Chapter 5 Extracranial Large Artery Atherothrombosis
Extracranial atherothrombotic disease is an important cause of stroke, and the management of asymptomatic occlusive disease poses additional challenges and opportunities for stroke prevention. Depending on the population studied, approximately 20% of strokes are attributed to large vessel extracranial disease.1 The prevalence of asymptomatic carotid stenosis greater than 50% stenosis is approximately 3% in the general population.2–4 There is a higher prevalence of extracranial atherosclerotic disease in whites compared with African Americans and other ethnic groups.5,6
ULTRASONOGRAPHY
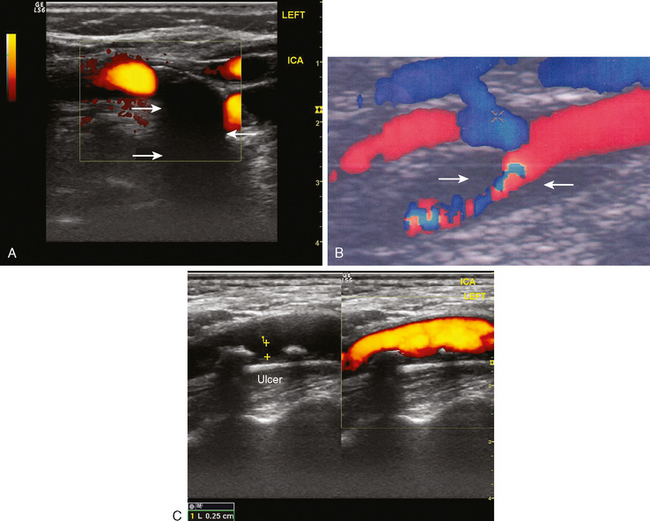
Determining the exact degree of stenosis remains the single most important goal of extracranial ultrasonography. However, plaque composition is an additional determinant of future stroke risk, particularly in the setting of symptomatic stenosis. Carotid plaques that are hypoechoic (i.e., have a dark intraplaque area), or ulcerated are of particular concern.7–11 These hypoechoic areas are likely to represent intraplaque hemorrhage, and these plaques should be considered unstable, thus having an increased thromboembolic potential. The presence of calcifications, in contrast, imparts relative plaque stability, and such plaques are less likely to be symptomatic12 (Figure 5-1).
Ultrasonography is technician-dependent and requires considerable skill and expertise. It is typically used as an initial screening tool, and thereby sensitivity is optimized at the expense of specificity-that is, it will tend to overestimate the degree of stenosis resulting in some false-positive results. Therefore a confirmatory test is necessary to improve the overall accuracy of the diagnostic approach.13 Ultrasound is less reliable in identifying carotid stenosis in moderate ranges (between 50% and 69%)14,15 and carotid occlusion.13
Because ultrasonography uses an increase in flow velocities as the main diagnostic criterion, the findings of ultrasonography in the setting of abnormal collateral flow patterns must be carefully interpreted. This situation often arises during insonation of a moderately stenotic plaque in patients with contralateral occlusion or high-grade stenosis. In these cases, ultrasound may overestimate the degree of stenosis of the moderately stenotic plaque,16,17 and the findings on ultrasonography must be cautiously reviewed and confirmed with other imaging modalities.
It is also important to realize that ultrasonography only visualizes the proximal portion of the carotid artery, and pathology involving the distal cervical segment may be unrecognized.
