Chapter 59 Extreme Lateral Infrajugular Transcondylar Approach for Resection of Skull Base Tumors
The extreme lateral infrajugular transcondylar (ELITE) approach was born out of the anatomic constraints and operative challenges presented by the cranial base. The transcondylar approach was first devised as an avenue for access to the foramen magnum and ventral medulla.1,2 In 1986, Heros3 described the extreme lateral inferior suboccipital approach for access to vertebral and vertebrobasilar artery lesions. The ELITE approach is a special transcondylar and transjugular tubercle skull base approach developed and elaborated in Germany and Japan in 1987.4,5 After years of refinement, this has become a very useful and well-described approach for this region of the skull base.
Paragangliomas of the temporal bone were first identified as a unique entity in 1945.6 Attempts at characterization and ultimate surgical resection were initially fraught with high morbidity and mortality because of their location amidst complex anatomic structures. Primary treatment with radiation therapy soon became the mainstay for therapy with the hopes of limiting complications. Radiation therapy has been shown to be superior to observation of these lesions; however, the long-term risks of malignancy and radiation morbidity limit its success.7–10 Definitive surgical resection remains the mainstay for attempted cure.
Attempts at surgical resection and cure led to the development of tumor classification strategies to help guide surgical planning and to improve communication of results. Two main classification schemes were devised and remain in use today, primarily in the otology/neurotology literature—the Fisch11 and Glasscock-Jackson classifications.12 The Fisch scheme is as follows:
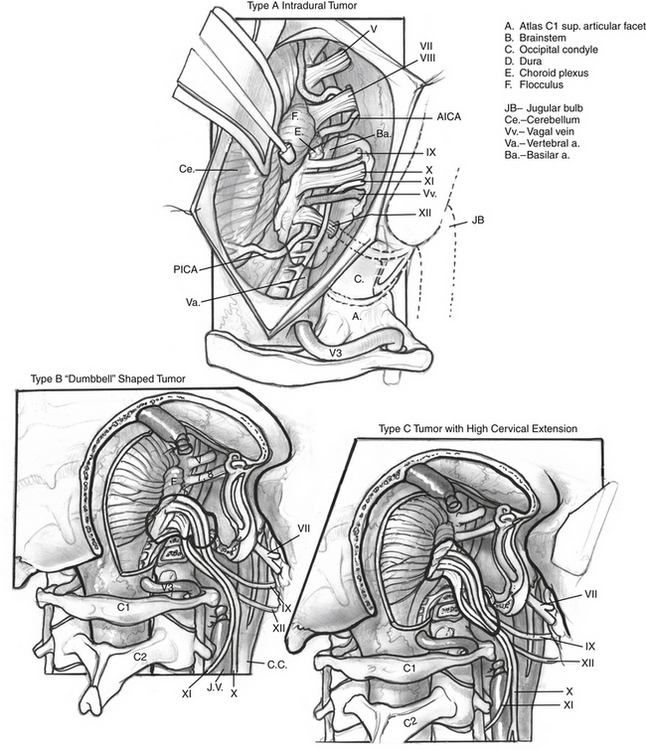
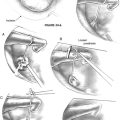
The authors have used a simpler classification of tumors in this location, and modifications and additions to the ELITE approach—including transsigmoid, transjugular, infrajugular, and high cervical approaches—are tailored to provide needed exposure for tumor resection (Fig. 59-1):
SURGICAL PROCEDURE
A preoperative arteriogram and embolization are performed 48 hours before the surgical procedure.13 Facial nerve monitoring is carried out in all cases. The surgeon may consider additional monitoring, including auditory brainstem evoked potentials, somatosensory evoked potentials, motor evoked potentials, and monitoring of CN X, XI, and XII. An electromyographic endotracheal tube can be used for CN X monitoring, and electrodes placed directly into the sternocleidomastoid muscle and the tongue can be used for CN XI and XII monitoring. Before the incision, perioperative antibiotics and corticosteroids are administered.
