target without significantly modifying its physical characteristics, the power of the laser must be high enough to produce an appropriate effect when it reaches the target. Once this emitted laser light strikes tissue, it can be reflected, scattered, absorbed or transmitted. While reflection is not a very useful property in medicine, there are some applications where scattering can be employed to achieve therapeutic goals. The most useful current medical applications are absorption and transmission.
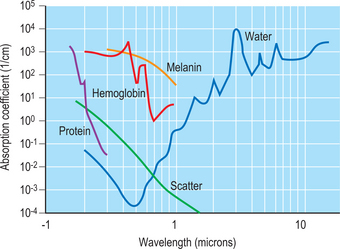
Our current concept of what happens when laser light and tissue interact was articulated by Anderson and Parrish1 in 1984 in their landmark paper describing selective photothermolysis. Specific components in tissue absorb light more intensely at some wavelengths than at others. These components are known as chromophores. For example, absorption by water peaks at 2900 nm and at 10600 nm. The erbium laser emits at 2940 nm and the carbon dioxide laser emits at 10600 nm. The incident energy from both of these lasers is rapidly absorbed by water in the most superficial layers of skin where it is converted to thermal energy. If the laser energy is sufficiently high, it will vaporize the water and in doing so will thereby remove or ablate the tissue. By controlling the incident energy and the duration of application, precise amounts of skin can be removed with limited collateral thermal damage.
Another example of this concept of absorption can be seen in the choice of an argon laser emitting at 514 nm (in the green part of the visible spectrum) where water absorption is very low and melanin absorption is relatively high. Energy emitted from this laser passes through the fluid medium of the eye to be absorbed by the chromophore melanin in the retinal pigment epithelium. The thermal energy generated by this effort can be useful in treating some pathology such as diabetic retinopathy. Figure 22-1 shows the different chromophores and their wavelengths of absorption. The goal of laser skin resurfacing is to vaporize or ablate surface tissue while minimizing thermal damage adjacent to the site of application. The obvious first step is to build the laser with enough power to reach the heat of vaporization, which in the case of water is 100°C. How efficiently this process of vaporizing tissue proceeds will be determined largely by the shape of the laser beam and the time of application. Since most medical lasers emit in either a true Gaussian shape or something similar to it, the most useful bell-shaped configuration will have the energy of the beam highly concentrated in the central portion of the beam. The objective in ablative resurfacing lasers is to exceed the energy of vaporization for the broadest area of the beam possible and to minimize the undesirable formation of thermally devitalized but intact skin on the margins of the beam. In prosaic terms, a beam configuration that looks more like a ‘high top hat’ than a ‘beret’ is desirable.
Duration of application of the laser beam can be a confusing parameter to understand. Conduction of any energy away from the site of origin/application requires time. When the water in tissue is the chromophore or target in tissue, the light energy is converted to thermal energy. If the laser energy is applied for a short enough time, thermal energy will be largely dissipated at the site. However if the energy is applied long enough, then the thermal energy will be conducted to the adjacent tissues. If this phenomenon occurs over a long enough time, significant thermal devitalization of the surrounding tissue is possible.2 Thermal burns can be produced that can dramatically alter the healing process, to the extent of scarring. This period of time before significant conduction occurs is defined as the thermal relaxation time of tissue. For skin, that time is about 400 microseconds (400 μsec). The challenge is to build a laser with enough power to treat a fairly large area of skin with a ‘short burst.’
An alternative to a single pulse is a scanner that will trace a small focused beam over the target rapidly enough to produce a desirable effect and still not expose the target area for longer than the thermal relaxation time of the tissue. Of course at the sides and depths of the beam, some subablative energy will exist which will produce a residual of thermally devitalized tissue beyond the area of application. Actually, this thermal residual can be helpful because it produces cauterization of small vessels which otherwise might bleed thereby obscuring the field.3 The effect of ablative laser applications can be predicted. The common denominator, at least among lasers of the same wavelength, is fluence which is the amount of energy applied per unit area. Fluence is measured in joules/cm2.