MR AND CT ANGIOGRAPHY
In general clinical practice, the findings on ultrasonography are often confirmed with an alternate imaging technique. MR and CT angiography have essentially replaced catheter angiography as the confirmatory test. The advantages MR and CT angiography are that they are noninvasive and readily available. The sensitivity and specificity for both techniques exceeds 80% to 90%.13,15,18,19 For the majority of patients, this approach (i.e., ultrasonography and CT/MR angiography combined) will correctly identify the true degree of stenosis. However, it continues to be controversial if noninvasive techniques are reliable enough to make decisions about surgical intervention, particularly if the degree of stenosis is in the moderate (50%–69%) range.15
CT angiography has recently experienced a revival with the advent of multislice detectors and development of sophisticated postimaging software. Advantages of CT over MR angiography lie in its acquisition speed. Even though few data are available with these newer techniques, they are likely to improve further the diagnostic accuracy of CT angiography.19
MR angiography is widely used at present but has some inherent limitations. There is a tendency for overestimation of the degree of stenosis because of sampling error. Therefore MR angiography may be more valuable as a screening tool rather than to confirm that stenosis is actually present.20 The specificity and sensitivity can be improved with gadolinium administration.20,21
Diagnostic accuracy of noninvasive imaging is generally improved if results of ultrasonography and MR or CT angiography are concordant.13
CATHETER CEREBRAL ANGIOGRAPHY
Catheter cerebral angiography remains the gold standard in determining the degree of stenosis and identifying surgical candidates. However, even in recent clinical trials, there has been a trend in favor of noninvasive testing, particularly in asymptomatic patients, given the risks of catheter angiography.22 The risk of stroke may be as high as 1.2% in asymptomatic patients; however, other reports have shown a reduced risk of serious complications in general practice.23,24
Less well recognized are subclinical infarcts detected by diffusion-weighted MR imaging after diagnostic cerebral angiography. Such lesions are present in up to 20% of patients and are the result of silent microembolism,25 but might produce subtle neuropsychiatric manifestations that go undetected.
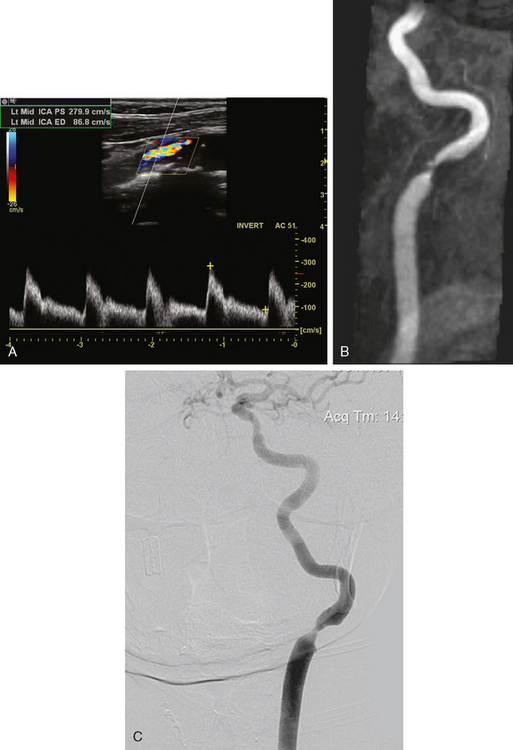
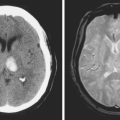
A 75-year-old hypertensive diabetic woman developed right-sided weakness. Extracranial ultrasonography showed a right ICA occlusion and left ICA high-grade stenosis meeting diagnostic criteria for stenosis exceeding 70% of the luminal diameter. MR angiography of the neck showed a high-grade left internal stenosis and confirmed the right internal carotid occlusion. On cerebral angiography, the left carotid stenosis was carefully measured to be 60% (Figure 5-2). The external carotid artery was occluded.
