Chapter 31 Extraintestinal Endosonography (including Celiac Block)
Lung Cancer
Lung cancer is the leading cause of cancer death in the United States in men and women and has an overall 5-year survival rate of 15%.1,2 Treatment decisions are based on the location and extent of the tumor. The presence of extrapulmonary metastasis is crucial because patients without mediastinal involvement are potential candidates for resection. The distinction between non–small cell lung cancer (NSCLC), which accounts for 80% of tumors, and small cell lung cancer (SCLC), which accounts for 20% of tumors, is important because of the more aggressive nature of SCLC. SCLC is usually classified as limited or extensive disease, although the criteria for these two categories remain controversial.3–5 Although the TNM (primary tumor, regional nodes, metastases) staging system traditionally has not been used in staging SCLC, it is expected to be included in the forthcoming seventh edition of the TNM classification of malignant tumors.6–9 Metastatic disease is detected in 80% of SCLC cases at the time of diagnosis and tends to spread quickly so that surgery is considered less often in SCLC compared with NSCLC. Although highly responsive to radiotherapy and chemotherapy, SCLC usually recurs within 2 years.
In comparison, half of NSCLC cases are localized or locally advanced and can be treated by surgery, the cornerstone of therapy for NSCLC, or with adjuvant therapy with or without resection.10–12 NSCLC, which includes adenocarcinoma, squamous cell cancer, and large cell cancer, continues to be staged using the 2002 International Staging System, which is unchanged from the 1997 revision (Box 31.1).12–14 This section focuses on EUS applications in the diagnosis and staging of NSCLC, although much of what is covered can be applied to SCLC.
Box 31.1
International Staging System for Lung Cancer, 1997 Revision
Primary Tumor (T)
Nodal Involvement (N)
Data from Greene FL, Page DL, Fleming ID, et al, editors: AJCC (American Joint Committee on Cancer) cancer staging manual, ed 6, New York, 2002, Springer-Verlag, pp 167–174; and Mountain CF: Revisions in the International System for Staging Lung Cancer. Chest 111:1710–1717, 1997.
Staging and Staging Modalities
Mediastinal lymph node metastases are present in nearly half of all patients with NSCLC. Accurate staging of NSCLC is crucial in determining treatment options because the detection of mediastinal lymph node metastasis preoperatively has therapeutic implications. In the absence of distant metastasis, the documentation of mediastinal metastasis is probably the most common deterrent to cure.15–26 The TNM staging system used for lung cancer (see Box 31.1) designates ipsilateral peribronchial, intrapulmonary, or ipsilateral hilar lymph nodes as N1 disease and ipsilateral mediastinal and subcarinal lymph node involvement as N2 disease. Although N2 disease is potentially resectable, most patients with N2 disease receive multimodality treatment. Contralateral lymph node involvement of mediastinal or hilar nodes or either ipsilateral or contralateral scalene or supraclavicular lymph nodes is designated N3 disease, which precludes resection (Table 31.1 and Fig. 31.1, and see Box 31.1).12–14,26,27
| Nodal Station | Anatomic Landmarks |
|---|---|
| N2 NODES: ALL N2 NODES LIE WITHIN THE MEDIASTINAL PLEURAL ENVELOPE | |
| 1. Highest mediastinal nodes | Nodes lying above horizontal line at the upper rim of the brachiocephalic (left innominate) vein where it ascends to the left, crossing in front of the trachea at its midline |
| 2. Upper paratracheal nodes | Nodes lying above horizontal line drawn tangential to the upper margin of the aortic arch and below the inferior boundary of the No. 1 nodes |
| 3. Prevascular and retrotracheal nodes | Prevascular and retrotracheal nodes may be designated 3A and 3B; midline nodes are considered to be ipsilateral |
| 4. Lower paratracheal nodes | Lower paratracheal nodes on the right lie to the right of the midline of the trachea between horizontal line drawn tangential to the upper margin of the aortic arch and line extending across the right main bronchus at the upper margin of the upper lobe bronchus and contained within the mediastinal pleural envelope; lower paratracheal nodes on the left lie to the left of the midline of the trachea between horizontal line drawn tangential to the upper margin of the aortic arch and line extending across the left main bronchus at the level of the upper margin of the left upper lobe bronchus, medial to the ligamentum arteriosum and contained within the mediastinal pleural envelope. |
| Researchers may wish to designate the lower paratracheal nodes as No. 4s (superior) and No. 4i (inferior) subsets for study purposes; No. 4s nodes may be defined by a horizontal line extending across the trachea and drawn tangential to the cephalic border of the azygos vein; No. 4i nodes may be defined by the lower boundary of No. 4s and the lower boundary of No. 4 as described previously | |
| 5. Subaortic (aortopulmonary window) | Subaortic nodes are lateral to the ligamentum arteriosum or the aorta or left pulmonary artery and proximal to the first branch of the left pulmonary artery and lie within the mediastinal pleural envelope |
| 6. Paraaortic nodes (ascending aorta or phrenic) | Nodes lying anterior and lateral to ascending aorta and the aortic arch or the innominate artery, beneath line tangential to the upper margin of the aortic arch |
| 7. Subcarinal nodes | Nodes lying caudal to the carina of the trachea but not associated with the lower lobe bronchi or arteries within the lung |
| 8. Paraesophageal nodes (below carina) | Nodes lying adjacent to the wall of the esophagus and to the right or left of the midline, excluding subcarinal nodes |
| 9. Pulmonary ligament nodes | Nodes lying within the pulmonary ligament, including nodes in the posterior wall and lower part of the inferior pulmonary vein |
| N1 NODES: ALL N1 NODES LIE DISTAL TO THE MEDIASTINAL PLEURAL REFLECTION AND WITHIN THE VISCERAL PLEURA | |
| 10. Hilar nodes | Proximal lobar nodes, distal to the mediastinal pleural reflection and the nodes adjacent to the bronchus intermedius on the right; radiographically, hilar shadow may be created by enlargement of both hilar and interlobar nodes |
| 11. Interlobar nodes | Nodes lying between the lobar bronchi |
| 12. Lobar nodes | Nodes adjacent to the distal lobar bronchi |
| 13. Segmental nodes | Nodes adjacent to the segmental bronchi |
| 14. Subsegmental nodes | Nodes around the subsegmental bronchi |
From Mountain CF, Dresler CM: Regional lymph node classification for lung cancer staging. Chest 11:1718–1723, 1997.
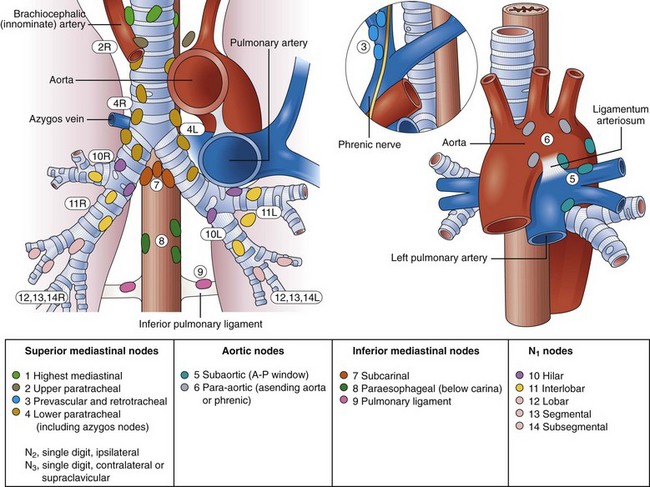
Fig. 31.1 Lymph node stations.
(From Mountain CF, Dresler CM: Regional lymph node stations for lung cancer staging. Chest 111:1718–1723, 1997.)
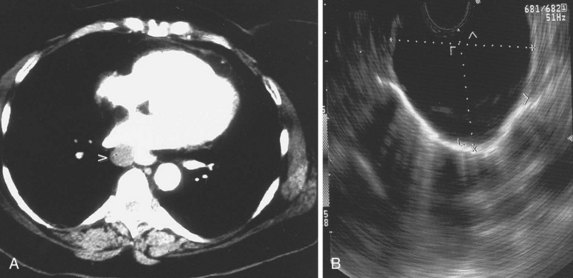
Various techniques are currently available to diagnose and stage lung cancer, including plain radiography, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), endobronchial ultrasound (EBUS), and EUS. CT scan of the chest is the current standard by which mediastinal lymphadenopathy is detected. Generally, lymph nodes larger than or equal to 1 cm on chest CT scan are considered abnormal. A review of previously published studies reveals an accuracy of CT staging of the mediastinum of 52% to 88%.28–38 This variation has been attributed to the wide range of correlation of lymph node size to the presence of malignant involvement. Although the general trend is increased risk for metastasis correlating with increasing lymph node size, lymph node size is not an accurate criterion for assessing risk. Problems associated with size as a criterion include the inability to differentiate inflammatory or reactive lymph nodes from malignant involvement. In one study, 37% of mediastinal lymph nodes that ranged in size from 2 to 4 cm were benign,38 and 40% of enlarged nodes in another series were not cancerous.39 Similarly, normal-sized lymph nodes can contain foci of cancer. McKenna and colleagues40 found no correlation between the presence of mediastinal nodal metastases and nodal size. Metastases may be found in 21% of normal-sized nodes.41
MRI may be slightly superior to CT in the detection of mediastinal disease,42 and PET has been shown to be superior to CT for staging for the mediastinum.43,44 PET does not rely on an arbitrary cutoff of size to diagnose malignant nodes but detects the increased glycolytic rate in metabolically active tumors. In a meta-analysis, PET had a sensitivity of 79% and a specificity of 91% compared with CT, which had sensitivity and specificity of 60% and 77%, for the detection of mediastinal disease.43 In another meta-analysis by Toloza and colleagues,44 the performance characteristics of CT, PET, and EUS for staging the mediastinum in NSCLC were compared. PET was more accurate than CT or EUS for detecting mediastinal metastases with a sensitivity of 84% and a specificity of 89% for PET compared with CT (sensitivity 57% and specificity 82%) and EUS (sensitivity 78% and specificity 71%). However, PET is limited for small lesions (≤1 cm), has false-negative results in tumors with low metabolic activity, and has false-positive results in benign lesions such as granulomatous disease. Although PET has a relatively high sensitivity, because of the importance and implications of staging, specificity is still too low, and pathologic staging is still generally sought.45–47
Fritscher-Ravens and associates48 performed a prospective comparison of CT, PET, and EUS for the detection of metastatic lymph nodes metastases in patients with lung cancer being considered for operative resection. After bronchoscopic evaluation, CT, PET, and EUS were performed to evaluate potential mediastinal involvement with bronchoscopic biopsy and cytology–proven (n = 25) or radiologically suspected (n = 8) lung cancer before surgery. Surgical histology was used as the “gold standard” and revealed NSCLC in 30 patients, neuroendocrine tumor in 1 patient, and benign disease in 2 patients. With respect to the correct prediction of mediastinal lymph node stage, the sensitivities of CT, PET, and EUS were 57%, 73%, and 94%; specificities were 74%, 83%, and 71%; and accuracies were 67%, 79%, and 82%. Results of PET could be improved when combined with CT (sensitivity 81%, specificity 94%, accuracy 88%). The specificity of EUS (71%) was improved to 100% by fine needle aspiration (FNA) cytology. The authors concluded that no single imaging method alone was conclusive in evaluating potential mediastinal involvement. They also suggested that CT may be necessary to evaluate the pretracheal region and the rest of the thorax and that PET may be valuable to detect distant metastases.