Laser safety
Laser safety is a requirement for all physicians. There are many regulatory levels in laser medicine: federal, state, local, departmental, and institutional. Before offering laser resurfacing or using any laser tool in practice, be sure to check with each level for safety standards. Federal regulations are outlined by the Occupational Safety Health & Administration (OSHA, www.osha.gov) in the form of general industry standards, directives, and compliance letters. Laser safety standards are specifically further delineated by the American National Standards Institute (ANSI). ANSI Z136.1-2000 document covers the general safe use of lasers. ANSI Z136.3-1996 document provides standards for the safe use of lasers in health care facilities. This document is most often used by regulatory groups and in litigation. Most states also maintain regulations as adjuncts to OSHA and ANSI standards, which may be even more rigorous.
Much has been written about plumes from lasers and cautery devices that contain viral and potentially mutagenic materials, hazardous gases, and bloodborne pathogens.4 Avoidance of such contaminants is obviously important. These plume hazards can be mitigated by laser specific surgical masks and smoke evacuators. The evacuator should be positioned within 2 cm of the smoke production for its highest efficiency. Specific laser masks should also be employed. The standard surgical mask has a 5 micron filter. Laser surgical masks have filtering capacity of 0.1 microns or less.
Table 22-1 Clinical laser safety checklist for intraoperative ablative facial laser resurfacing
| Prevent oxygen from pooling under drapes during the case (wet towels are helpful) |