CLINICAL FEATURES OF CAROTID ATHEROTHROMBOSIS
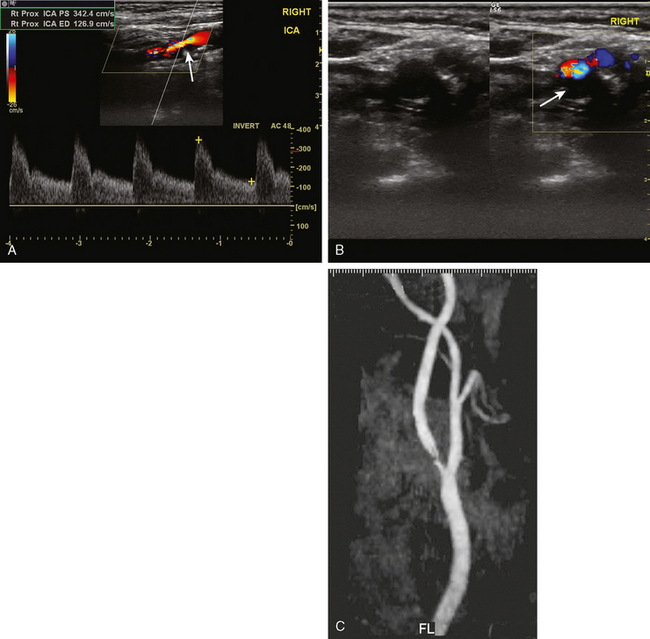
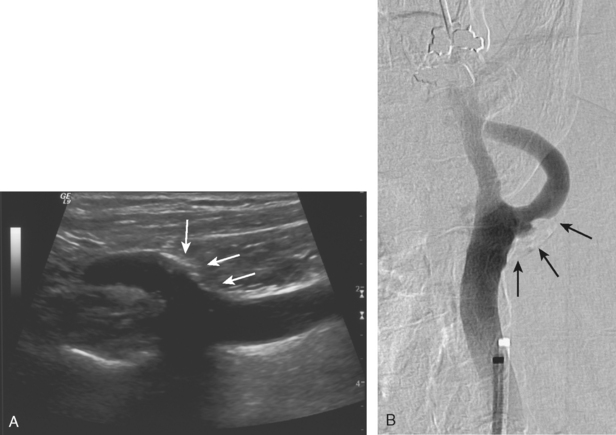
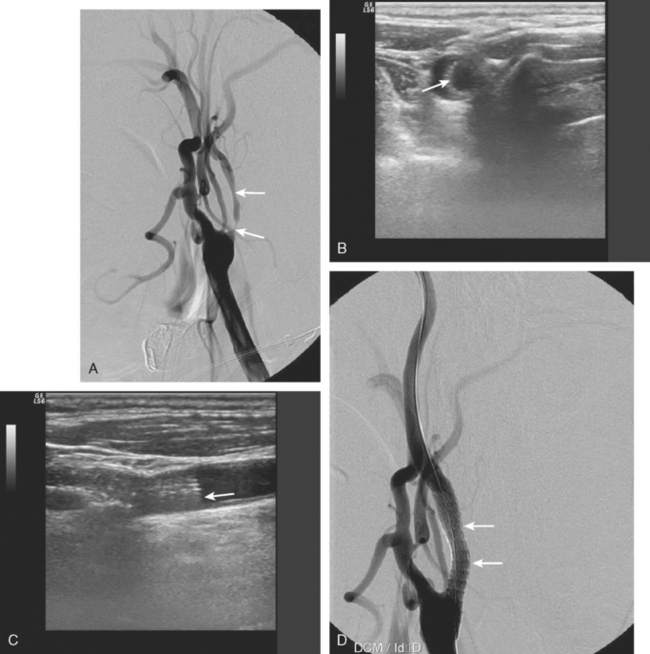
A 76-year-old man with a prior history of hypertension developed three episodes of sudden transient left hand and forearm numbness lasting 10 to 15 minutes over the previous week. His neurological examination showed no abnormalities. A harsh right carotid bruit was heard on neck auscultation. He underwent an emergent carotid ultrasound, which revealed a hypoechoic plaque at the right internal carotid origin causing high-grade stenosis of the lumen. MR angiography of the neck performed later confirmed these findings (Figure 5-3). He was admitted to a local hospital and placed on antithrombotic therapy. The following morning, he developed left-sided facial droop, dysarthria, and left-arm paralysis. He underwent an emergent endarterectomy and recovered without residual deficits.
This case illustrates the clinical hallmarks of large arterial atherothrombosis:
A 55-year-old smoker underwent a routine ocular examination and was found to have a Hollenhorst plaque in her right eye. She denied any visual symptoms. Carotid ultrasound revealed an ulcerated plaque at the origin of the right ICA causing no more than 30% to 40% stenosis (Figure 5-4). She was placed on aspirin, and her vascular risk factors were treated intensively.