Whenever enlarged lymph nodes are seen in the mediastinum on chest CT scan, standard practice is to perform a lymph node biopsy for more accurate staging. The traditional methods for performing a lymph node biopsy are via CT or bronchoscopy or both. Bronchoscopy with FNA is commonly used to evaluate suspicious paratracheal, hilar, and subcarinal lymph nodes seen on CT.49–52 The role of bronchoscopy in the diagnosis and staging of NSCLC is well established and has a sensitivity of approximately 60%.53–59 Bronchoscopy is unable to access the aortopulmonary window or the inferior mediastinal nodes, however. CT-guided biopsy of the mediastinum is limited by overlying vascular and bony structures. When the lymph node status is not determined with CT or bronchoscopy or both, mediastinoscopy and in some cases limited thoracotomy are performed to clarify the disease stage.37,60–62 However, these procedures are more invasive and require general anesthesia and inpatient recovery, increasing the time, cost, and risk of the staging process.63
Endoscopic Ultrasound
The advent of EUS has made it possible to image the GI tract and surrounding extraluminal structures such as the mediastinum with precise resolution. EUS has played an increasingly important role as an accurate and safe method for staging patients with NSCLC.64–85 With the advent of transesophageal EUS-guided FNA, suspicious posterior mediastinal lymph nodes, including aortopulmonary window, subcarinal, and inferior (below carina) paraesophageal nodes, can be sampled. Tracheal air artifact generally precludes reliable assessment of the anterior mediastinum lesions, pretracheal nodes, and upper paratracheal nodes; however, the advent of EBUS technology seems to be minimizing this limitation. The use of EUS and EBUS technology can enhance the overall accuracy for detecting mediastinal lymph node metastasis.
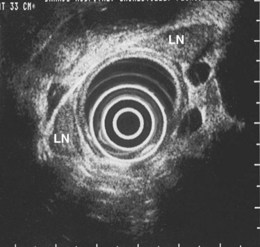
The results of an early pilot study evaluating the role of EUS in 17 patients with lung cancer found EUS to be very accurate at detecting mediastinal lymphadenopathy with an overall accuracy of 71% versus 41% for CT.83 However, the capability of performing EUS-guided FNA was unavailable during this initial study. Sampling of suspicious lymph is essential except in N1 nodes. In the 1990s, several prospective studies evaluated the accuracy of EUS, EUS-guided FNA, and chest CT scan in detecting and staging mediastinal lymph node metastasis in patients with NSCLC based on correlation with surgical staging.69,70,75 Gress and colleagues75 reported a study consisting of patients with NSCLC and enlarged mediastinal lymph nodes (>1 cm) seen on chest CT scan. EUS-guided FNA was performed for suspicious contralateral posterior mediastinal or subcarinal lymph nodes. EUS criteria used to differentiate benign from malignant lymph nodes resulted in an accuracy of 84% compared with 49% for CT. The sensitivity and specificity of CT was 64% and 35%, which compared with a sensitivity and specificity of 86% and 83% for EUS. The addition of transesophageal EUS–guided FNA improved the overall accuracy of lymph node staging to 96% with a sensitivity of 93% and a specificity of 100%. The combination of CT and EUS did not improve overall accuracy above that for EUS alone for detecting lymph node involvement. However, the addition of CT aided in evaluating the extent of the lung cancer, detecting distant metastasis not seen by EUS and evaluating anterior and pretracheal nodes, which are not imaged by EUS. EUS was best at accurately detecting mediastinal lymph node metastasis in the aortopulmonary window (station 5), subcarinal (station 7), and paraesophageal (station 8) regions (see Fig. 31.1).
These findings are similar to the studies reported by Giovannini and colleagues73 and Silvestri and coworkers,74 who reported sensitivities of 81% and 89% and specificities of 100% each. A recent meta-analysis by Micames and colleagus67 showed a pooled sensitivity of 83% and a pooled specificity of 97% for EUS-FNA staging of NSCLC.
Although still not approaching the “gold standard” of mediastinoscopy and lymph node biopsy, EUS-FNA has greatly improved minimally invasive staging of NSCLC. As noted earlier, where EUS fails is in the anterior mediastinum where tracheal air artifact generally precludes reliable assessment of the anterior mediastinum lesions, pretracheal nodes, and upper paratracheal nodes. In an attempt to view the anterior mediastinum, radial EBUS was initially developed in the 1990s. There was limited use of the technology, however, because it was impossible to perform real-time guided FNA of lesions. In more recent years, convex, linear probe technology has been developed and now allows for real-time EBUS-guided transbronchial needle aspiration (TBNA). A systematic review of EBUS-TBNA showed that it was a safe and effective modality to aid in the staging of NSCLC with a sensitivity ranging from 85% to 100% without reported complications.68 Gu and colleagues69 performed a meta-analysis of studies aimed at measuring EBUS-TBNA accuracy and found a 93% sensitivity and 100% specificity in detecting mediastinal lymph node metastasis. Given the high sensitivity and specificity of both EUS-FNA and EBUS-FNA, many investigators have been seeking to perform a complete, “medical mediastinoscopy,” with the hope of avoiding the more traditional, invasive staging, including mediastinoscopy, of NSCLC before operative intervention.
The largest study to date was performed by Wallace and colleagues,70 who compared the use of TBNA, EUS-FNA, and EBUS-TBNA using pathologic confirmation of malignancy or benign disease as the diagnostic standard. This group found EBUS-FNA to be more sensitive, with a higher negative predictive value than TBNA. More importantly, they found the combination of EBUS-FNA with EUS-FNA to have a sensitivity of 93%, a positive predictive value of 100%, and a negative predictive value of 97%. These results suggest that EBUS-FNA may complement EUS-FNA and possibly could provide near-complete, minimally invasive staging of the mediastinum in patients with NSCLC. These combined modalities may eventually eliminate the need for mediastinoscopy or other invasive surgical procedures for staging purposes.
Endoscopic Ultrasound Technique for Imaging the Mediastinum
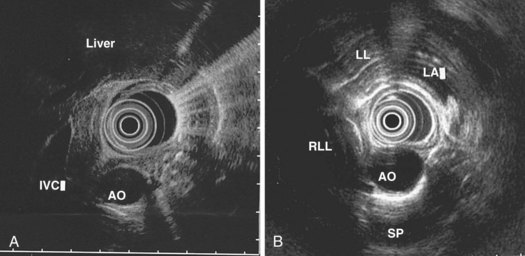
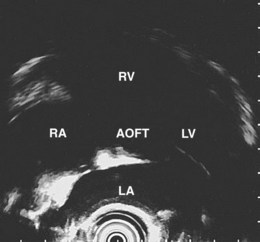
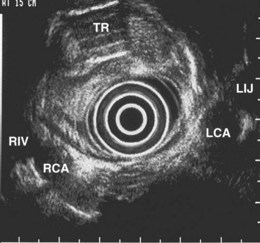
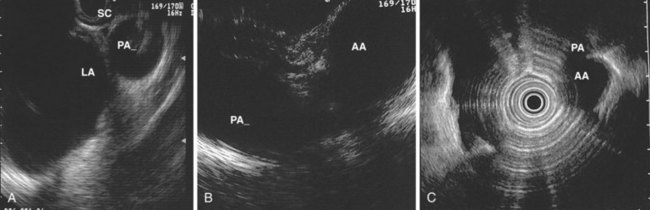
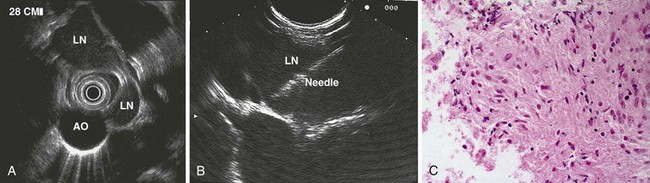
The right lung appears as hyperechoic rings emanating from the 9 o’clock position, whereas the left lung appears at the 2 o’clock position. In the midesophagus, the right and left bronchi are easily demarcated by the hyperechoic rings (echogenic air) seen at the 11 o’clock and 1 o’clock positions. The two bronchi join together to form the trachea normally at 27 to 28 cm from the incisors. The azygos vein can be seen coming into position to the right of the aorta and moves anterior to the spine and toward the right lung. As the endoscope is withdrawn further, the azygos vein can be seen to move forward and extend anteriorly into the superior vena cava. The ascending aorta can be difficult to trace because this structure runs deep to the hilar structures (pulmonary vessels), and because of air within the bronchi and trachea, the ascending aorta is often not fully imaged. In the proximal esophagus, the aortic arch is identified on the left and moves rightward and anteriorly across the screen. In the cervical esophagus, above the level of the aortic arch, the carotid vessels and, occasionally, the thyroid gland can be seen (Figs. 31.2, 31.3, and 31.4).
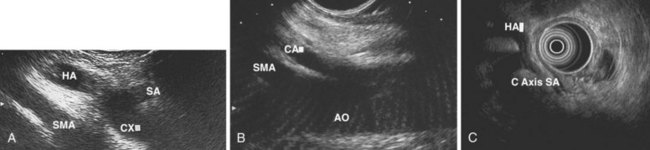
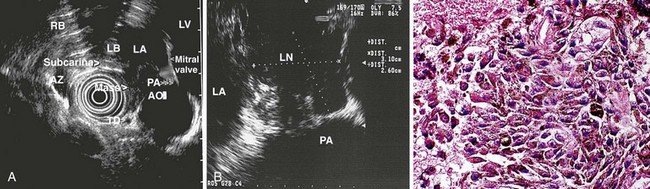
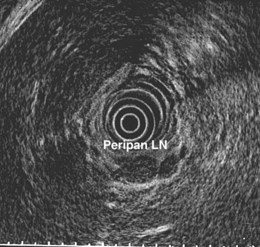
Evaluation of the mediastinum using linear EUS requires rotation of the echoendoscope every few centimeters for a thorough evaluation. As in radial EUS, vascular structures provide the major landmarks for orientation, and the home base structure is the descending aorta, which is first located approximately 35 cm from the incisors. The echoendoscope is rotated initially clockwise (right) bringing structures anterior to the esophagus into view and then counterclockwise (left) bringing posterior structures into view. The left atrium is found by rotating the shaft of the scope 180 degrees in the distal esophagus to midesophagus until a large, echolucent structure is seen within which the mitral valve leaflets are located. By tipping the scope upward and with slight withdrawal, the subcarinal lymph node station is located immediately beneath the endoscope at approximately 27 cm between the left atrium and right pulmonary artery. The aortopulmonary window is located by following the descending aorta cephalad to the arch and pushing the endoscope in again about 2 cm. The endoscope is turned 90 degrees clockwise and tipped up slightly until a cross-sectional view of the aortic arch and the more distally located left pulmonary artery are seen. The area between these structures is known as the “AP window.” Another potentially important area for FNA of lymph nodes is the celiac axis. This area is located by finding the abdominal aorta at the level of the GE junction and the takeoff of the celiac artery with the superior mesenteric artery just distal to this (Figs. 31.5 and 31.8).