| Smoke/plume evacuator |
| Metal eye shields for patient, protective lenses for staff |
| Anodized Laser safe instruments (e.g. David-Baker lid clamp, Jaeger plate, Desmarres lid retractor) |
| Test laser on wet tongue blade before applying to skin to confirm it is co-axial with the aiming beam |
Preoperative evaluation and patient selection
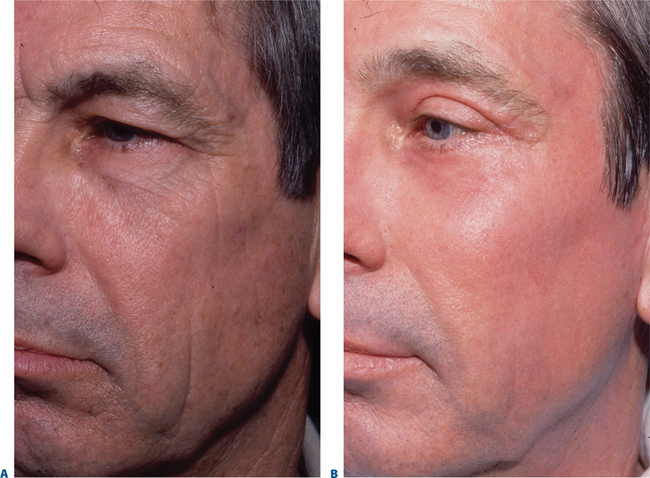
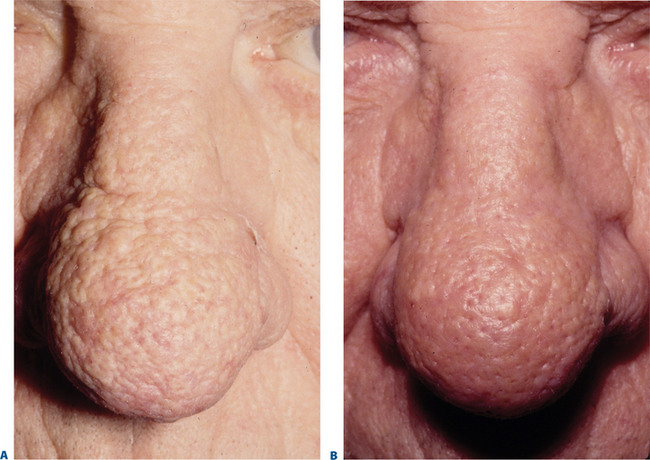
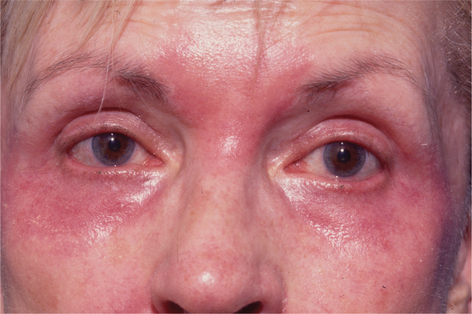
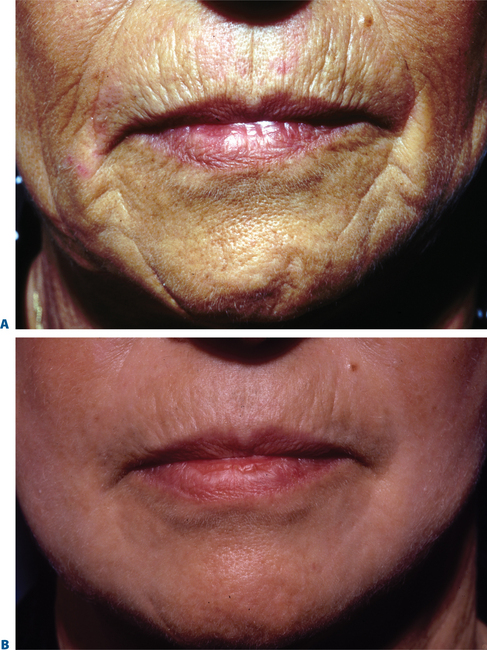
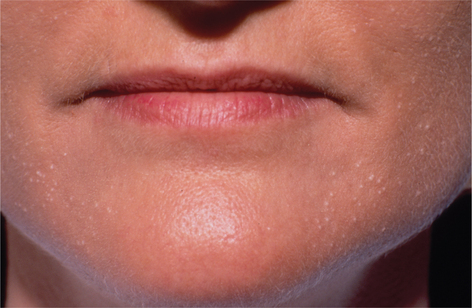
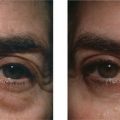
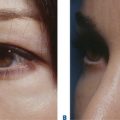
Facial resurfacing is an elective cosmetic procedure. Counseling patients about realistic expectations and postoperative care is critical before undertaking any such procedure. Special care should be taken to understand which areas of the face are of concern to the patient. Indications for facial laser resurfacing include facial rhytids, skin laxity (Fig. 22-2), dyschromia, atrophic acne scarring, rhinophyma (Fig. 22-3) and scar revision (Fig. 22-4). Benign skin growths may also be ablated; however, all suspicious skin lesions must be biopsied first. Education of the patient before the procedure is crucial. Show the prospective patient a photo album of pictures taken daily of actual patients who have undergone laser skin resurfacing during their first two postoperative weeks. This review can be most helpful in preparing the patient for what can be a challenging postoperative recuperation.
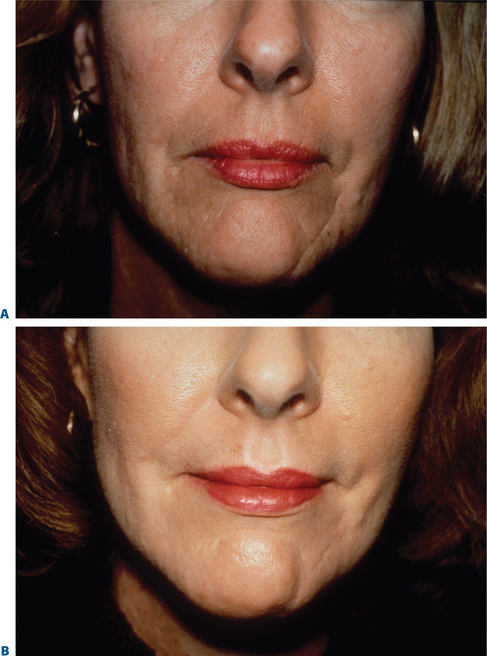
Figure 22-4 Scar modification, another application of resurfacing. A, Preoperative; B, postoperative.