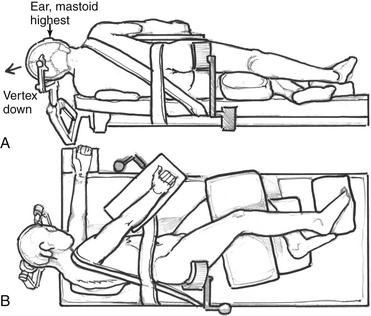
Patient positioning and incision differ for the dorsolateral and anterolateral modifications. For the dorsolateral ELITE procedure, the patient is placed in a lateral decubitus position (Fig. 59-2A and B). For the anterolateral approach, the patient is positioned in a supine fashion with the head displaced away from the side of the lesion. A shoulder roll is placed to aid with the high cervical portion of the procedure and to raise the shoulder on the side of the lesion. Obese patients and patients with very short necks may be placed in a lateral position if necessary. The patient should be padded and secured for ease of rotation of the table throughout the case.
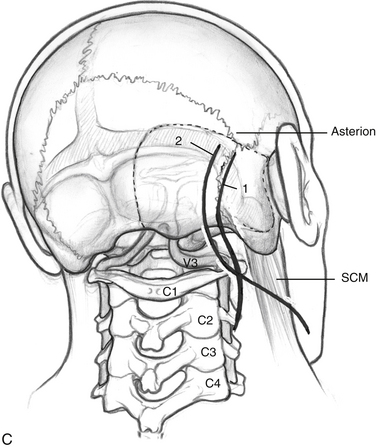
Incisions are shown in Figure 59-2C. For the dorsolateral approach, a lazy S incision is used, 1 to 2 cm posterior to the mastoid bone, and extending inferiorly along the hairline. For the anterolateral ELITE procedure, a retroauricular curvilinear C-shaped, or question mark–shaped, skin incision is begun approximately 2 to 3 cm posterior to the upper border of the ear. Inferiorly, this incision is carried down into the neck, traversing the border of the sternocleidomastoid muscle (SCM) and running parallel to the body of the mandible, approximately two fingerbreadths below. The skin flaps are raised. For the dorsolateral approach, the SCM is retracted anteriorly; for the anterolateral approach, the SCM is retracted posteriorly. The inferior edge is raised in the subplatysmal plane. Superiorly, the temporoparietal fascia is elevated as a separate flap under the skin flap, after it is dissected off the bone with the periosteum.
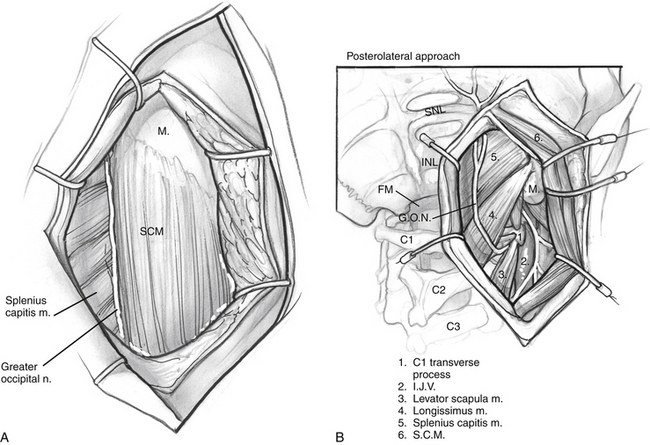
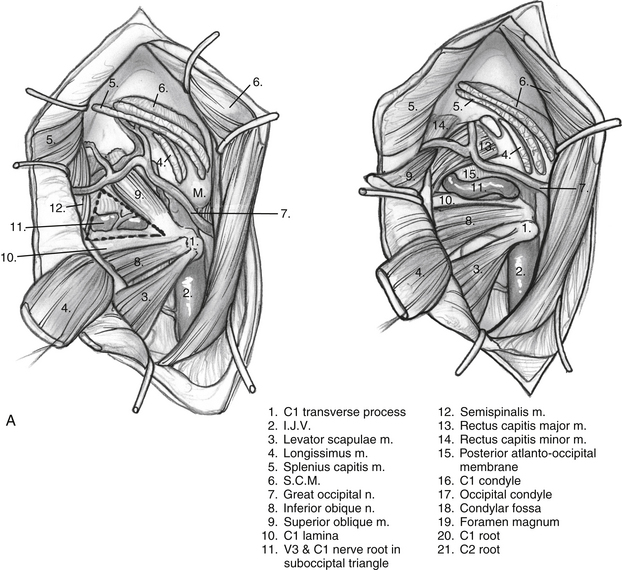