Techniques for Staging Non–Small Cell Lung Cancer with Endoscopic Ultrasound
The preparation of the patient is the same as for standard endoscopy. Prophylactic antibiotics are not administered unless recommended by the American Heart Association or the American Society of Gastrointestinal Endoscopy because EUS-guided FNA of mediastinal lesions is not associated with significant bacteremia. However, prophylactic quinolone antibiotics are recommended for FNA of cystic mediastinal lesions, which is similar to the recommendations for pancreatic cystic lesions and perirectal lesions.86–93 After informed consent is obtained and conscious sedation is administered (we have found propofol to be effective), the instrument is advanced into the stomach, and the celiac axis is imaged. The probe is slowly withdrawn to the GE junction and then cephalad using radial scanning images generally obtained with 7.5-MHz frequencies at each 1-cm interval while keeping the aorta at the 5 o’clock or 6 o’clock position. All mediastinal lymph nodes seen are “mapped” by location according to the American Thoracic Society classification scheme (see Box 31.1, Table 31.1, Fig. 31.1, and video on expertconsult.com).12–14
An objective determination is made as to whether the mediastinal lymphadenopathy detected by EUS is consistent with benign or malignant status according to previously reported studies using the same criteria.82,87,94–100 EUS criteria used to diagnose malignant lymph nodes are round shape, sharp distinct borders, hypoechoic texture, and a short-axis diameter greater than 5 mm. Each of these parameters should be present for a lymph node to be considered as potentially malignant; however, FNA has significantly improved the sensitivity and specificity of detection of malignant lymph nodes.73–75,84,85,87,100,101
Technique for Performing Endoscopic Ultrasound–Guided Fine Needle Aspiration
Mediastinal Lymph Nodes
The EUS-guided FNA biopsy technique was initially developed for use with the linear array instrument and has been described elsewhere.95–97,102,103 The unique viewing angle of the linear array transducer allows for observation of the needle as it exits the biopsy channel and enables direction of the needle tip into the target lesion. A similar technique using a radial scanning echoendoscope has been reported; however, serious complications have been described via this technique, and it is not recommended.94,103
EUS-guided FNA involves the insertion of the FNA catheter device through the accessory channel of the echoendoscope followed by deployment of the needle under EUS guidance into the lymph node to be sampled. The handle mechanism is secured to the accessory port, and if the instrument has an elevator, the elevator should be fully released into the down position to allow easy passage of the needle. The elevator can be used during the biopsy to direct the needle gently into the lesion. Doppler is used to identify surrounding vascular structures. The FNA needle is slowly advanced toward the target lesion. With certain needles, it helps if the stylet is withdrawn a few millimeters (2 to 3 mm), and the needle and the stylet are then directed into the target. When the needle has entered the lesion, the stylet is advanced (to clear the needle) and then removed. The endosonographer or assistant applies suction to the catheter system using a 5-mL or 10-mL Luer-Lok syringe. Suction is followed by “in-and-out” movements of the catheter after firmly locking the needle-catheter system to the appropriate depth so that the needle is not advanced beyond a desired depth. Typically, we make 7 to 10 gradual in-and-out movements within the lesion. Before removing the needle, the negative pressure is released slowly, the needle is removed from the lesion, and subsequently the needle system is unscrewed from the echoendoscope. It has been suggested to perform EUS-guided FNA of lymph nodes without the use of suction because suction may result in a bloody sample that may be more difficult for the cytopathologist to examine.100
Preliminary cytology findings are obtained immediately during the FNA procedure by a cytopathologist or cytotechnologist present during the study. We recommend having a cytopathologist or cytotechnologist present during the EUS-guided FNA portion of the procedure because it can improve the efficiency of the technique. If a cytopathologist or cytotechnologist is unavailable, two to three passes should be taken by the endosonographer for lymph nodes (or liver metastases) and five to six passes should be taken for masses (similar to pancreatic masses) to ensure adequate cellularity in more than 90% of cases.100,104 However, this approach is associated with a 10% reduction in definitive cytologic diagnoses, increased time and risk, and potentially the need for additional needles.104
The FNA sample obtained is prepared for reviewing using Diff-Quik stain (HARLECO; EMD Chemicals, Inc, Gibbstown, NJ) applied to the slide containing the deposited specimen or fixed with ethanol. Additional passes are made until a positive cytology or adequate tissue sample is obtained. When lymphoma is suspected, added material is collected if possible and placed in a preservative solution (GIBCO RPMI Media 1640; Life Technologies, Carlsbad, CA) for subsequent flow cytometry and immunocytochemistry as indicated.105,106 If an infection is suspected, a culture media can be used.
Other Malignant Mediastinal Disease
The most important indication for mediastinal imaging is the detection or staging (or both) of lung cancer. There are several reports of EUS-guided FNA in the cytologic diagnosis of mediastinal metastases from various extrathoracic malignancies in which EUS has played a pivotal role in detection or staging. These include metastatic pancreatic, esophageal, gastric, colon, laryngeal, germ cell, renal cell, breast, and ovarian cancers.76,77,79–83,107–110
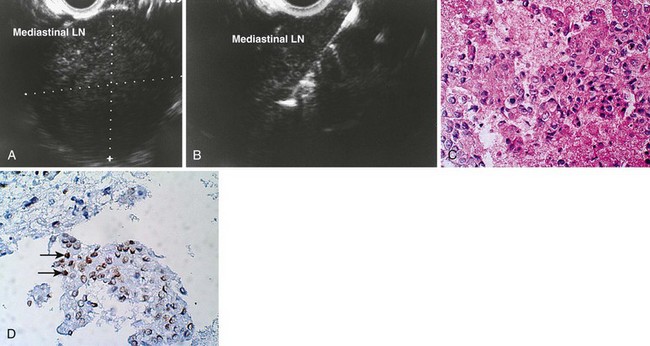
Most of the experience with EUS in patients with lymphoma has been in the setting of gastric lymphoma, although more recent studies support the use of EUS-FNA with flow cytometry for the diagnosis of mediastinal lymphoma as well.80,111–113 In a study by Fritscher-Ravens and colleagues, 153 patients with mediastinal lymphadenopathy undergoing EUS-guided FNA, lymphadenopathy originating from the lung was present in greater than 80% of patients without a previous cancer diagnosis, whereas recurrence of extrathoracic sites was the major cause of mediastinal lymphadenopathy in patients with a previous malignancy.80 Benign lesions and treatable second cancers were found in a significant minority of patients.
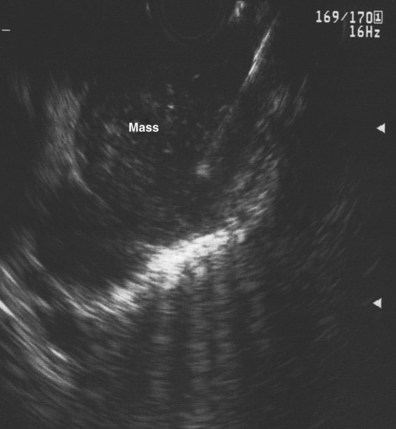
Devereaux and colleagues110 retrospectively reviewed a large, single-center experience with EUS-guided FNA for the diagnosis of mediastinal mass or lymphadenopathy in the absence of known pulmonary malignancy. In this report, 49 patients were analyzed; a malignant process was diagnosed in 22 of 49 (45%), and a benign process was found in 24 of 49 (49%). These included four patients with previously undiagnosed lung cancer, whereas metastatic breast carcinoma was the most frequent (6 of 22 [27%]) lesion. EUS-guided FNA was diagnostic in 46 of 49 (94%) patients. Catalano and coworkers107 reported a multicenter study of 62 patients in which FNA results were classified as benign or infectious, malignant pulmonary, and malignant mediastinal (lymphoma, metastatic malignancy); EUS-guided FNA was diagnostic in 90% of cases. Panelli and associates115 reported a series of 33 patients with mediastinal masses, which represented 2.3% of 1447 upper EUS examinations over a 5-year period. EUS-guided FNA was performed in 25 of 33 (76%), of which 22 (67%) ultimately were determined to be malignant. Wiersema and coworkers82 reported a series of 82 patients with mediastinal lymphadenopathy, and EUS-guided FNA after other nonsurgical techniques failed to provide a diagnosis or could not be used. The sensitivity and specificity of EUS-guided FNA were 96% and 100%. In addition, a meta-analysis identified 76 studies, 32 of which described EUS-guided FNA, which confirm a high sensitivity and specificity for EUS-guided FNA in the diagnosis of mediastinal lymphadenopathy.116 These studies suggest that in the absence of accessible extrathoracic disease lesions or as an alternative, EUS-guided FNA is a useful technique for the cytodiagnosis of extrathoracic cancers that are metastatic to the mediastinum (Fig. 31.9).
Nonmalignant Mediastinal Disease
Although commonly present in patients with suspected or known pulmonary malignancy, mediastinal lymph nodes are also present in patients with benign diseases such as histoplasmosis, tuberculosis, and sarcoidosis.80,110,117–121 In addition, benign cystic structures such as congenital foregut cysts account for approximately 20% of mediastinal masses.93
Wiersema and coworkers117 described three patients with dysphagia from compression of the esophagus by mediastinal masses. EUS showed that the masses were enlarged lymph nodes with anechoic areas thought to represent caseating necrosis. The EUS-guided FNA finding of reactive lymphocytes along with a positive complement fixation titer was instrumental in making the diagnosis of mediastinal histoplasmosis. Savides and colleagues119 described 11 patients with dysphagia who had a midesophageal submucosal mass or stricture. EUS findings consisted of large, matted posterior mediastinal lymph nodes in all patients. The diagnosis of histoplasmosis was supported by the EUS finding of lymph node calcifications in seven of these patients, symptomatic improvement in response to antifungal medication in all seven patients who were treated, and a mean follow-up of 20.5 months in which none of the patients developed signs of a malignancy.
EUS has been reported to be an accurate and simple method for the diagnosis of sarcoidosis, a systemic granulomatous disease with a predilection for the lung and mediastinal lymph nodes.106,107,120–123 In most situations, the recommended procedure is a transbronchial biopsy, which has a diagnostic yield of 40% with one biopsy and 90% when four biopsy specimens are obtained.122 If the transbronchial approach is unsuccessful, more invasive diagnostic procedures such as mediastinoscopy or lung biopsy may be used.
Several more recent reports have described the utility of EUS-guided FNA in the diagnosis of sarcoidosis manifesting with mediastinal lymphadenopathy.106,107,120–122,124 Mishra and coworkers120 described seven patients with mediastinal lymphadenopathy in which EUS helped confirm the diagnosis of sarcoidosis. Nodes were 1.8 to 6 cm in the long axis and were elongated and triangular and described as draping around the esophagus. In a retrospective review of 21 consecutive patients, Gress and colleagues123 found EUS with FNA to have a sensitivity of 86% in diagnosing sarcoidosis in patients with mediastinal lymphadenopathy, but they failed to identify reliable EUS characteristics (shape, size, echogenicity, or homogenicity pattern) specific for the disease.
A study reporting the results of EUS-guided FNA evaluation in 19 patients with suspected sarcoidosis revealed enlarged mediastinal lymph nodes (mean size 2.4 cm) located subcarinally (n = 15), in the aortopulmonary window (n = 12), and in the lower posterior mediastinum (n = 5).107 The nodes were described as isoechoic or hypoechoic, with “atypical” vessels in five cases. The aspirate obtained using EUS-guided FNA was adequate in all patients and contained blood in excess of normal in some, which was believed to be indicative of a high degree of vascularity. Cytology showed epithelioid cell granuloma formation, and cultures for mycobacteria were negative in all of the patients except one, in whom the final diagnosis was tuberculosis. The specificity and sensitivity of EUS-guided FNA in the diagnosis of sarcoidosis were 94% and 100%. Fritscher-Ravens and coworkers80 found 2 patients with tuberculosis in 101 patients without a history of cancer who had EUS-guided FNA of mediastinal lymph nodes. This study highlights the need for acid-fast staining and culture to exclude tuberculosis in cases of noncaseating granulomas. In addition, there is potential for EUS-guided FNA in the cytologic diagnosis of intraabdominal and pancreatic sarcoidosis (Figs. 31.10 and 31.11).122,123,125,126
Approximately 4% of regional lymph nodes of carcinomas have noncaseating epithelioid granulomas so that a presumptive diagnosis of sarcoidosis or another granulomatous disease should be made only after careful exclusion of malignancy and close follow-up.127 Detectable mediastinal lymph nodes may also be present in normal subjects and are often found in patients undergoing EUS examinations for various indications (Fig. 31.12).82,128 It has been postulated that detectable mediastinal lymph nodes in asymptomatic individuals may be related to prior histoplasmosis or other pulmonary infections.119 Devereaux and colleagues,110 in their retrospective study of 49 patients with mediastinal masses in the absence of known pulmonary malignancy, found a benign process in 24 of 49 (49%) including 8 patients with histoplasmosis, 1 patient with sarcoid, 2 patients with leiomyoma, 2 patients with duplication cyst, 1 patient with teratoma, and 10 patients with benign lymph node cytology on EUS-guided FNA.