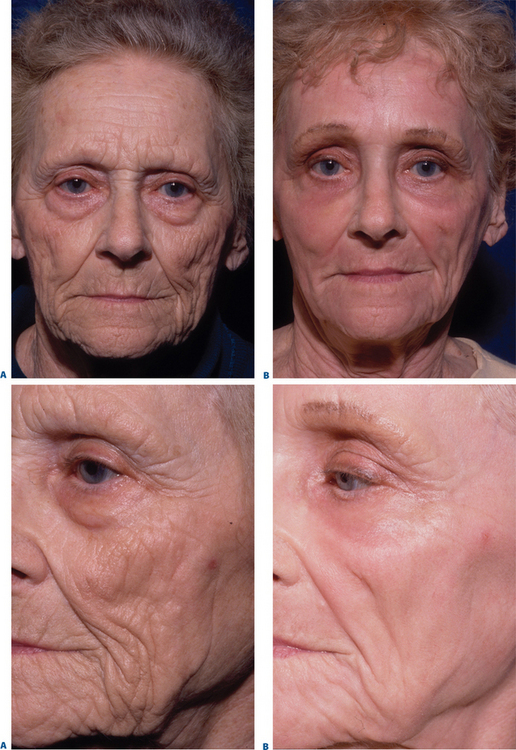
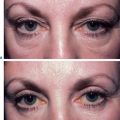
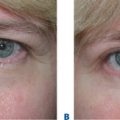
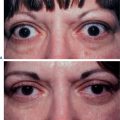
Complications and how they will be treated should also be discussed. Photographs must be taken to document preoperative anatomy. Both the patient and practitioner must have appropriate expectations of results. Emphasize that the goal is to modify and not erase. Laser facial resurfacing can usually improve severely photoaged skin to a moderate level, and a moderately photoaged skin to a minimal level and so on.5 Laser resurfacing is also a fine complementary procedure to other surgical modalities. Figures 22-5 and 22-6 show a patient who underwent full face resurfacing combined with a brow lift, midface lift, and four-lid blepharoplasty.
A routine medical and dermatologic history should be obtained. The skin type must be noted as part of the normal physical exam. Fitzpatrick Class IV or darker has an increased risk of pigmentation change and scarring. Oral retinoids within the previous 1 to 2 years may have depleted the density of pilosebaceous appendages. These structures provide the reservoir for reepithelialization. Active acne should be treated with oral antibiotics before resurfacing. Tobacco abuse may cause delayed healing which could lead to hypertrophic scarring. Table 22-2 shows contraindications to resurfacing.
| Unrealistic expectations |
| Oral retinoids (accutane) within previous 1 year (reduces reservoir from which re-epithelialization occurs) |
| Vitiligo – relative contraindication |
| Active skin infection |
| Ectropions – avoid vigorous lower lid treatment |
| Non-facial skin – especially neck or chest, because of paucity of appendages decreases the speed of re-epithelialization and increases possibility of scarring |
| History of deep peels – relative contraindication |
| History of hypertrophic scarring – relative contraindication |
| History of keloids – relative contraindication |
| History of skin radiation (may have caused decrease in appendages) |
| Pregnancy |
A pretreatment consultation should include pro-viding prescriptions for complication prophylaxis. A common protocol includes an anti-herpetic, beginning 1 day preoperatively and continued until fully epithelialized (7–10 days).6 Most experienced surgeons also prescribe an oral antibiotic. More controversial are topical retinols or alphahydroxy acids in the immediate preoperative period which may prime the skin for healing, and the routine use of hydroquinones postoperatively to decrease possibility of hyperpigmentation.