The anterior flap is now reflected, and the posterior auricular muscle should be visible behind the external auditory canal. Inferiorly, the greater auricular nerve should be seen crossing the SCM approximately 3 cm below the mastoid tip. This nerve can be preserved for use as an interposition graft, if needed. The posterior flap is displaced, and the posterolateral neck muscles are reflected to expose the mastoid more fully. This group of muscles is composed of three layers (Fig. 59-3). The superficial layer is composed of the SCM and splenius capitis muscles. The middle layer consists of the longissimus capitis and semispinalis capitis. These muscles are reflected to expose the deep layer, which consists of the rectus capitis posterior major, the obliquus capitis superior, and the obliquus capitis inferior muscles. These muscles form the suboccipital triangle (Fig. 59-4A). The styloid diaphragm also is identified in this region. Beneath that diaphragm runs the occipital artery as it courses beneath the posterior belly of the digastric muscle (Fig. 59-4B).
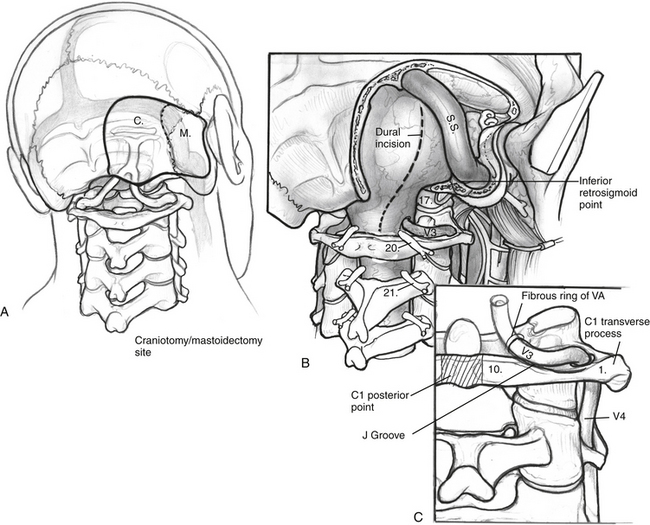
It is essential to identify the V3 segment of the vertebral artery to avoid injury during this exposure. V3 can be identified in one of three ways: by dissection of the suboccipital triangle, by identification of the vertebral sulcus or J groove of the C1 lamina, or by use of a Doppler probe (Fig. 59-5). For identification of the suboccipital triangle, the deep layer of muscles mentioned previously serves as the guide to this anatomic region. The rectus capitis posterior major inserts superiorly on the nuchal line and attaches inferiorly on the spinous process of C2. The obliquus capitis inferior muscle inserts superiorly on the transverse processes of C1 and inferiorly on the spinous process of C2. The obliquus capitis superior attaches superiorly at the temporo-occipital suture and inferiorly on the transverse process of C1. The suboccipital triangle is opened by detaching the superior and inferior oblique muscles off of the transverse process of C1 and reflecting them posteriorly. The rectus capitis posterior major is reflected posteriorly as well, after freeing it from the inferior nuchal line; this helps identify the C1 lamina and the vertebral artery.