EUS is often useful in distinguishing cystic lesions from solid lesions in the mediastinum, whereas chest CT scan can have limited utility in providing this distinction.91,129–134 Wildi and coworkers91 reported on a retrospective review of the results of EUS in 20 patients with suspected mediastinal cysts. The features used to classify cysts were as follows: Benign simple cysts appear as anechoic or hypoechoic smooth, spherical structures with well-defined thin walls; esophageal duplication cysts are adherent to the esophagus, whereas cysts originating from the airways are designated as bronchogenic cysts. Cysts that do not fall into either category are termed nonspecific duplication cysts. A layered wall structure supports the diagnosis of duplication cyst but is not mandatory. Simple cysts include mesothelial cysts, lymphogenous cysts, and thoracic duct cysts. Simple cysts do not have a layered wall and do not have a connection to the airways or esophagus and are termed nonspecific simple cysts. When solid tissue is seen within the fluid, the cysts are considered complex (e.g., benign cystic teratoma, thymic cyst), and the diagnosis of a benign simple cyst is excluded.
In 19 of 20 patients reported by Wildi and coworkers,91 definite diagnosis of a mediastinal cyst was established by EUS (12 anechoic, 6 hypoechoic, 1 anechoic with small echoic foci). In only 4 of 18 cases was CT (17 cases) or MRI (1 case) diagnostic of a cyst. In three cases with mixed echo features, EUS-guided FNA was performed with administration of prophylactic antibiotics. In a fourth case, without prior prophylactic administration of an antibiotic, FNA was performed in a solid-appearing duplication cyst misdiagnosed by EUS as extensive lymphadenopathy. Mediastinitis subsequently developed requiring thoracotomy, in which an infected bronchogenic cyst was diagnosed. The authors concluded that aspiration of cysts should be avoided in lesions with clearly anechoic features because of the risk of infection, whereas FNA should be considered for hypoechoic lesions (when a cyst cannot be clearly distinguished from a solid tumor), but prophylactic antibiotics should be administered (Fig. 31.13).
Adrenal and Renal Lesions
EUS can provide early excellent images of the left adrenal gland. The right adrenal gland is also accessible by EUS, and its routine evaluation may be feasible, although it is more difficult to visualize than the left adrenal gland, and the procedure is not routinely performed.135 The left adrenal gland is more difficult to view than the right gland when imaging with transabdominal ultrasound.136–138
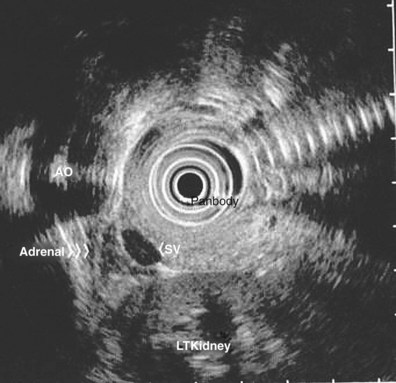
When imaging the left adrenal gland, the echoendoscope is advanced into the proximal stomach, and the aorta is identified just below the GE junction. The splenic vein is imaged by advancing the transducer forward with a clockwise rotation. Following the splenic vein laterally, the splenic hilum is found by further clockwise rotation and slight withdrawal. The left kidney is imaged by advancing the scope from the splenic hilum with a slight counterclockwise rotation. The left adrenal gland lies just below the splenic vein, between the left kidney (superior and medial to the kidney) and the aorta. In a study by Chang and colleagues,138 the average long-axis dimension of the adrenal gland was 2.5 cm, and the short-axis dimension was 0.8 cm. The adrenal gland as imaged on EUS is homogeneous and hypoechoic with two basic morphologic types: seagull shape and elliptical shape. Occasionally, the adrenal gland can appear as both shapes in the same patient with a slight change in the orientation of the EUS probe tip.138 In some patients, the central region of the gland may appear more echogenic than the peripheral region (Fig. 31.14).
Incidental benign adrenal lesions, so-called adrenal incidentalomas, are commonly found on CT scans performed for various indications. Unless unequivocally benign, biopsy of these lesions should be performed in certain scenarios. CT scans performed in the staging work-up of lung cancer reveal that more than 16% of patients have adrenal masses on screening examination.138–143 Metastasis to the adrenal glands as the cause of isolated mass lesions occurs in 32% to 93% of cases of NSCLC as determined by FNA cytology.141,144 In autopsy series of NSCLC, adrenal metastases are found in 59% of cases.145,146
EUS-guided FNA may provide an alternative to percutaneous aspiration technology in the evaluation of adrenal lesions. EUS-guided biopsy of an adrenal mass has been reported in a patient in whom CT-guided FNA was unsuccessful.147 Chang and associates147 reported the identification of the left adrenal gland in 97% of 31 consecutive patients undergoing EUS for known pulmonary or GI malignancies. In one patient with a history of lobectomy for lung cancer, staged as T1N0, a follow-up CT scan showed interval enlargement of an adrenal mass from 2.5 cm to 4 cm. A previous CT-guided FNA was negative for malignancy. An EUS-guided FNA of the left adrenal gland was performed and revealed metastatic disease. A more recent case series also suggests that the right adrenal gland may be more accessible by EUS-guided FNA than previously believed.135 Given the clinical impact of an adrenal metastasis, routine assessment of the left and possibly right adrenal glands in patients with lung cancer is recommended during the EUS evaluation.147
EUS of the right and left kidneys is possible because of the immediate approximation between the GI lumen and the kidneys. The right kidney can be imaged by placing the transducer in the second portion of the duodenum and rotating laterally. The left kidney can be imaged from the body of the stomach, posterior to the spleen, as described previously. The left kidney often is easier to show than the right kidney. The kidneys have a hyperechoic central medulla, a hypoechoic outer cortex, and a thin echogenic capsule (Fig. 31.15).
Approximately 85% of renal masses detected on CT are renal cell carcinomas, and 15% of CT-detected renal masses are benign lesions.148,149 Biopsy of malignant-appearing masses that are resectable should not be routinely performed. Biopsy of a solitary renal mass is generally accepted in cases with a known primary extrarenal malignancy when the presence of a metastasis would alter management.148,150 Biopsy specimens of renal masses have traditionally been obtained under either transabdominal ultrasound or CT guidance with a sensitivity of 62% to 100% and a specificity of 0% to 100% in the diagnosis of renal cell carcinoma.114,148 No data currently exist regarding the frequency with which renal masses are identified during upper EUS examinations. A few case reports exist showing the possibility of making a diagnosis of renal cell carcinoma using EUS-guided FNA148,151; however further experience is required before the establishment of EUS-guided FNA as a modality for performing renal biopsy.
Ascites and Pleural Fluid
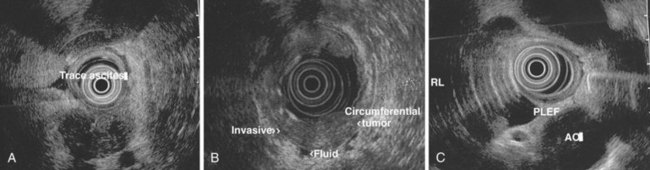
The identification of malignant pleural effusion or malignant ascites is diagnostic of advanced disease in various malignancies. EUS seems to be more sensitive than CT in the detection of small amounts of ascites and pleural fluid. Drainage of pleural fluid or ascites at the time of EUS is possible and can be helpful if positive for malignancy. The technique of EUS-guided FNA of ascites or pleural fluid is similar to the technique applied to other lesions. Seeding of malignant cells into the fluid through the GI tract is a concern. The site of the needle penetration in the GI lumen must not be involved with tumor (Fig. 31.16).
Chang and associates147 first reported the detection of malignant fluid via EUS-guided FNA of pleural effusion and ascites in patients with gastric cancer. In a larger retrospective study, these same investigators reported the utility of EUS for the detection of ascites and EUS-guided FNA in 571 consecutive patients who underwent upper EUS for various indications.152 EUS detected ascites in 85 (15%) patients, whereas CT detected ascites in 14 (18%) of the 79 patients who underwent CT scanning before EUS. Of 85 patients, 31 underwent EUS-guided FNA paracentesis, and malignant ascites was diagnosed in 5 of these patients. More recently, investigators have been looking at whether detection of low-volume ascites by EUS is a good predictor of outcomes in esophageal cancer.139,153 These studies have shown that patients with ascites detected only by EUS examination have a shorter survival than patients without low-volume ascites. However, patients with ascites whose tumors were still deemed surgically resectable had the same survival rate as patients without ascites. These findings have been supported by similar studies that have described the clinical significance of peritoneal fluid detected by EUS.154,155
Liver Lesions
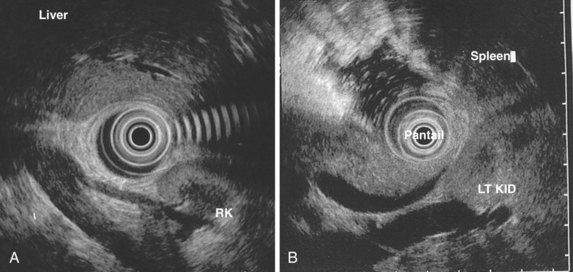
EUS provides very good imaging of the left lobe of the liver and a significant portion of the right lobe of the liver. The left lobe and hilum of the liver are examined from the gastric body and fundus. The tip of the echoendoscope is placed in the gastric antrum; then, slowly withdrawing the echoendoscope, the tip is deflected up and rightward. When the liver comes into view, the instrument is rotated to evaluate portions of the liver. The right lobe of the liver is best imaged from the duodenum but can also be seen from the antrum. Liver lesions near the second or third portion of the duodenum, peripheral lesions near the dome of the diaphragm, and lesions in the inferior portion of the right lobe of the liver can be difficult to visualize (see Figs. 31.2 and 31.15).156
CT-guided and ultrasound-guided FNA of liver lesions have been reported to have sensitivities of 83% to 93% for the detection of malignant disease.138,140–143 Although EUS has not traditionally been used in evaluation of the liver, early experience suggests that EUS-guided FNA is comparable to CT-guided FNA in terms of safety and diagnostic utility for hepatic lesions.156–161 Nguyen and associates156 conducted a prospective study in which 574 consecutive patients with a history or suspicion of GI or pulmonary tumor who were undergoing upper EUS examinations underwent EUS evaluation of the liver. They found small focal liver lesions that were undetected with conventional CT. Of patients, 14 (2.4%) were found to have focal liver lesions (five right lobe, nine left lobe) and underwent EUS-guided FNA. The median largest diameter of the liver lesions was 1.1 cm (range 0.8 to 5.2 cm), and the mean number of passes per lesion was 2.0 (range 1 to 5 passes). Of the 15 liver lesions sampled via EUS-guided FNA, 15 were malignant and 1 was benign. Before EUS, CT depicted liver lesions in only 3 of 14 (21%) patients; in most patients, CT was performed within 2 months of the EUS procedure. In seven patients, the initial diagnosis of cancer was made by means of EUS-guided FNA of the liver. There were no immediate or late complications.
Rarely, percutaneous FNA of the liver has been associated with tumor seeding, intrahepatic hematoma, and hemorrhage.162–166 As postulated by Nguyen and coworkers,156 EUS-guided FNA has the possible advantage over the percutaneous route of shorter insertion length of the needle if the liver lesion is deep to the skin surface. With EUS-guided FNA, there is continuous visualization of the needle tip, which helps to minimize risk for bleeding when the procedure is performed in conjunction with color flow and Doppler ultrasound. tenBerge and colleagues161 reported a multicenter study of 167 cases of EUS-guided FNA of the liver. Complications were reported in six (4%) cases, including death in one patient with an occluding biliary stent and biliary sepsis, bleeding (one case), fever (two cases), and pain (two cases). In 23 of 26 patients, EUS-guided FNA helped diagnose malignancy after nondiagnostic FNA with transabdominal ultrasound guidance. EUS was able to localize an unrecognized primary tumor in 17 of 33 (52%) cases after CT showed only liver metastases. This study highlighted the fact that adequate biliary drainage is recommended in the setting of cholangitis before or coincident with the FNA procedure. EUS-guided FNA of liver lesions seems to be safe and effective and may become a more useful diagnostic method for liver lesions.