Operative techniques for ablative facial resurfacing
Laser selection
The energy emitted by the CO2 laser is not as completely absorbed as that from the erbium laser. The practical result is the existence of non-ablative fluences at the depths and edges of the application. The laser energy at these margins is largely absorbed by collagen. In this process, collagen visibly contracts or tightens which seems to provide a scaffold for new collagen development.7,8 The ‘long pulse’ erbium lasers can create a similar effect by following the first ablative pulse sequentially with a second non ablative pulse that leaves a significant thermal residual.9 Some machines (e.g. Derma-K) have combined the two wavelengths to accomplish the same objective of hemostasis and collagen shrinkage. The beam from both lasers can be focused. This manipulation allows a lower powered laser to achieve higher fluences (remember: fluence = Joules/cm2).
Safety reminders
All surgical personnel must wear protective eyewear. Surgical instruments must be laser impermeable and non-reflective to avoid possible ricochets. Free flowing oxygen should be avoided to prevent risk of a flash fire.
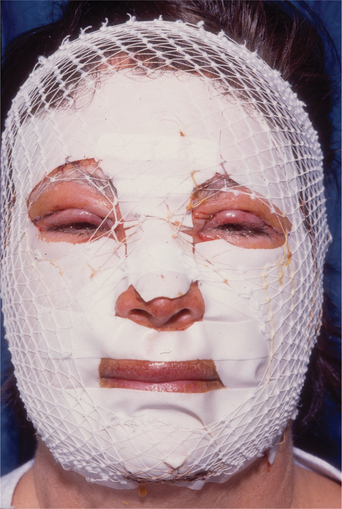
Postoperative care for ablative laser resurfacing
Immediately after surgery, an occlusive or semiocclusive wound environment must be initiated. One possibility is a biosynthetic dressing10 (e.g. Flexzan) (Fig. 22-7). As an alternative, petrolatum ointment may be applied. Topical antibiotics should be avoided, especially during the early phases of wound healing, because the epithelium has been removed by the surgical procedure thereby reducing the barrier effect of the epidermis. If a biosynthetic dressing is used, it is usually removed on postoperative day 1–3 by ‘soaking it off’ in a long shower. Once the dressing is removed, vigorous open wound care is begun using soaks and ointments. Aquaphor is a common choice although many other healing balms have been advocated including Crisco and Vaseline. The most important objective is keeping the wound moist which promotes rapid re-epithelialization. Soaks containing a weak acid (1 teaspoon of white vinegar/cup of tap water produces a concentration of about 0.25% acetic acid) will ‘cut’ the residual ointment and be a weak antibacterial. The soaks should be applied as ‘sopping wet’ 6–8 times/day. Using clean paper towels provides an alternative to the accumulation of soiled hand towels. After soaking for 15–30 minutes, reapply the barrier ointment. The treated skin should not be allowed to ‘dry out.’ A yellowish serous transudate will be prominent on the treated dermal surface for 3–5 days. Keeping the wounds clean will help to prevent accumulation of a nutrient bed for fungal or bacterial infection. Expect moderate erythema and some edema, especially in periorbital and perioral zones.
Pain is usually minimal. Many patients need only over-the-counter medicines. Most physicians prescribe an anti-herpetic for prophylaxis because the trauma of the surgery may reactivate dormant herpes simplex virus which could lead to a herpetic infection. Oral antibiotics may also be prescribed. The patient should be seen frequently in the first 10 days to monitor for infection or other complications. It is important to assess how meticulous the patient is with their post-operative skin care. Since this early recovery period is a vulnerable one for the patient, emotional support and encouragement are important during this time.