MECHANISMS OF INFARCTION
Atherothrombotic Embolism
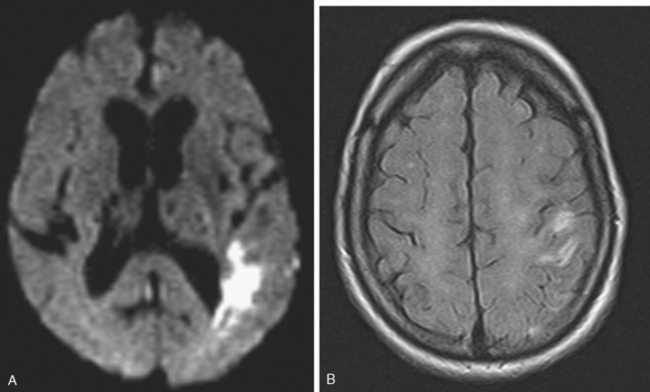
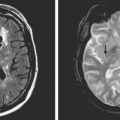
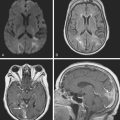
A 68 year-old man with history of hypertension, dyslipidemia, and diabetes developed sudden onset of left arm and leg numbness. On examination, he had diminished left-arm sensation and sensory extinction. Brain MRI showed an acute infarct in the right parietal lobe on diffusion-weighted imaging sequence. Fluid-attenuated inversion recovery (FLAIR) showed a prior infarct in right middle cerebral territory (Figure 5-5). Carotid ultrasound and MR angiography revealed high-grade stenosis of the right ICA artery at its origin.
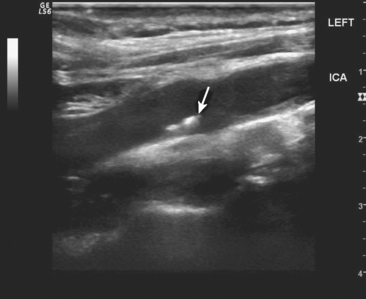
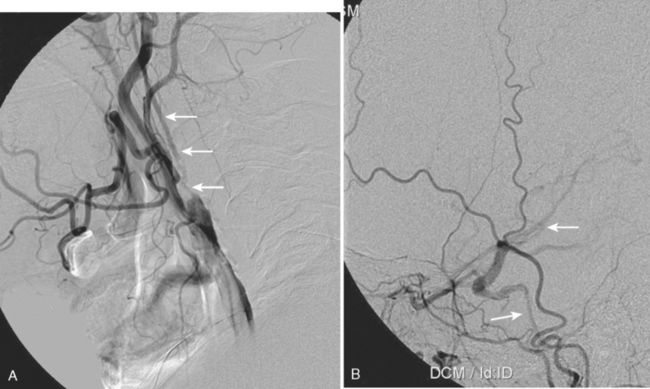
A carotid ultrasound showed plaque formation in the left carotid bulb, which produced no alteration of Doppler flow pattern and was estimated to cause no more than 50% stenosis of the luminal diameter (Figure 5-6). Subsequently, he underwent a catheter cerebral angiogram, which confirmed the presence of an ulcerated left carotid bulb plaque, causing no significant stenosis. Given the repetitive nature of his symptoms restricted to a single vascular distribution, it was felt that his symptoms were most likely explained by repeated thromboembolism from the proximal ICA plaque. Consequently, he was treated with left carotid endarterectomy. A hemorrhagic ulcerated plaque was found at the time of the operation. He recovered well from surgery and has been symptom-free for the following 2 years.
A 68-year-old man with hypertension was seen at a stroke referral center for a past history of left-sided cortical stroke, which occurred more than 1 year earlier. On follow-up Duplex examination, he was found to have a severely narrowed right ICA with a dampened flow pattern (Figure 5-7). MR angiography suggested occlusion of the right cervical carotid artery. Catheter cerebral angiogram demonstrated a proximal high-grade stenosis with a severely narrowed and collapsed ICA distal to the stenosis. He was deemed to have a functional occlusion and treated with medical therapy.