Miscellaneous
The close proximity of the intestinal tract to abdominal organs raises the possibility of EUS-guided FNA of idiopathic abdominal masses. Catalano and coworkers167 retrospectively evaluated the diagnostic accuracy of EUS-guided FNA of abdominal masses of unknown cause and its impact on subsequent evaluation. From five tertiary referral centers, 34 patients with idiopathic abdominal masses underwent EUS-guided FNA after evaluation including CT or transabdominal ultrasound, or both. CT showed an intraabdominal mass in all patients. Four patients had a history of intraabdominal cancer (two cervical, one ovarian, one colon), but these cancers were considered to be in remission. A final diagnosis for the mass lesions was established in all patients by various methods, including EUS-guided FNA, surgery, autopsy, or long-term follow-up. Abdominal masses were classified into three categories: infectious, benign or inflammatory, and malignant. EUS-guided FNA established a tissue diagnosis in 29 of 34 (85%) patients: infectious, 80% including abscess and infected pseudocyst; benign or inflammatory, 67% including hematoma or postsurgical inflammatory mass, leiomyoma, and sarcoidosis; and malignant, 91% including sarcoma, lymphoma, hepatoma, adenocarcinoma of unknown primary, ovarian cancer, transitional bladder carcinoma, uterine or cervical cancer, recurrent colon cancer, neuroendocrine tumor, paraganglionoma, metastatic lung cancer, and prostate cancer. EUS-guided FNA was instrumental in directing subsequent evaluation in 29 (85%) patients and therapy in 26 (77%). The number of fine needle passes needed for adequate tissue sampling was lower for nonmalignant (2.2 to 3.2 passes) versus malignant diseases (4.6 passes). A perirectal abscess developed in one patient and was treated successfully with antibiotics.
Cases using EUS-guided FNA to diagnose a schwannoma of the mediastinum168 and a retroperitoneal neurilemoma have been described.169 In addition, studies by Erickson and Tretjak170 and Anand and colleagues171 have helped show that EUS-guided FNA is an effective method with a high sensitivity and specificity for diagnosing and altering patient management of nonpancreatic lesions adjacent to the GI tract.
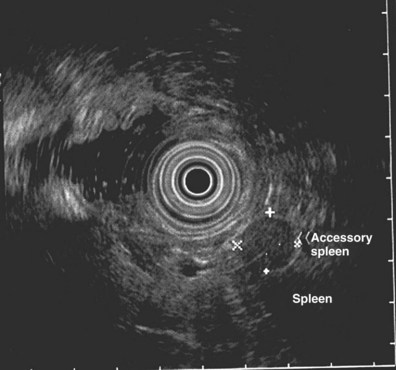
Accessory spleen may be a potential cause of misinterpretation on EUS. Barawi and colleagues172 described the EUS features of accessory spleen in 10 (8 accessory spleen, 2 lobulated spleen) patients. The mean size of these lesions was 2.7 cm × 3.1 cm. Nine accessory spleens were round, and one was oval. All were located inferolateral to the pancreatic tail and medial to the spleen. All of these lesions had a sharp and regular outer margin and homogeneous echo texture; four were hypoechoic, and six were hyperechoic. CT scan may be helpful in confirming the presence of lobulated spleen and accessory spleen (Fig. 31.17).
Fritscher-Ravens and associates173 described EUS-guided FNA of splenic lesions in 12 patients when other modalities were inconclusive (n = 5), not attempted because of small size (0.9 to 1.4 cm; n = 4), or considered dangerous (adjacent to the splenic hilum or located peripherally; n = 3). The lesions ranged in size from 0.8 to 4.2 cm (median 1.4 cm). A positive diagnosis was made in 10 of 12 patients (83%); cytology was inadequate in 1 patient. Bacteriology was positive for Staphylococcus aureus and Serratia in one patient each and for Mycobacterium tuberculosis in two patients. Diagnoses included lymphoma, sarcoidosis, abscesses, tuberculosis, metastatic colon cancer, and infarction (in one patient). One patient experienced pain after the procedure, but no hematoma was shown on subsequent ultrasound examination. Since this study, other studies have been performed supporting the possibility that EUS-guided FNA of splenic masses is feasible and safe.174,175
Complications
EUS-guided FNA is a relatively safe procedure compared with CT-guided FNA, bronchoscopy with transbronchial FNA, mediastinoscopy, or open or exploratory procedures. Generally, when complications occur, these are usually mild and self-limited. In reports and more recent prospective studies that have addressed complications of EUS-guided FNA, rare complications included endoscope-induced perforations, febrile episodes (after FNA of pancreatic cystic lesions), hemorrhage, pancreatitis, and pneumoperitoneum; false-positive diagnoses have been described.79,89,92,93,158,176–180 As alluded to previously, the role of prophylactic antibiotics for EUS-guided FNA is unclear. The general practice has been to administer antibiotics to any patient undergoing FNA of cystic pancreatic or perirectal lesions. Barawi and colleagues86 studied 108 consecutive EUS-guided FNA cases that did not show bacteremia at 30 minutes and 60 minutes after the procedure. However, the study by Van de Mierop and colleagues93 revealed a 19% incidence of bacteremia with EUS-guided FNA of solid lesions.
Endoscopic Ultrasound–Guided Celiac Plexus Block and Celiac Plexus Neurolysis
Pain resulting from pancreatic cancer and chronic pancreatitis is often difficult to manage. Many approaches have been used to treat these patients, including narcotic analgesia, antidepressants, pancreatic enzymes, octreotide, denervation procedures (most commonly CPB), and various palliative or decompression or drainage procedures.180–192 The effectiveness of these therapies is not only highly variable but also often controversial, especially in the treatment of chronic pancreatitis. Opioid analgesics are probably used most often and can treat pain effectively, but they are associated with numerous side effects, including constipation, delirium, nausea, and the potential for addiction in patients with chronic pancreatitis.193,194 Nonpharmacologic methods of pain control may improve quality of life and minimize drug-related side effects.193
The celiac plexus lies anterior to the aorta at the level of the celiac artery. Most of the sensory nerves returning from the pancreas and other intraabdominal viscera pass through the celiac ganglion and splanchnic nerves. Interruption of these fibers may lessen pain in pancreatic malignancies and in chronic pancreatitis.195 CPB, a temporizing treatment, most commonly refers to injection of a steroid and long-acting local anesthetic into the celiac plexus to control pain associated with chronic pancreatitis. In contrast, CPN generally refers to injection of alcohol or phenol, a more permanent agent, into the celiac axis area.195 This technique induces a chemical splanchnicectomy that ablates the nerve fibers that transmit pain and is used in patients with pancreatic cancer; however, neural regrowth may limit the effect.195 In practice, the terms CPB and CPN are often interchangeable.
The efficacy of CPN for the treatment of cancer pain has been shown in numerous studies. The benefit seems to be similar independent of the method used with pain control in 70% to 90% of patients up to 3 months after the procedure.196–201 CPB and CPN have traditionally been performed via various percutaneous (most common posterior route) and surgical approaches and most recently under endoscopic guidance.202 Of the nonsurgical approaches, EUS offers the most direct access to the celiac plexus. Wiersema and coworkers193,195,203 recognized the anatomic advantage that EUS provides in visualizing the celiac region and were successful in performing transgastric EUS-guided CPN with results similar to the more traditional approaches.
The timing of CPN relative to pain onset seems to predict response. In one study, CPN was more effective when the block was performed early after pain onset.197 This result was postulated to be related to involvement of visceral and somatic nerves late in the disease and pain apparently deriving mainly from the celiac plexus early on.195,197 More recently, it has been proposed that direct injection into the celiac ganglia, multiple injections in the area of the ganglia, or bilateral injections around the celiac ganglia are safe and may be more beneficial in providing sustained pain relief.204–206 These studies are contradictory, however, and better prospective trials are needed to determine if these approaches make an improvement over the standard technique of EUS-guided CPB.
Studies suggest that CPB may have a role in the treatment of pain related to chronic pancreatitis.207,208 In a study of 18 patients with chronic pancreatitis, reduction in pain was noted in 50% (5 of 10) of EUS-guided CPB compared with 25% (2 of 8) of CT-guided blocks.207 The benefit persisted for 24 weeks in 30% of responders. A cost comparison showed a $200 saving for EUS-guided CPB compared with CT-guided CPB. Another report of 90 patients by the same investigators found a significant improvement in overall pain scores in 55% at 4 weeks and 8 weeks of follow-up.208 A persistent benefit beyond 24 weeks was observed in only 10% of patients. Pain relief was more likely in older patients (>45 years old) and patients who had not had previous surgery for chronic pancreatitis. Further studies are needed to clarify what role EUS-guided CBP will play in the management of painful chronic pancreatitis.
Technique for Performing Endoscopic Ultrasound–Guided Celiac Plexus Block and Celiac Plexus Neurolysis
The celiac artery is readily visualized with a curvilinear array EUS scope and provides the important landmark structure when performing EUS-guided CPB and CPN. For practical purposes, the celiac ganglia is located at the origin of the celiac artery, although its exact location has been described anywhere from 1 cm to 9 mm inferior to the artery takeoff (the right ganglion is most commonly 6 mm inferior and the left ganglion is most commonly 9 mm inferior to the celiac artery origin).195,197 Although the celiac ganglia themselves can be visualized in some cases, the celiac axis is more routinely identified by its relative position to the artery. The proximity of the celiac ganglia to the posterior gastric wall ensures an accurate passage of the needle into the ganglia, minimizing the risk of complications and potentially increasing the effectiveness.
Two techniques are available: one in which the injection is performed by rotating to each side of the celiac trunk and one in which the entire injection is performed just anterior and cranial to the celiac artery takeoff.193,195 For CPB, 10 mL of preservative-free bupivacaine (0.25%; Abbott Laboratories, Abbott Park, IL) and 1 mL of triamcinolone (40 mg; Fujisawa USA, Deerfield, IL) are injected on both sides of the celiac trunk. The aspiration test is repeated before each steroid injection. A 3-mL saline flush is performed before needle withdrawal on each side. For CPN, 10 mL of bupivacaine (0.25%) is injected followed by 10 mL of dehydrated 98% absolute alcohol. Before alcohol injection, the 10-second aspiration test is repeated. The needle is flushed with 3 mL of saline before withdrawal of the needle. The procedure is repeated on the opposite side of the aorta. With alcohol injection, an echo-dense cloud is typically seen, but this is not usually seen with steroid injection.
The examination, performed on an outpatient basis, is usually completed in 30 minutes. Recovery time is usually 2 hours. Before discharge, orthostatic blood pressure changes should be checked, and patients should be counseled regarding potential complications (see video on expertconsult.com).
Complications
CPB and CPN are generally effective, safe, and well-tolerated procedures. The three most common complications are transient hypotension (20% to 40%), transient diarrhea (4% to 38%), and transient increase in pain (9%), which are expected in CPB performed via any route.195,198,209 Interruption of the plexus can result in a sympathetic blockade.210 Clinical manifestations of sympathetic blockade can include diarrhea and hypotension resulting from a relative unopposed visceral parasympathetic activity. Mesenteric vasodilation accounts for the hypotension, which resolves in approximately 2 days. Diarrhea and increase in baseline pain are also usually limited to 2 days. Less common complications include unilateral paresis or paraplegia, pneumothorax, loss of sphincter function, retroperitoneal bleeding, renal puncture, and prolonged gastroparesis.193,195,198,199,209 In addition, cephalic spread of the neurolytic agent may result in involvement of the cardiac nerves and plexus.211 Although not unique to EUS-guided CPN, EUS may decrease the incidence of complications because the needle does not traverse the paraspinal region or somatic nerves or traverse the diaphragm and pleural space.180,193,195 Another benefit with EUS-guided CPN is the ability to perform the block as part of a single procedure that may include tumor staging and FNA.212 Infectious complications are uncommon but potentially serious. In a series of 90 patients, only 1 patient developed an infectious complication (peripancreatic abscess), which resolved with a 2-week course of antibiotics.208 The authors reasoned that there may have been a predisposition to infection owing to gastroduodenal colonization with bacteria because the patient was taking a proton pump inhibitor. They suggested that prophylactic antibiotics should be considered in patients who are receiving acid suppression. The bactericidal nature of ethanol seems to minimize this risk for infection, and some experts do not routinely use antibiotics in this setting, regardless of concurrent acid suppression.193
1 Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. Ca Cancer J Clin. 2003;53:5-26.