Complete re-epithelialization should occur around day 7 to 10 and will be manifested by smooth pink skin. It will take a month for the epithelium to regain its full thickness. Make-up and other cosmetics can be slowly introduced, usually one at a time to facilitate identification of potential irritants. Inform patients that contact dermatitis (Fig. 22-8) can occur even to topicals that they have successfully used in the past. Sun block of at least SPF 30 should be used. Acne and milia can also occur after the epidermis has been re-established. The new epidermis is thin, so avoid unnecessary chemicals, perfumes, or abrasive scrubs for the first few weeks. The skin is usually pink for 8–12 weeks (Figs 22-5, 22-6). New collagen and elastin fiber production may continue for up to 12 months after resurfacing. Figure 22-9 represents one year post-op follow-up results. As with other anti-aging procedures, most results should be longstanding.
Complications of ablative laser resurfacing
Bacterial cellulitis can occur in the first 1–2 weeks following resurfacing.11 Typical signs are redness, pain, infectious exudates, and even foul odor. Gram stain, cultures, and sensitivities are necessary in all cases. IV antibiotics may be needed depending on severity. If bacterial infection does occur, polymicrobial infections are not uncommon. Staph, strep, and pseudomonas are the typical pathogens. Some surgeons advocate pretreatment prophylaxis with antibiotics such as cephalosporin.
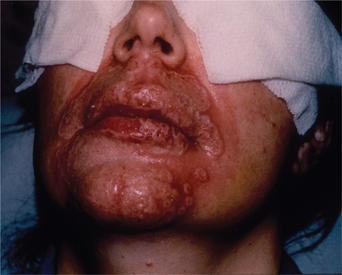
Herpetic and fungal infections can be seen in the same time frame. It can be difficult to properly identify herpetic infection in the early postoperative stage because herpetic dermatitis usually presents with epithelial signs. The typical vesicular stage is not seen when the epidermis is gone. A row or crop of raw, red lesions may be observed instead. Significant scarring can occur if untreated. Figure 22-10 represents a severe herpetic infection following perioral resurfacing. Fungal infections, usually candida, appear as soft white plaques with erythema and satellite lesions. Diagnostic evidence with KOH prep can be vital to identification and differentiation. Prophylaxis with a single dose of fluconazole may be prudent.
Acne may occur after resurfacing. An oral tetracycline, such as Minocin, may be prescribed. Milia are often present in the first 3–4 weeks postoperative (Fig. 22-11). Treatment with low concentrations of retinoic acid, alphahydroxy acids, or glycolic peels is usually beneficial.
Post-inflammatory hyperpigmentation occurs transiently in about 30 percent of resurfacing patients (Fig. 22-12). It typically presents in the first 3–4 weeks. It is more common in Fitzpatrick Type III to VI skin types.12 Melanin builds up in macrophages and production is increased during the postoperative period. There are many treatment options, including observation (resolves usually within 6 months), topical bleaching agents (i.e. hydroquinones), topical steroids, sun avoidance and sun block (SPF 30 and higher). Some surgeons pretreat those at risk for hyperpigmentation with hydroquinones and possibly topical steroids.
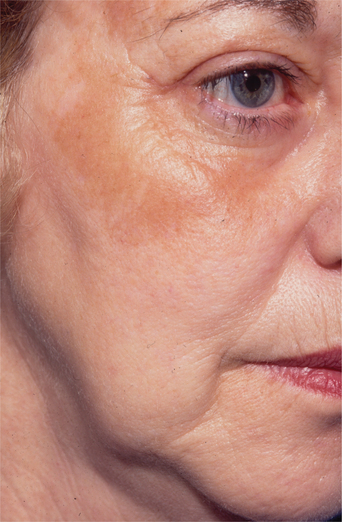
Figure 22-12 Post-inflammatory hyperpigmentation may present in patients with Fitzpatrick skin types III to VI.
Hypopigmentation occurs in less than 1 percent of treated patients. It is thought to be caused by an overall reduction in melanocytes following resurfacing. Patients who have undergone previous deep chemical peels or dermabrasion are most at risk. Unfortunately, there is no simple or reliable treatment. Cosmetic camouflage may be the most practical answer.