Hemodynamic Infarction
Cerebral infarction may also occur as a result of perfusion failure, and the prevalence of hemodynamic mechanisms may have been underestimated.36,37 Such types of infarctions are typically associated with large arterial vessel occlusion or high-grade stenosis. Situations that may lead to changes in cerebral blood flow and perfusion pressure are often precipitating factors. This type of infarction tends to occur in watershed and border-zone areas, that is, the distal irrigation fields between the anterior and middle cerebral artery (anterior watershed), posterior and middle cerebral artery (posterior watershed), and the medullary penetrating and lenticulostriate arteries of the middle cerebral artery along the lateral and cephalad border of the lateral ventricle (internal border zone).38
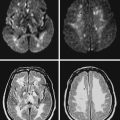
An 82-year-old man with history of hypertension and smoking had a documented right carotid occlusion. He presented to the consultation after awakening with left-sided weakness. A careful review of recent symptoms revealed that over the previous 2 to 3 weeks, he had experienced several episodes of lightheadedness and left-sided heaviness after getting up from the bed or a chair; each episode resolved within a few minutes. On several of those occasions, his left arm would shake uncontrollably for several seconds. The day prior to his stroke he had fallen on his left hip. His examination showed left hemiparesis. He had a large hematoma over his left hip. His hematocrit had decreased from 39% (his baseline) to 32%. Brain MRI showed infarction in the deep internal middle cerebral artery watershed distribution, which included the distal irrigation fields of the lenticulostriate and cortical penetrating medullary branches (Figure 5-8). Carotid ultrasound, MR angiography, and CT angiography confirmed occlusion of the ipsilateral ICA.
TREATMENT
Symptomatic Carotid Disease
Treatment of atherosclerotic disease comprises careful considerations of medical and surgical therapy. Surgical intervention has to be the primary consideration in symptomatic disease exceeding 70% stenosis because of the high risk of stroke recurrence on medical therapy.23,41 The risk of recurrent stroke on medical therapy is 26% in 2 years and is reduced to 9% with endarterectomy.23,41 The risk of subsequent stroke is greater with increasing degrees of stenosis.
Surgical therapy must be instituted rapidly because the risk of early reinfarction is high. In fact, the benefit of carotid endarterectomy in stroke prevention is significantly reduced if performed more than 2 weeks from symptom onset.28 In symptomatic carotid disease with 50% to 69% stenosis, the risk of recurrent infarction is lower and the benefit of surgical intervention is more modest.42 Other features such as patient sex, age, and plaque characteristics (ulcerations, intraplaque hemorrhage) may need to be taken into consideration to identify patients at increased risk for stroke recurrence.