2 Travis WD, Lubin J, Ries L, et al. United States lung carcinoma incidence trends: Declining for most histologic types among males, increasing among females. Cancer. 1996;77:2464-2470.
3 Argiris A, Murren JR. Staging and clinical prognostic factors for small-cell lung cancer. Cancer J. 2001;7:437-447.
4 Simon GR, Wagner H, American College of Chest Physicians. Small cell lung cancer. Chest. 2003;123(1 Suppl):259S-271S.
5 Micke P, Faldum A, Metz T, et al. Staging small cell lung cancer: Veterans Administration Lung Study Group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer. 2002;37:271-276.
6 Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:603-612.
7 Rusch V, Crowley JJ, Goldstraw P, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the N descriptors in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706-714.
8 Postmus PE, Brambilla E, Chansky K, et al. The IASLC Lung Cancer Staging Project: Proposals for revision of the M descriptors in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:686-693.
9 Groome PA, Bolejack V, Crowley JJ, et al. The IASLC Lung Cancer Staging Project: Validation of the proposals for revision of the T, N, and M descriptors and consequent stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:694-705.
10 Deslauriers J. Current surgical treatment of nonsmall cell lung cancer 2001. Eur Respir J Suppl. 2002;35:61s-70s.
11 Feins RH. Multi-modality treatment of non-small cell lung cancer. Surg Clin North Am. 2002;82:611-620.
12 Greene FL, Page DL, Fleming ID, et al, editors. AJCC (American Joint Committee on Cancer) cancer staging manual, ed 6. New York: Springer-Verlag. 2002:167-174.
13 Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710-1717.
14 Mountain CF. A new international staging system for lung cancer. Chest. 1986;89:225s-233s.
15 Barker JM, Silvestri GA. Lung cancer staging. Curr Opin Pulm Med. 2002;8:287-293.
16 Jett JR, Scott WJ, Rivera MP, et al. American College of Chest Physicians. Guidelines on treatment of stage IIIB non-small cell lung cancer. Chest. 2003;123(1 Suppl):221S-225S.
17 Robinson LA, Wagner HJr, Ruckdeschel JC. American College of Chest Physicians. Treatment of stage IIIA non-small cell lung cancer. Chest. 2003;123(1 Suppl):202S-220S.
18 Martini N, Baines MS, McCormick PM, et al. Surgical treatment in non-small cell carcinoma of the lung: The Memorial Sloan-Kettering experience. In: Hoogstraten B, Addis BJ, Hansen HH, et al, editors. Treatment of Lung Tumors. Heidelberg: Springer-Verlag; 1987:111-132.
19 Sandler AB, Buzaid AC. Lung cancer: A review of current therapeutic modalities. Lung. 1992;170:249-265.
20 Hatter J, Kohman LJ, Mosca RS, et al. Preoperative evaluation of stage I and stage II non-small cell lung cancer. Ann Thorac Surg. 1994;58:1738-1741.
21 Shields TW. Surgical therapy for carcinoma of the lung. Clin Chest Med. 1993;14:121-147.
22 Medina Gallardo JF, Borderas Naranjo F, Torres Cansino M, et al. Validity of enlarged mediastinal nodes as markers of involvement by non-small cell lung cancer. Am Rev Respir Dis. 1992;146:1210-1212.
23 Van Raemdonck DE, Schneider A, Ginsberg RJ. Surgical treatment for higher staged non-small cell lung cancer. Ann Thorac Surg. 1993;54:999-1013.
24 Naruke T, Goya T, Tsuchiva R, et al. The importance of surgery to non-small cell carcinoma of lung with mediastinal lymph node metastasis. Ann Thorac Surg. 1988;46:603-610.
25 Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surg Clin North Am. 1987;67:1037-1049.
26 Mountain CF. The biological operability of stage III non-small cell lung cancer. Ann Thorac Surg. 1985;40:60-64.
27 Glazer GM, Gross BH, Quint LE, et al. Normal mediastinal lymph nodes: Number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144:261-265.
28 Ingram CE, Belli AM, Lewars MD, et al. Normal lymph node size in the mediastinum: A retrospective study in two patient groups. Clin Radiol. 1989;40:35-39.
29 Mann H. CT in the management of lung cancer. Semin Ultrasound CT MR. 1988;9:40-52.
30 Verschakeln JA, Bogaert J, De Wever W. Computed tomography in staging for lung cancer. Eur Respir J Suppl. 2002;35:40s-48s.
31 Patterson GA, Ginsberg RJ, Poon PY, et al. A prospective evaluation of magnetic resonance imaging, computed tomography, and mediastinoscopy in the preoperative assessment of mediastinal node status in bronchogenic carcinoma. J Thorac Cardiovasc Surg. 1987;94:679-684.
32 Aronchick JM. CT of mediastinal lymph nodes in patients with non-small cell lung carcinoma. Radiol Clin North Am. 1990;28:573-581.
33 Glazer GM, Orringer MB, Gross GH, et al. The mediastinum in non-small cell lung cancer: CT-surgical correlation. AJR Am J Roentgenol. 1984;142:1101-1105.
34 Cybulsky IJ, Lanza LA, Ryan MB, et al. Prognostic significance of computed tomography in resected N2 lung cancer. Ann Thorac Surg. 1992;54:533-537.
35 Lewis JW, Pearlberg JL, Beute GH, et al. Can computed tomography of the chest stage lung cancer? Yes and no. Ann Thorac Surg. 1990;49:591-596.
36 Dales RE, Stark RM, Raman S. Computed tomography to stage lung cancer: Approaching a controversy using meta-analysis. Am Rev Respir Dis. 1990;141(5 Pt 1):1096-1101.
37 Staples CA, Muller NL, Miller RR, et al. Mediastinal nodes in bronchogenic carcinoma: Comparison between CT and mediastinoscopy. Radiology. 1988;167:367-372.
38 McLoud TC, Bourgouin PM, Greenberg RW, et al. Bronchogenic carcinoma: Analysis of staging in the mediastinum with CT by correlative lymph node mapping and sampling. Radiology. 1992;182:319-323.
39 Arita T, Matsumoto T, Kuramitsu T, et al. Is it possible to differentiate malignant mediastinal nodes from benign nodes by size? Reevaluation by CT, transesophageal echocardiography, and nodal specimen. Chest. 1996;110:1004-1008.
40 McKenna RJ, Libshitz HI, Mountain CF, et al. Roentgenographic evaluation of mediastinal nodes for pre-operative assessment in lung cancer. Chest. 1985;88:206-210.
41 Arita T, Kuramitsu T, Kawamura M, et al. Bronchogenic carcinoma: Incidence of metastases to normal sized lymph nodes. Thorax. 1995;50:1267-1269.
42 Miller JD, Gorenstein LA, Patterson GA. Staging: The key to rational management of lung cancer. Ann Thorac Surg. 1992;53:170-178.
43 Dwamena BA, Sonnad SS, Angobaldo JO, et al. Metastases from non-small cell lung cancer: Mediastinal staging in the 1990s—meta-analytic comparison of PET and CT. Radiology. 1999;213:530-536.
44 Toloza EM, Harpole L, McCrory DC. Noninvasive staging of non-small cell lung cancer: A review of the current evidence. Chest. 2003;123(1 Suppl):137S-146S.
45 Flickling W, Wallace MB. EUS in lung cancer. Gastrointest Endosc. 2002;56:S18-S21.
46 Wallace MB, Silvestri GA, Sahai AV, et al. Endoscopic ultrasound-guided fine needle aspiration for staging patients with carcinoma of the lung. Ann Thorac Surg. 2001;72:1861-1867.
47 Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343:254-261.
48 Fritscher-Ravens A, Bohuslavizki KH, Brandt L, et al. Mediastinal lymph node involvement in potentially resectable lung cancer: Comparison of CT, positron emission tomography, and endoscopic ultrasonography with and without fine-needle aspiration. Chest. 2003;123:442-451.
49 Schieppati E. Mediastinal lymph node puncture through the tracheal carina. Surg Gynecol Obstet. 1958;107:243-246.
50 Wang KP, Terry P, Marsh B. Bronchoscopic needle aspiration biopsy of paratracheal tumors. Am Rev Respir Dis. 1978;118:17-21.
51 Oho K, Kato H, Ogawa I, et al. A new needle for transfiberoptic bronchoscope use. Chest. 1979;76:492.
52 Wang KP. Flexible transbronchial needle aspiration biopsy for histologic specimens. Chest. 1985;88:860-863.
53 Shure D, Fedullo PF. Transbronchial needle aspiration in the diagnosis of submucosal and peribronchial bronchogenic carcinoma. Chest. 1985;88:49-51.
54 Harrow EM, Oldenburg FAJr, Lingenfelter MS, et al. Transbronchial needle aspiration in clinical practice: A five-year experience. Chest. 1989;96:1268-1272.
55 Schenk DA, Bower JH, Bryan CL, et al. Transbronchial needle aspiration staging of bronchogenic carcinoma. Am Rev Respir Dis. 1986;134:146-147.
56 Harrow E, Halber M, Hardy S, et al. Bronchogenic and roentgenographic correlates of a positive transbronchial needle aspiration in the staging of lung cancer. Chest. 1991;100:1592-1596.
57 Carlin BW, Harrell JH2nd, Fedullo PF. False-positive transcarinal needle aspirate in the evaluation of bronchogenic carcinoma. Am Rev Respir Dis. 1989;140:1800-1802.
58 Wang KP, Terry PB. Transbronchial needle aspiration in the diagnosis and staging of bronchogenic carcinoma. Am Rev Respir Dis. 1983;127:344-347.
59 Midthun DE, Cortese DA. Bronchoscopic needle aspiration and biopsy. In: Prakash UBX, editor. Bronchoscopy. New York: Raven Press; 1994:147-153.
60 Zwischenberger JB, Savage C, Alpard SK, et al. Mediastinal transthoracic needle and core lymph node biopsy: Should it replace mediastinoscopy? Chest. 2002;121:1165-1170.
61 Jolly PC, Hutchinson CH, Detterbeck F, et al. Routine computed tomographic scans, selective mediastinoscopy, and other factors in evaluation of lung cancer. J Thorac Cardiovasc Surg. 1991;102:266-271.
62 Merav AD. The role of mediastinoscopy and anterior mediastinotomy in determining operability of lung cancer: A review of published questions and answers. Cancer Invest. 1991;9:439-442.
63 Aabakken L, Silvestri GA, Hawes RH, et al. Cost-efficacy of endoscopic ultrasonography with fine-needle aspiration vs. mediastinotomy in patients with lung cancer and suspected mediastinal adenopathy. Endoscopy. 1999;31:707-711.
64 Lee N, Inoue K, Yamamoto R, et al. Patterns of internal echoes in lymph nodes in the diagnosis of lung cancer metastasis. World J Surg. 1992;16:986-993.
65 Sugimachi K, Ohno S, Fujishima H, et al. Endoscopic ultrasonographic detection of carcinomatous invasion of lymph nodes in the thoracic esophagus. Surgery. 1990;107:366-371.
66 Schuder G, Isringhaus H, Kubale B, et al. Endoscopic ultrasonography of the mediastinum in the diagnosis of bronchial carcinoma. Thorac Cardiovasc Surg. 1991;39:299-303.
67 Micames CG, McCrory DC, Pavey DA, et al. Endoscopic ultrasound-guided fine-needle aspiration for non-small cell lung cancer staging: A systematic review and metaanalysis. Chest. 2007;131:539-548.
68 Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: A systematic review. Eur Respir J. 2009;33:1156-1164.