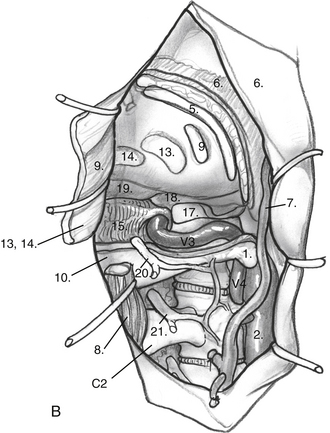
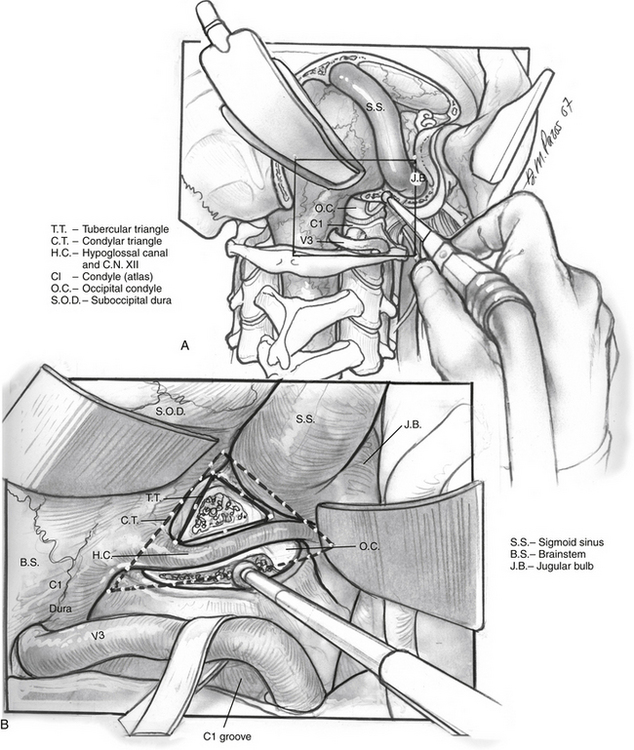
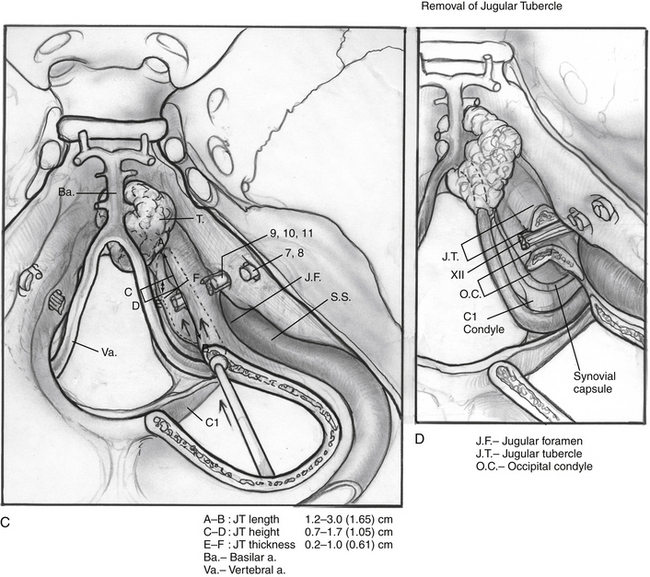
The posterior and medial one third of the occipital condyle is removed in front of the C1 dura, using a high-speed diamond drill. Resecting more than half of the condyle can cause instability of the craniovertebral junction, and stabilization should be considered on resection. As the condyle is removed, the surgeon proceeds through a cortical layer leading to a cancellous bony layer. Venous bleeding may be present and should be controlled with bone wax and Surgicel before proceeding. The next cortical layer overlies the hypoglossal canal. The hypoglossal nerve, branch of the ascending pharyngeal artery, and venous plexus of the canal are located within the canal. Because the canal sits superior to the condylar facet and inferior to the jugular tubercle and is directed slightly cephalad at a 60 degree anterolateral plane, skeletonization of the canal to its lateral extent indicates a one third resection of the superomedial portion of the occipital condyle. Removal of this portion of the condyle provides easier access to the ventral foramen magnum (Fig. 59-6A and B).