Asymptomatic Carotid Artery Stenosis
The management of asymptomatic carotid stenosis continues to be a challenge and remains controversial. The risk of first stroke on medical treatment in patients with greater than 60% asymptomatic carotid stenosis is low, approximately 2% annually, and with endarterectomy, it may be reduced to 1%.22,43 Aggressive management of vascular risk factors with newer antithrombotic, antihypertensive, and lipid-lowering agents may have reduced this risk of stroke with medical therapy even further in recent years.22,44,45
Carefully selected asymptomatic patients with greater than 60% stenosis benefit from endarterectomy compared with medical therapy.22,43 The benefits, however, are moderate, were not found for women, and are only evident with a low surgical complications rate of approximately 2%.22,43
Identifying a subgroup of asymptomatic patients that may be at higher risk of stroke would limit intervention to those patients likely to benefit the most and maximize the risk–benefit ratio of surgical intervention. Careful consideration must be given to age, sex, and life expectancy, because the benefits of surgery only become apparent over time. Additional factors that might identify populations at risk for first stroke are not entirely defined but may include presence of asymptomatic microemboli on transcranial Doppler,44,46 asymptomatic infarction in the distribution of the stenosis,47 plaque characteristics (ulcerations and intraplaque hemorrhage),7–11 and impaired vasomotor reactivity.48
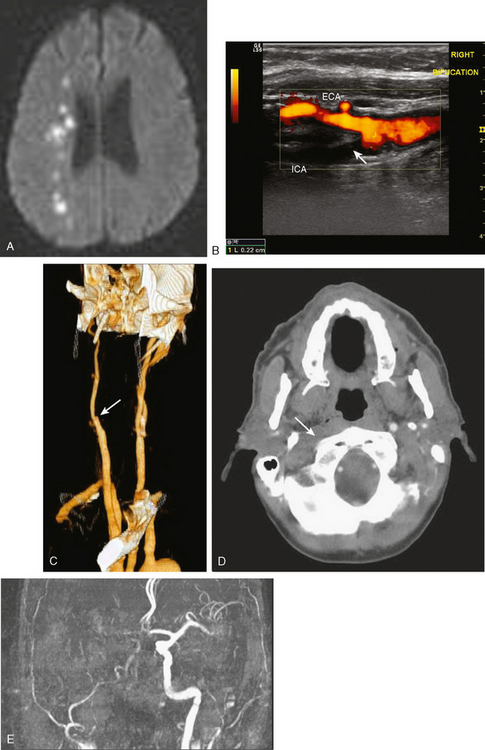
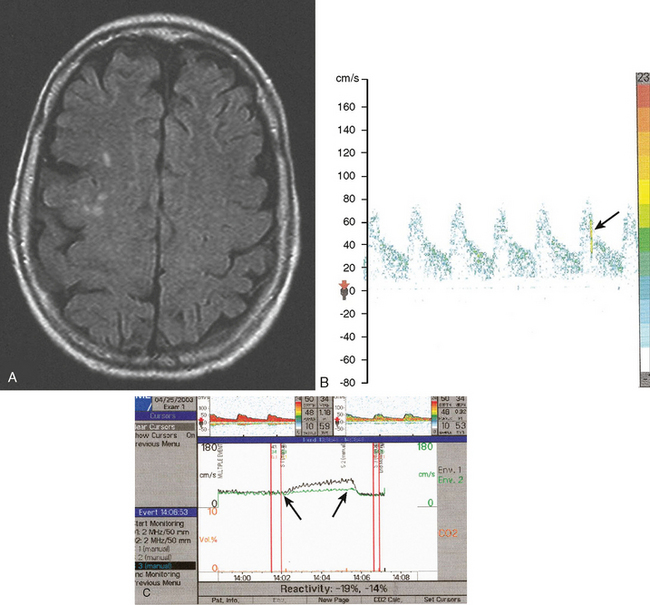
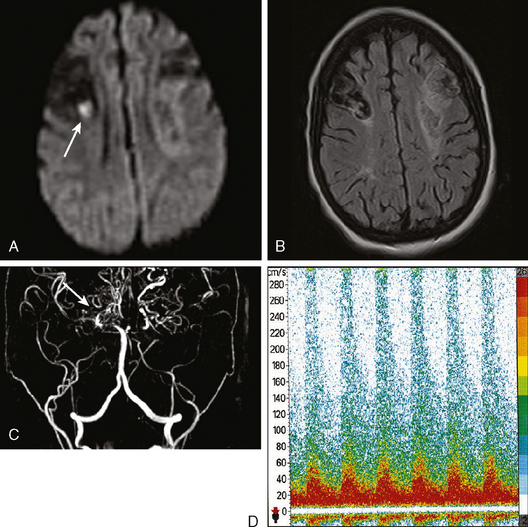
An asymptomatic 65-year-old diabetic and hypertensive active man was found to have a carotid bruit. An initial carotid ultrasound suggested a 50% to 60% carotid stenosis. He was followed with serial carotid ultrasonography, and 1 year later, the ultrasound performed in the same laboratory showed progression of the stenosis to 80% to 90%, caused by a larger hypoechoic, ulcerated plaque. This was subsequently confirmed by MR angiography of the neck, and MR angiography of the brain disclosed poor compensatory collateral flow in the affected hemisphere. Brain MRI showed several cortical FLAIR hyperintensities in the ipsilateral hemisphere, indicating subclinical infarctions. He had several microembolic signals on transcranial Doppler and impaired vasomotor reactivity (Figure 5-9). He was counseled on his future stroke risk as well as risks and benefits of medical versus surgical therapy. His stroke risk was felt to exceed the annual average of 2% generally applied to asymptomatic patients with greater than 60% carotid stenosis.