Table 22-3 Advantages and disadvantages of ablative laser resurfacing
| Advantages |
| Most aggressive modality for improving texture and laxity of aging skin |
| Can be tailored to patient needs |
| Unlike non-ablative applications, the ‘bottom’ of the treatment area can be seen, providing for more predictable yet safer efforts |
| Relatively quick procedure |
| Disadvantages |
| Healing period of epidermal regrowth with true downtime |
| Risk of scarring |
| Redness for several weeks |
| Risk of altered pigmentation |
| Risk of infection |
| Procedural discomfort |
Scarring may result as a rare complication in the resurfaced face.13 It is probably the most significant iatrogenic complication, but is preventable with proper technique. The resurfacing must not be performed too vigorously. Stay within the superficial reticular dermis. In addition, careful cleaning of the residual desiccated tissue during the resurfacing will avoid char.14 Char is formed by the carbonization of residual treated tissues. Char then becomes the chromophore for additional passes of the ablative resurfacing laser. When char becomes the target, there is a change in thermal dynamics from the temperature needed to vaporize water (100°C) to a much higher temperature. This additional thermal energy has the potential to produce side effects that may result in scars. Scarring can present unpredictably in certain skin types. The surgeon must be aware of previous unfavorable healing and or keloids. Treatment of scarring may consist of topical steroids, intralesional steroids, 585 nm pulse dye laser, or surgical excision. The risk of scarring can be best reduced by appropriate preoperative evaluation, safe surgical technique, close follow up of the patient after surgery, and good compliance with aftercare instructions.
1 Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524.
2 Ross EV, Domankevitz Y, Skrobal M, et al. Effects of CO2 laser pulse duration in ablation and residual thermal damage. Lasers Surg Med. 1996;19:123-129.
3 Kirschner RA. Cutaneous laser surgery with the CO2 laser. Surg Clin North Am. 1992;64:871-883.
4 Gloster HMJr., Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32(3):436-441.
5 Biesman BS. Cutaneous facial resurfacing with the carbon dioxide laser. Ophthal Surg Lasers. 1996;27:685-698.
6 Lowe NJ, Lask G, Griffin ME. Laser skin resurfacing: pre and posttreatment guidelines. Dermatol Surg. 1995;21(12):1017-1019.
7 Bass LS, Aston SJ: Shrinkage and thermal injury in human skin in vitro after resurfacing with carbon dioxide and erbium : YAG lasers. Lasers Surg Med 1997; (Suppl): 30 pp.
8 Cotton J, Hood AF, Gonin R, et al. Histologic evaluation of preauricular and postauricular human skin after high energy, short-pulse carbon dioxide laser. Arch Dermatol. 1996;132(4):425-428.
9 Gardner E, Reinisch L, Stricklin GP, et al. In vitro changes in non-facial human skin following CO2 laser resurfacing. Lasers Surg Med. 1996;19:379-387.
10 Pozner JN, Ramirez OM, Weinstein C: Experience with the use of semipermeable dressing following laser resurfacing. Lasers Surg Med 1997 (Suppl): 60 pp.
11 Goldman MP, Fitzpatrick RE, Smith SR, et al: Infections complicating pulsed CO2 laser resurfacing for photoaged facial skin. Laser Surg Med 1997; (Suppl): 43 pp.
12 Ho C, Nguyen Q, Lowe NJ, et al. Laser resurfacing in pigmented skin. Dermatol Surg. 1995;21(12):1035-1037.
13 Nanni CA, Alster TS: Complications of CO2 laser resurfacing. Laser Surg Med 1997; (Suppl): 53 pp.
14 Ross EV, Glatter RD, Duke D, et al: Effects of pulse and scan stacking in CO2 laser skin resurfacing. Laser Surg Med 1997; (Suppl): 61 pp.