69 Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta-analysis. Eur J Cancer. 2009;45:1389-1396.
70 Wallace MB, Pascual JMS, Raimondo M, et al. Minimally invasive endoscopic staging of suspected lung cancer. JAMA. 2008;299:540-546.
71 Kondo D, Imaizumi M, Abe T, et al. Endoscopic ultrasound examination for mediastinal lymph node metastases of lung cancer. Chest. 1990;98:586-593.
72 Aibe T, Ito T, Yoshida T, et al. Endoscopic ultrasonography of lymph nodes surrounding the upper GI tract. Scand J Gastroenterol. 1986;123(Suppl):164-169.
73 Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171-177.
74 Silvestri GA, Hoffman BJ, Bhutani MS, et al. Endoscopic ultrasound with fine-needle aspiration in the diagnosis and staging of lung cancer. Ann Thorac Surg. 1996;61:1441-1446.
75 Gress FG, Savides TJ, Sandler A, et al. Endoscopic ultrasonography, fine-needle aspiration biopsy guided by endoscopic ultrasonography, and computed tomography in the preoperative staging of non-small-cell lung cancer: A comparison study. Ann Intern Med. 1997;127(8 Pt 1):604-612.
76 Hunerbein M, Ghadimi BM, Haensch W, et al. Transesophageal biopsy of mediastinal and pulmonary tumors by means of endoscopic ultrasound guidance. J Thorac Cardiovasc Surg. 1998;116:554-559.
77 Janssen J, Johanns W, Luis W, et al. Clinical value of endoscopic ultrasound-guided transesophageal fine needle puncture of mediastinal lesions. Dtsch Med Wochenschr. 1998;123:1402-1409.
78 Serna DL, Aryan HE, Chang KJ, et al. An early comparison between endoscopic ultrasound-guided fine-needle aspiration and mediastinoscopy for diagnosis of mediastinal malignancy. Am Surg. 1998;64:1014-1018.
79 Williams DB, Sahai AV, Aabakken L, et al. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single center experience. Gut. 1999;44:720-726.
80 Fritscher-Ravens A, Sriram PV, Bobrowski C, et al. Mediastinal lymphadenopathy in patients with or without previous malignancy: EUS-FNA-based differential cytodiagnosis in 153 patients. Am J Gastroenterol. 2000;95:2278-2284.
81 Wiersema MJ, Vazquez-Sequeiros E, Wiersema LM. Evaluation of mediastinal lymphadenopathy with endoscopic US-guided fine-needle aspiration biopsy. Radiology. 2001;219:252-257.
82 Wiersema MJ, Hassig WM, Hawes RH, et al. Mediastinal lymph node detection with endosonography. Endoscopy. 1993;39:788-793.
83 Hawes RH, Gress F, Kesler KA, et al. Endoscopic ultrasound versus computed tomography in the evaluation of the mediastinum in patients with non-small cell lung cancer. Endoscopy. 1994;26:784-787.
84 Wallace MB, Silvestri GA, Sahai AV, et al. Endoscopic ultrasound-guided fine needle aspiration for staging patients with carcinoma of the lung. Ann Thorac Surg. 2001;72:1861-1867.
85 Fritscher-Ravens A, Soehendra N, Schirrow L, et al. Role of transesophageal endosonography-guided fine-needle aspiration in the diagnosis of lung cancer. Chest. 2000;117:339-345.
86 Barawi M, Gottlieb K, Cunha B, et al. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189-192.
87 Faigel DO. EUS in patients with benign and malignant lymphadenopathy. Gastrointest Endosc. 2001;53:593-598.
88 Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095.
89 O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474.
90 Savides TJ, Master SS. EUS in rectal cancer. Gastrointest Endosc. 2002;56(Suppl):S12-S18.
91 Wildi SM, Hoda RS, Fickling W, et al. Diagnosis of benign cysts of the mediastinum: The role and risks of EUS and FNA. Gastrointest Endosc. 2003;58:362-368.
92 Levy MJ, Norton ID, Wiersema MJ, et al. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57:672-678.
93 Van de Mierop F, Buorgeois S, Hiel M, et al. Bacteremia after EUS guided puncture: A prospective analysis [abstract]. Gastrointest Endosc. 1999;49:A13100.
94 Ikenberry S, Gress FG, Savides TA, et al. Fine-needle aspiration of posterior mediastinal lesions guided by radial scanning endosonography. Gastrointest Endosc. 1996;43:605-610.
95 Wiersema M, Hawes R, Tao LC, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35-39.
96 Rex DK, Tarver RD, Wiersema MJ, et al. Endoscopic transesophageal fine needle aspiration of mediastinal masses. Gastrointest Endosc. 1991;37:465-468.
97 Wiersema MJ, Kochman ML, Chak A, et al. Real-time endoscopic ultrasound-guided fine needle aspiration of a mediastinal lymph node. Gastrointest Endosc. 1993;39:429-431.
98 Catalano MF, Sivak MVJr, Rice T, et al. Endosonographic features predictive of lymph node metastases. Gastrointest Endosc. 1994;40:442-446.
99 Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration of diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479.
100 Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441-447.
101 White PJr, Ettinger DS. Tissue is the issue: Is endoscopic ultrasonography with or without fine-needle aspiration biopsy in the staging of non-small-cell lung cancer an advance? Ann Intern Med. 1997;127(8 Pt 1):643-645.
102 Villman P, Hancke S, Hendrickson FW, et al. Endosonographic guided fine needle aspiration biopsy of malignant lesions in the upper gastrointestinal tract. Endoscopy. 1993;25:523-527.
103 Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound guided fine needle aspiration biopsy utilizing linear array and radial scanning endosonography: Results from a large single center experience. Gastrointest Endosc. 1997;45:243-250.
104 Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184-190.
105 Wiersema MJ, Gatzimos K, Nisi R, et al. Staging of non-Hodgkin’s gastric lymphoma with endosonography-guided fine-needle aspiration biopsy and flow cytometry. Gastrointest Endosc. 1996;44:734-736.
106 Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine-needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491.
107 Catalano MF, Nayar R, Gress F, et al. EUS-guided fine needle aspiration in mediastinal lymphadenopathy of unknown etiology. Gastrointest Endosc. 2002;55:863-869.
108 Fritscher-Ravens A, Sriram PV, Topalidis T, et al. Endoscopic ultrasonography-guided fine-needle cytodiagnosis of mediastinal metastases from renal cell cancer. Endoscopy. 2000;32:531-535.
109 Hahn M, Faigel DO. Frequency of mediastinal lymph node metastases in patients undergoing EUS evaluation of pancreaticobiliary masses. Gastrointest Endosc. 2001;54:331-335.
110 Devereaux BM, Leblanc JK, Yousif E, et al. Clinical utility of EUS-guided fine-needle aspiration of mediastinal masses in the absence of known pulmonary malignancy. Gastrointest Endosc. 2002;56:397-401.
111 Mehra M, Tamhane A, Eloubeidi MA. EUS-guided FNA combined with flow cytometry in the diagnoses of suspected or recurrent intrathoracic or retroperitoneal lymphoma. Gastrointest Endosc. 2005;62:508-513.
112 Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485-491.
113 Palazzo L, Roseau G, Ruskone-Fourmestraux A, et al. Endoscopic ultrasonography in the local staging of primary gastric lymphoma. Endoscopy. 1993;25:502-508.
114 Dechet CB, Sebo T, Farrow G, et al. Prospective analysis of intraoperative frozen needle biopsy of solid renal masses in adults. J Urol. 1999;162:1282-1285.
115 Panelli F, Erickson RA, Prasad VM. Evaluation of mediastinal masses by endoscopic ultrasound and endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2001;96:401-408.
116 Puli SR, Batapati KRJ, Bechtold ML, et al. Endoscopic ultrasound: Its accuracy in evaluating mediastinal lymphadenopathy? A meta-analysis and systematic review. World J Gastroenterol. 2008;14:3028-3037.
117 Wiersema MJ, Chak A, Wiersema LM. Mediastinal histoplasmosis: Evaluation with endosonography and endoscopic fine-needle aspiration biopsy. Gastrointest Endosc. 1994;40:78-81.
118 Hainaut P, Monthe A, Lesage V, et al. Tuberculous mediastinal lymphadenopathy. Acta Clin Belg. 1998;53:114-116.
119 Savides TJ, Gress FG, Wheat LJ, et al. Dysphagia due to mediastinal granulomas: Diagnosis with endoscopic ultrasonography. Gastroenterology. 1995;109:366-373.
120 Mishra G, Sahai AV, Penman ID, et al. Endoscopic ultrasonography with fine-needle aspiration: An accurate and simple diagnostic modality for sarcoidosis. Endoscopy. 1999;31:377-382.
121 Fritscher-Ravens A, Sriram P, Topalidis T. Diagnosing sarcoidosis using endosonography-guided fine-needle aspiration. Chest. 2000;118:928-935.
122 Annema JT, Veselic M, Rabe KF. Endoscopic ultrasound-guided fine needle aspiration for the diagnosis of sarcoidosis. Eur Respir J. 2005;25:405-409.
123 Michael H, Ho S, Pollack B, et al. Diagnosis of intra-abdominal and mediastinal sarcoidosis with EUS-guided FNA. Gastrointest Endosc. 2008;67:28-34.
124 Larsen SS, Krasnik M, Vilmann P, et al. Endoscopic ultrasound guided biopsy of mediastinal lesions has a major impact on patient management. Thorax. 2002;57:98-103.
125 Garcia C, Kumar V, Sharma OP. Pancreatic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1996;13:28-32.
126 Limaye A, Paauw D, Raghu G, et al. Sarcoidosis associated with recurrent pancreatitis. South Med J. 1997;90:431-433.
127 Brincker H. Sarcoid reactions in malignant tumors. Cancer Treat Rev. 1986;13:147-156.
128 Tio TL, Tytgat GNJ. Endoscopic ultrasonography in analyzing peri-intestinal lymph node abnormality. Scand J Gastroenterol. 1986;21(Suppl 123):158-163.
129 Van Dam J, Rice TW, Sivak MVJr. Endoscopic ultrasonography and endoscopically guided needle aspiration for the diagnosis of upper gastrointestinal tract foregut cysts. Am J Gastroenterol. 1992;87:762-765.
130 Geller A, Wang KK, DiMagno EP. Diagnosis of foregut duplication cysts by endoscopic ultrasonography. Gastroenterology. 1995;109:838-842.
131 Bhutani MS, Hoffman BJ, Reed C. Endosonographic diagnosis of an esophageal duplication cyst. Endoscopy. 1996;28:396-397.
132 Faigel DO, Burke A, Ginsberg GG, et al. The role of endoscopic ultrasound in the evaluation and management of foregut duplications. Gastrointest Endosc. 1997;45:99-103.
133 Bondestam S, Salo JA, Salonen OL, et al. Imaging of congenital esophageal cysts in adults. Gastrointest Radiol. 1990;15:279-281.
134 Mendelson DS, Rose JS, Efremidis SC, et al. Bronchogenic cysts with high CT numbers. AJR Am J Roentgenol. 1983;140:463-465.
135 Eloubeidi MA, Morgan DE, Cerfolio RJ, et al. Transduodenal EUS-guided FNA of the right adrenal gland. Gastrointest Endosc. 2008;67:522-527.
136 Marchal G, Gelin J, Verbeken E, et al. High-resolution real-time sonography of the adrenal glands: A routine examination? J Ultrasound Med. 1986;5:65-68.
137 Zappasodi F, Derchi LE, Rizzatto G. Ultrasonography of the normal adrenal glands: A study using linear-array real-time equipment. Br J Radiol. 1986;59:759-764.
138 Chang KJ, Erickson RA, Nguyen P. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration of the left adrenal gland. Gastrointest Endosc. 1996;44:568-572.
139 Twine CP, Barry JD, Blackshaw GR, et al. Prognostic significance of endoscopic ultrasound-defined pleural, pericardial or peritoneal fluid in oesophageal cancer. Surg Endosc. 2009;23:2229-2236.