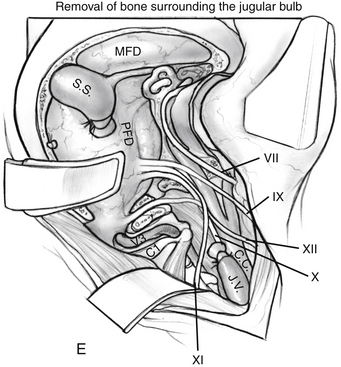
Dissection continues superiorly toward the jugular tubercle. The jugular tubercle is a smooth osseous protrusion on either side of the occipital bone.14 The base of the tubercle is defined by the hypoglossal canal, and it extends anteriorly 20 mm toward the clivus. Reduction of the tubercle permits access to the anterior clivus. Because the tubercle lies medial to the jugular foramen, CN IX, X, and XI are at risk as the tubercle is drilled out. Risk of injury can be minimized by drilling within a core of the tubercle and working anteriorly, deeper toward the clivus, minimizing heat and potential stretch injury to the nerves. Drilling should be carried 20 to 25 mm deep toward the clival junction. A 3 mm smooth diamond burr is ideal here, and can then be sized down to a 2 mm burr. Remaining thin bone can be dissected; inner cortical bone can be preserved to avoid damage to CN IX and X. It is important to have flat access from the lateral edge of the foramen magnum under CN IX, X, and XI to the inferior clivus and ventral medullary area (Fig. 59-6C-E). Between the jugular bulb and the C1 transverse process, the small rectus lateralis muscle must be removed. Occasionally, the C1 transverse process is shaved to expose the V3-V4 genu for wider exposure. In this process, care should be taken not to injure CN XI.
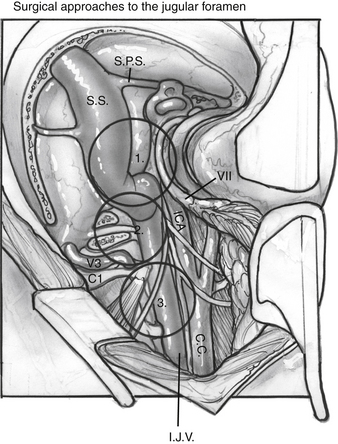
Adequate exposure of the sigmoid sinus, jugular bulb and IJV, and all lower cranial nerves should allow for preservation of vital functions and easy identification of the tumor within these structures. Figure 59-7 shows the exposure of these structures provided by this approach. The approach used for resection of large glomus jugulare tumors includes (1) the suprajugular, retrofacial, infralabyrinthine approach; (2) the infrajugular-transcondylar approach; and (3) the high cervical approach.
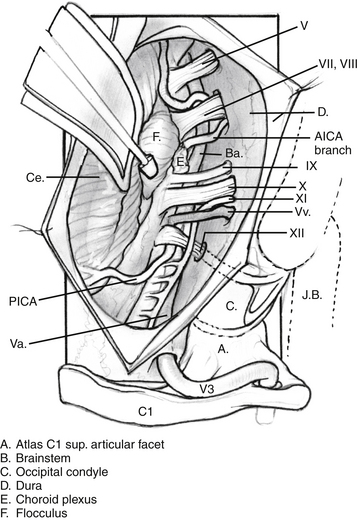
The intradural portion of the tumor is addressed next. The dural incision is curvilinear and is placed several millimeters posterior to the sigmoid sinus. It is carried inferiorly to the point where the vertebral artery pierces the dura. The dura is reflected back with multiple tacking sutures. With sufficient drilling of the occipital condyle and jugular tubercle, the accessory nerve is readily identifiable. Dissection can expose the medullary branch of the accessory nerve, the vagal vein and vagus nerve, and the glossopharyngeal nerve. The tumor is dissected free from the cranial nerves after the jugular foramen is inspected for further extension. Blood supply from the posterior inferior cerebellar artery can be cauterized (Fig. 59-8).
The wound is irrigated, and reconstruction of the cranial base is performed to obtain a watertight dural closure. Options include fascia for smaller defects, a pericranial flap, or a free flap reconstruction for larger defects. The use of vascularized myofascial flaps for reconstruction is thought to reduce the incidence of postoperative CSF leak.15 Abdominal fat should be harvested to fill the mastoid defect. Lumbar drainage is used in most cases.