Carotid Revascularization
New options for revascularization are currently emerging. Carotid endarterectomy is a proven and effective technique with a low complication rate. It will be difficult to improve further on the safety record and durability of carotid endarterectomy. There are, however, situations that increase the perioperative complication rate of carotid endarterectomy. Such scenarios include contralateral high-grade stenosis or occlusion, radiation induced carotid stenosis, inaccessible high cervical carotid bifurcation, restenosis after endarterectomy, and medical comorbidities, particularly poor cardiopulmonary status.51
Carotid artery stenting is a treatment option in those settings; however, it is increasingly applied for the treatment of carotid stenosis in general practice. A clear benefit of carotid stenting over carotid endarterectomy has not yet been demonstrated,51–53 and carotid endarterectomy should continue to be considered the gold standard intervention for the treatment of carotid stenosis.
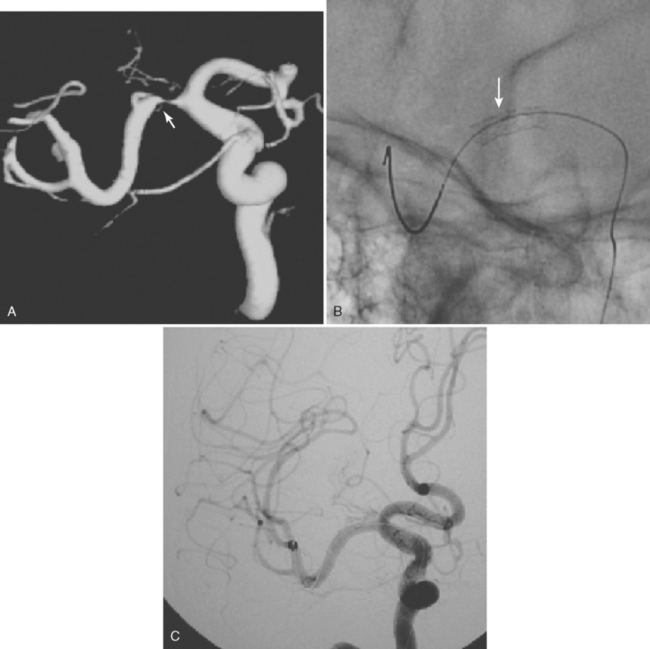
A 55-year-old man developed an episode of right-arm numbness and weakness. The patient had a prior history of neck radiation for lymphoma. Carotid ultrasonography revealed high-grade left carotid stenosis, which was later confirmed angiographically. The prior history of radiation was considered a relative indication for stent placement rather than endarterectomy (Figure 5-10).
VERTEBRAL ARTERY ATHEROSCLEROSIS
As in carotid disease, the important mechanisms of infarction are artery-to-artery embolism and hemodynamic mechanisms.54 Hemodynamic mechanisms are further dependent on the patency of the contralateral vertebral artery, which may either be affected by atherosclerosis or congenitally hypoplastic.