140 Harper PG, Houang M, Spiro SG, et al. Computerized axial tomography in the pretreatment assessment of small cell carcinoma of the bronchus. Cancer. 1981;47:1775-1780.
141 Pagani JJ. Non-small cell carcinoma adrenal metastasis: Computed tomography and percutaneous needle biopsy in their diagnosis. Cancer. 1984;53:1058-1060.
142 Sandler MA, Pearlberg JL, Madrazo BL, et al. Computed tomographic evaluation of the adrenal gland in the preoperative assessment of bronchogenic carcinoma. Radiology. 1982;145:733-736.
143 Whittlesey D. Prospective computed tomographic scanning in the staging of bronchogenic cancer. J Thorac Cardiovasc Surg. 1988;95:876-882.
144 Oliver TW, Bernardino ME, Miller JI, et al. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984;153:217-218.
145 Abrams HL, Spiro R, Goldstein N. Metastasis in carcinoma: Analysis of 1000 autopsied cases. Cancer. 1950;3:74-85.
146 Englemen RM, McNamara WL. Bronchogenic carcinoma: A statistical review of two hundred twenty-four autopsies. J Thorac Surg. 1954;27:227-237.
147 Chang KJ, Albers CG, Nguyen P. Endoscopic ultrasound-guided fine needle aspiration of pleural and ascitic fluid. Am J Gastroenterol. 1995;90:148-150.
148 Farrell JL, Brugge WR. EUS-guided fine-needle aspiration of a renal mass: An alternative method for diagnosis of malignancy. Gastrointest Endosc. 2002;56:450-452.
149 Davis CJ. Pathology of renal neoplasms. Semin Roentgenol. 1987;22:233-240.
150 Wood BJ, Khan MA, McGovern F, et al. Imaging guided biopsy of renal masses: Indications, accuracy and impact on clinical management. J Urol. 1999;161:1470-1474.
151 Artifon EL, Lopes RI, Kumar A, et al. Endoscopic ultrasound facilitates histological diagnosis of renal cell cancer. J Endourol. 2008;22:2447-2450.
152 Nguyen P, Chang K. GE EUS in the detection of ascites and EUS-guided paracentesis. Gastrointest Endosc. 2001;54:336-339.
153 Sultan J, Robinson S, Hayes N, et al. Endoscopic ultrasonography-detected low-volume ascites as a predictor of inoperability for oesophagogastric cancer. Br J Surg. 2008;95:1127-1130.
154 Pollack BJ, Chak A, Canto M, et al. Endoscopic ultrasonography in the detection of malignant ascites: Is it a marker of peritoneal carcinomatosis? [abstract]. Gastrointest Endosc. 1996;43:A549.
155 Canto M, Gislason G. Is extraluminal fluid at endoscopic ultrasonography an accurate marker of peritoneal carcinomatosis? A prospective study [abstract]. Gastrointest Endosc. 1998;47:AB142.
156 Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357-361.
157 Crowe DR, Eloubeidi MA, Chieng DC, et al. Fine-needle aspiration biopsy of hepatic lesions: Computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180-185.
158 Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095.
159 Bentz JS, Kochman ML, Faigel DO, et al. Endoscopic ultrasound-guided real-time fine-needle aspiration: Clinicopathologic features of 60 patients. Diagn Cytopathol. 1998;18:98-109.
160 Fritscher-Ravens A, Schirrow L, Atay Z, et al. Endosonographically controlled fine needle aspiration cytology—indications and results in routine diagnosis. Gastroenterology. 1999;37:343-351.
161 tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862.
162 Livraghi T, Damascelli B, Lombardi C, et al. Risk in fine-needle abdominal biopsy. J Clin Ultrasound. 1983;11:77-81.
163 Edoute Y, Ben-Haim S, Brenner B, et al. Fatal hemoperitoneum after fine-needle aspiration of a liver metastasis. Am J Gastroenterol. 1992;87:358-359.
164 Glaser KS, Weger AR, Schmid KW, et al. Is fine-needle aspiration of tumours harmless? Lancet. 1989;1:620.
165 Kowdley KV, Aggarwal A, Sachs PB. Delayed hemorrhage after percutaneous liver biopsy: Role of therapeutic angiography. J Clin Gastroenterol. 1994;19:50-53.
166 Vergara V, Garripoli A, Marucci MM, et al. Colon cancer seeding after percutaneous fine needle aspiration of liver metastasis. J Hepatol. 1993;18:276-278.
167 Catalano MF, Sial S, Chak A, et al. EUS-guided fine needle aspiration of idiopathic abdominal masses. Gastrointest Endosc. 2002;55:854-858.
168 McGrath KM, Ballo MS, Jowell PS. Schwannoma of the mediastinum diagnosed by EUS-guided fine needle aspiration. Gastrointest Endosc. 2001;53:362-365.
169 Okada N, Hirooka Y, Itoh A, et al. Retroperitoneal neurilemoma diagnosed by EUS-guided FNA. Gastrointest Endosc. 2003;57:790-792.
170 Erickson RA, Tretjak Z. Clinical utility of endoscopic ultrasound and endoscopic guided fine needle aspiration in retroperitoneal neoplasms. Am J Gastroenterol. 2000;95:1188-1194.
171 Anand D, Barroeta JE, Gupta PK, et al. Endoscopic ultrasound guided fine needle aspiration of non-pancreatic lesions: An institutional experience. J Clin Pathol. 2007;60:1254-1262.
172 Barawi M, Bekal P, Gress F. Accessory spleen: A potential cause of misdiagnosis at EUS. Gastrointest Endosc. 2000;52:769-772.
173 Fritscher-Ravens A, Mylonaki M, Pantes A, et al. Endoscopic ultrasound-guided biopsy for the diagnosis of focal lesions of the spleen. Am J Gastroenterol. 2003;98:1022-1027.
174 Eloubeidi MA, Varadarajulu S, Eltoum I, et al. Transgastric endoscopic ultrasound-guided fine-needle aspiration biopsy and flow cytometry of suspected lymphoma of the spleen. Endoscopy. 2006;38:617-620.
175 Iwashita T, Yasuda I, Tsurumi H, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy for splenic tumor: A case series. Endoscopy. 2009;41:179-182.
176 Gress F, Michael H, Gelrud D, et al. EUS-guided fine-needle aspiration of the pancreas: Evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56:864-867.
177 Wallace MB, Hawes RH, Sahai AV, et al. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: Safety and effect on patient management. Gastrointest Endosc. 2000;51:309-313.
178 Schwartz DA, Unni KK, Levy MJ, et al. The rate of false-positive results with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2002;56:868-872.
179 Reidenberg MM, Portenoy RK. The need for an open mind about the treatment of chronic nonmalignant pain. Clin Pharmacol Ther. 1994;55:367-369.
180 Fugere F, Lewis G. Coeliac plexus block for chronic pain syndromes. Can J Anaesth. 1993;40:954-963.
181 Hastings RH, McKay WR. Treatment of benign chronic abdominal pain with neurolytic celiac plexus block. Anesthesiology. 1991;75:156-158.
182 Leung JW, Bowen-Wright M, Aveling W, et al. Coeliac plexus block for pain in pancreatic cancer and chronic pancreatitis. Br J Surg. 1983;70:730-732.
183 Arner S, Myerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11-23.
184 McQuay HJ, Tramer M, Nye BA, et al. A systematic review of antidepressants in neuropathic pain. Pain. 1996;68:217-227.
185 Malfertheiner P, Mayer D, Buchler M, et al. Treatment of pain in chronic pancreatitis by inhibition of pancreatic secretion with octreotide. Gut. 1995;36:450-454.
186 Halgreen H, Pederson NT, Worning H. Symptomatic effect of pancreatic enzyme therapy in patients with chronic pancreatitis. Scand J Gastroenterol. 1986;21:104-108.
187 Malesci A, Gaia E, Fioretta A, et al. No effect of long-term treatment with pancreatic extract on recurrent abdominal pain in patients with chronic pancreatitis. Scand J Gastroenterol. 1995;30:392-398.
188 Mossner J, Secknus R, Meyer J, et al. Treatment of pain with pancreatic extracts in chronic pancreatitis: Results of a prospective placebo-controlled multicenter trial. Digestion. 1992;53:54-66.
189 Ferrer-Brechner T. Anesthetic management of cancer pain. Semin Oncol. 1985;12:431-437.
190 Stone HH, Chauvin EJ. Pancreatic denervation for pain relief in chronic alcohol associated pancreatitis. Br J Surg. 1990;77:303-305.
191 Bradley EL3rd. Long-term results of pancreaticojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153:207-213.
192 Markowitz JS, Rattner DW, Warshaw AL. Failure of symptomatic relief after pancreatojejunal decompression for chronic pancreatitis: Strategies for salvage. Arch Surg. 1994;129:374-379.
193 Levy MJ, Wiersema MJ. EUS-guided celiac plexus neurolysis and celiac plexus block. Gastrointest Endosc. 2003;57:923-930.
194 Zenz M, Strumpf M, Tryba M. Long-term oral opioid therapy in patients with chronic nonmalignant pain. J Pain Symptom Manage. 1992;7:69-77.
195 Hoffman BJ. EUS-guided celiac plexus block/neurolysis. Gastrointest Endosc. 2002;56(4 Suppl):S26-S28.
196 Wong GY, Brown DL. Transient paraplegia following celiac plexus block for cancer pain. Reg Anesth. 1995;20:352-355.
197 Ischia S, Ischia A, Polati E, et al. Three posterior percutaneous celiac plexus block techniques: A prospective, randomized study in 61 patients with pancreatic cancer pain. Anesthesiology. 1992;76:534-540.
198 Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: A meta-analysis. Anesth Analg. 1995;80:290-295.
199 Lillemoe KD, Cameron JL, Kaufman HS, et al. Chemical splanchnicectomy in patients with unresectable pancreatic cancer: A prospective randomized trial. Ann Surg. 1993;217:447-455.
200 Polati E, Finco G, Gottin L, et al. Prospective randomized double-blinded trial of neurolytic coeliac plexus block in patients with pancreatic cancer. Br J Surg. 1998;85:199-201.
201 Kawamata M, Ishitani K, Ishikawa K, et al. Comparison between celiac plexus block and morphine treatment on quality of life in patients with pancreatic cancer. Pain. 1996;64:597-602.
202 Mercadante S, Nicosia F. Celiac plexus block: A reappraisal. Reg Anesth Pain Med. 1998;23:37-48.
203 Wiersema MJ, Wiersema LM. Endosonography-guided celiac plexus neurolysis. Gastrointest Endosc. 1996;44:656-662.
204 Levy MJ, Topazian MD, Wiersema MJ, et al. Initial evaluation of the efficacy and safety of endoscopic ultrasound-guided direct ganglia neurolysis and block. Am J Gastroenterol. 2008;103:98-103.
205 LeBlanc JK, DeWitt J, Johnson C, et al. A prospective randomized trial of 1 versus 2 injections during EUS-guided celiac plexus block for chronic pancreatitis pain. Gastrointest Endosc. 2009;69:835-842.
206 Sahai AV, Lemelin V, Lam E, et al. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: A comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326-329.
207 Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound- and computed tomography-guided celiac plexus bloc for managing chronic pancreatitis pain. Am J Gastroenterol. 1999;94:900-905.
208 Gress F, Schmitt C, Sherman S, et al. Endoscopic ultrasound-guided celiac plexus block for managing abdominal pain associated with chronic pancreatitis: A prospective single center experience. Am J Gastroenterol. 2001;96:409-416.
209 Davies DD. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86:264-266.
210 Patt RB, Reddy S. Spinal neurolysis for cancer pain. Ann Acad Med. 1994;23:216-220.
211 Hardy PA, Wells JC. Coeliac plexus block and cephalic spread of injectate. Ann R Coll Surg Engl. 1989;71:48-49.
212 Naresh T, Gunaratnam NT, Sarma AV, et al. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316-324.