COMPLICATIONS
Complications of cranial base surgery for tumors of the jugular foramen are well described. Several series have reported common complications, including CSF leak and wound infection, with an incidence of approximately 5% to 10% for each case.16,17 Facial nerve and hearing deficits are common, and outcomes vary significantly depending on preoperative function, approach, and tumor extent. Lower cranial nerve deficit, often producing aspiration and voice disorder, was the most common surgical complication in one large series, at almost 10%.15 Other complications include cerebrovascular accident, meningitis, pulmonary embolus, hydrocephalus, and death. These more severe complications are rare, occurring in about 1% of cases.16,17 Craniovertebral instability is a concern with over-resection of the condyle; however, with preservation of at least 50% of the condyle, stabilization should be unnecessary.18
RESULTS
Liu and colleagues18 reported surgical outcomes from resection of glomus jugulare tumors in 30 patients using the ELITE procedure. Mean follow-up was 28.5 months. Total resection was achieved in 73%, and near-total resection was achieved in 20% of patients. Eight patients (27%) had worsened facial nerve palsy postoperatively, and four of those patients maintained a permanent deficit. Two patients with permanent facial paralysis underwent partial anterior transposition of CN VII, and two required an interposition cable graft because of tumor invasion of the nerve. Four patients developed sensorineural hearing loss; all were patients with significant intradural tumor. Cochlear function was thought to be compromised secondary to any of various factors, including traction on the cerebellum or CN VIII, or violation of the cochlea during bone dissection or tumor resection. Of patients, 30% had worsened CN IX and X function, six with permanent deficit, and three patients went on to laryngoplasty to improve swallowing and voice function. There were five CN XI and three CN XII pareses. There were no CSF leaks and no operative mortality. No patients required stabilization of the occipitocervical junction.
One advantage of the ELITE surgical approach is tumor access with minimal manipulation of the facial nerve. It is recognized that long facial nerve rerouting results in interruption of the deep petrosal artery, leading to perigeniculate ischemia. There is also a chance of mechanical trauma with mobilization of the nerve from the narrow labyrinthine segment and manipulation of the epineurium in the internal auditory canal.19 The key to preserving facial function is minimal dissection, and translocation only as necessitated by the tumor and the approach. Preservation of the periosteum of the bone surrounding the facial nerve can help to preserve the stylomastoid artery, which supplies the mastoid segment of the facial nerve. We describe a select anterior translocation only as necessary to gain access to the vertical C7 (infratemporal) portion of the carotid artery.
Although the approaches used by many surgeons require facial nerve rerouting, more conservative facial nerve management has been advocated, at least for some tumors without extensive anterior involvement. Pensak and Jackler20 described the fallopian bridge technique, and reported usage in a large percentage of glomus jugulare tumors in their practices. In this technique, the facial nerve is skeletonized and left in situ, and the external auditory canal is not violated. This approach cannot be used when there is anterior extension of tumor to the carotid genu, but is not contraindicated by the presence of an intradural tumor component. They report outcomes in 35 patients using this technique, with a House-Brackmann grade I result in 92% at 6 months after surgery.
Results of glomus jugulare tumor resection using the ELITE approach may be compared with reports of series using other approaches. Pareschi and coworkers21 described their experience with 52 glomus jugulare tumors. Of those tumors, 42 were at least Fisch classification type C, and 37 underwent surgery, with an overall resection rate of 96%. Overall, 33 patients required an infratemporal fossa approach with 5 requiring A and B types. Two patients required second-stage petro-occipital transsigmoid approach. The external and middle ear was sacrificed in all cases. Facial nerve function at 1 year was House-Brackmann grade I or II in 51% of patients, and grade III or better in 84%. The facial nerve was transposed in 70% of patients, and preserved anatomically in 80%. The glossopharyngeal and vagus nerves were preserved anatomically in 48% and 35%. The authors noted that most patients have some difficulty with dysphagia, but there were no reported pneumonias. Twelve patients required vocal cord medialization. CSF leaks were not reported; however, three patients developed a subcutaneous CSF collection that was managed conservatively.
Jackson and colleagues22 reported results for 182 lateral skull base surgeries for glomus jugulare tumors. Of those patients, 21% were class I, 20% were class II, 35% were class III, and 23% were class IV. Total surgical control was reported as 85%. Their series included 66 patients with intracranial extent, 44 of which had pars nervosa involvement requiring resection of CN IX through XII. Jackson and colleagues22 noted that one or more new cranial nerve deficits arose in 59% of patients. Complete tumor removal was possible without any cranial nerve resection in 31%. CSF leak was reported in 3 of 66 cases after 1987, when complex reconstruction of the cranial base began in their practice.
The Otology Group16 described their management of the facial nerve in lateral skull base surgery for benign tumors. Their outcomes are reported as good (House-Brackmann grade I-II), fair (House-Brackmann grade III-IV), and poor (House-Brackmann grade V-VI). The patients in whom the nerve was left in situ had normal function. Procedures with short translocation preserved good function in 92%, while procedures with long translocation preserved good function in 66%. Extreme translocation resulted in good function in only 38% of patients. Patients with resected and grafted nerves had much poorer results, as would be expected.
Oghalai and associates23 reported on results of surgical treatment using a transjugular craniotomy for resection of a series of 28 jugular foramen tumors with intracranial extent, including 6 glomus jugulare tumors. A fallopian bridge technique of facial nerve dissection, which leaves the vertical portion of the facial nerve in situ, was used when possible. Of the 24 patients with normal or near-normal preoperative facial nerve function, function was preserved (House-Brackmann grade I-II) postoperatively in 22. Patients who did not require facial nerve translocation did not experience deterioration of function. Tumor access required ear canal closure in a few (21%) cases. Of patients, 60% had good preoperative hearing levels (defined as American Academy of Otolaryngology–Head and Neck Surgery class A or B), and 41% had good hearing postoperatively. All nine patients who were spared destructive otologic procedures (ear canal closure, translabyrinthine or transcochlear procedures) maintained good hearing postoperatively. A 53% incidence of complete lower cranial nerve palsy was identified in the patient population preoperatively, and new lower cranial nerve deficits were noted in 27%.
In the series reported by Fisch and colleagues and cited by Moe and colleagues,17 132 patients with glomus jugulare tumors were reviewed. Most (n = 119) of the patients had advanced tumors (class C and D); 81 had intradural extension. Total tumor removal was reported in 83% of the total population. Of patients, 84% required an advanced infratemporal fossa approach, 72% required an anterior displacement of the facial nerve, and approximately 15% required facial nerve resection, with either grafting or anastomosis. Results with anterior displacement were favorable, with good function (House-Brackmann grade I-II) retained in 88% of patients. Preservation of the lower cranial nerves for class C and D tumors varied depending on class and nerve. CN VIII and IX were most at risk and had preservation rates between 20% and 50%. CN X, XI, and XII fared better, with preservation rates of 60% to 80%. In the reported follow-up, 10% of patients had aspiration. Ten percent were noted to have CSF leaks postoperatively.
DISCUSSION
Good facial nerve function is important for quality of life, and injury to the facial nerve carries significant morbidity. Classically, large glomus jugulare tumor resection required rerouting of the facial nerve and possibly resection of the nerve to complete surgical resection. Numerous techniques have been described to translocate the facial nerve.19 The ELITE approach may use a slight anterior translocation if needed to gain access to the petrous portion of the carotid artery. Anterior dislocation of the mandible can produce symptoms of trismus, pain, malocclusion, and first bite syndrome.16 The transcondylar transtubercular approach allows the surgeon to work posterior to the angle of the mandible without displacement, and the petrous portion of the carotid artery can still be accessed without the corresponding morbidity. In a similar manner, this exposure prevents violation of the external ear canal, sparing the patient a conductive hearing loss.
Access to the lower clivus is difficult to achieve. It is necessary to displace the petrous portion of the carotid anteriorly and translocate the lower cranial nerves inferiorly. Lower cranial nerve morbidity is common during glomus jugulare surgery because these nerves are intimately related to the tumor.24 Preserving the dural covering of the lower cranial nerves as they are displaced can help minimize this morbidity.
1. Seeger W. Atlas of Topographical Anatomy of the Brain and Surrounding Structures. Vienna: Springer-Verlag; 1978.
2. Bertalanffy H., Seeger W. The dorsolateral, suboccipital, transcondylar approach to the lower clivus and anterior portion of the craniocervical junction. Neurosurgery. 1991;29:815-821.
3. Heros R.C. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
4. Fukushima T., Day J.D. Manual of Skull Base Dissection. Pittsburgh: AF-Neurovideo Inc; 1996.
5. Fukushima T, Sameshima T, Friedman AH (eds): Exercise 10. In Manual of Skull Base Dissection. 2nd ed. Raleigh, NC, AF-Neurovideo, 2004.
6. Rosenwasser H. Carotid body-like tumor of the middle ear and mastoid bone. Arch Otolaryngol. 1945;41:64-67.
7. Gottfried O.N., Liu J.K., Couldwell W.T. Comparison of radiosurgery and conventional surgery for the treatment of glomus jugulare tumors. Neurosurg Focus. 2004;17:E4.
8. Pollock B.E. Stereotactic radiosurgery in patients with glomus jugulare tumors. Neurosurg Focus. 2004;17:E10.
9. Lim M., Gibbs I.C., Adler J.R.Jr., Chang S.D. Efficacy and safety of stereotactic radiosurgery for glomus jugulare tumors. Neurosurg Focus. 2004;17:E11.
10. Carrasco V., Rosenman J. Radiation therapy of glomus jugulare tumors. Laryngoscope. 1993;103(11 Pt 2 Suppl 60):23-27.
11. Fisch U., Mattox D. Classification of glomus temporale tumors. In: Microsurgery of the Skull Base. New York: Thieme Medical Publishers; 1988:149-153.
12. Jackson C.G. Glomus tympanicum and glomus jugulare tumors. Otolaryngol Clin. 2001;34:5.
13. LaRouere M.J., Zappia J.J., Wilner H.I., et al. Selective embolization of glomus jugulare tumors. Skull Base Surg. 1994;4:21-25.
14. Mintelis A., Sameshima T., Bulsara K.R., et al. Jugular tubercle: Morphometric analysis and surgical significance. J Neurosurg. 2006;105:753-757.
15. Ramina R., Maniglia J.J., Fernandes Y.V., et al. Tumors of the jugular foramen: Diagnosis and management. Operative Neurosurg. 2005;57(1 Suppl):59-68.
16. Manolidis S., Jackson G., Von Doersten P.G., et al. Lateral skull base surgery. The Otology Group experience. Skull Base Surg. 1997;7:129-137.
17. Moe K.S., Li D., Linder T.E., et al. An update on the surgical treatment of temporal bone paraganglioma. Skull Base Surg. 1999;9:185-194.
18. Liu J.K., Sameshima T., Couldwell W.T., Fukushima T. The combined transmastoid retro- and infralabyrinthine transjugular transcondylar transtubercular high cervical approach for resection of glomus jugulare tumors. Operative Neurosurg. 2006;59:115-125.
19. Parhizkar N., Hiltzik D.H., Selesnick S.H. Facial nerve rerouting in skull base surgery. Otolaryngol Clin North Am. 2005;38:685-710.
20. Pensak M.L., Jackler R.K. Removal of jugular foramen tumors: The fallopian bridge technique. Otolaryngol Head Neck Surg. 1997;117:586-591.
21. Pareschi R., Righini S., Destito D., et al. Surgery of glomus jugulare tumors. Skull Base Surg. 2003;13:149-157.
22. Jackson C.G., McGrew B.M., Forest J.A., et al. Lateral skull base surgery for glomus tumors: Long-term control. Otol Neurotol. 2001;22(3):377-382.
23. Oghalai J.S., Leung M.K., Jackler R.K., McDermott M.W. Transjugular craniotomy for the management of jugular foramen tumors with intracranial extension. Otol Neurotol. 2004;25:570-579.
24. Sen C., Hauge K., Kacchara R., et al. Jugular foramen: Microscopic anatomic features and implications for neural preservation with reference to glomus tumors involving the temporal bone. Neurosurgery. 2001;48:838-848.