The vertebral artery origin may be assessed with ultrasound and CT and MR angiography. However, only a few studies have reported on the diagnostic accuracy of these imaging modalities. A sensitivity of approximately 70% and specificity exceeding 90% has been reported for the diagnosis of proximal vertebral artery disease with ultrasonography compared with the gold standard of cerebral angiography.55,56 A similar sensitivity and specificity has been reported for CT and MR angiography for stenosis of the vertebral artery origin.57–59
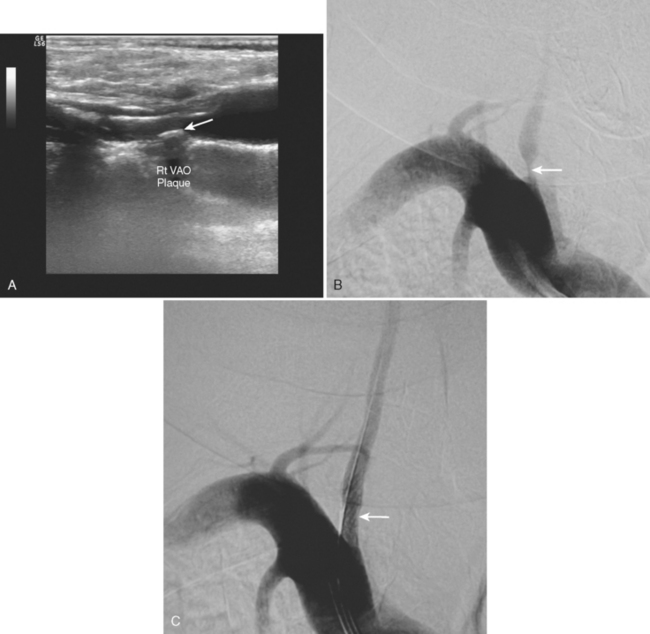
A 65-year-old hypertensive man developed recurrent symptoms of vertigo, diplopia, and hemibody numbness. Initial vascular evaluation with ultrasound reported antegrade flow bilaterally in both vertebral arteries insonated in the midcervical area. MR angiography of the neck showed normal vertebral arteries but did not include their most proximal segments. The patient’s symptoms persisted despite several changes in antithrombotic regimen. Repeated complete vertebral ultrasonography suggested a high-grade vertebral artery-origin stenosis. This finding was later confirmed by catheter angiography. A stent was placed at the vertebral artery origin, and the patient had no further symptoms (Figure 5-11).
1 Sacco RL, Ellenberg JH, Mohr JP, Tatemichi TK, Hier DB, Price TR, Wolf PA. Infarcts of undetermined cause: the NINCDS Stroke Data Bank. Ann Neurol. 1989;25:382-390.
2 Mathiesen EB, Joakimsen O, Bonaa KH. Prevalence of and risk factors associated with carotid artery stenosis: the Tromso Study. Cerebrovasc Dis. 2001;12:44-51.
3 Ghilardi G. Carotid stenotic-obliterative lesions. Distribution in 16,379 subjects 45-75 years of age. Minerva Cardioangiol. 1994;42:345-350.
4 Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging. 2007;17:19-47.
5 Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: A review. Stroke. 1986;17:648-655.
6 Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H, Litt B. Race and sex differences in the distribution of cerebral atherosclerosis. Stroke. 1996;27:1974-1980.
7 Polak JF, Shemanski L, O’Leary DH, Lefkowitz D, Price TR, Savage PJ, et al. Hypoechoic plaque at us of the carotid artery: an independent risk factor for incident stroke in adults aged 65 years or older. Cardiovascular Health Study. Radiology. 1998;208:649-654.
8 Sterpetti AV, Schultz RD, Feldhaus RJ, Davenport KL, Richardson M, Farina C, Hunter WJ. Ultrasonographic features of carotid plaque and the risk of subsequent neurologic deficits. Surgery. 1988;104:652-660.
9 Russell DA, Wijeyaratne SM, Gough MJ. Changes in carotid plaque echomorphology with time since a neurologic event. J Vasc Surg. 2007;45:367-372.
10 Mathiesen EB, Bonaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the Tromso Study. Circulation. 2001;103:2171-2175.
11 Bluth EI, Kay D, Merritt CR, Sullivan M, Farr G, Mills NL, et al. Sonographic characterization of carotid plaque: detection of hemorrhage. AJR Am J Roentgenol. 1986;146:1061-1065.
12 Grogan JK, Shaalan WE, Cheng H, Gewertz B, Desai T, Schwarze G, et al. B-mode ultrasonographic characterization of carotid atherosclerotic plaques in symptomatic and asymptomatic patients. J Vasc Surg. 2005;42:435-441.
13 Long A, Lepoutre A, Corbillon, Branchereau A. Critical review of non- or minimally invasive methods (duplex ultrasonography, MR- and CT-angiography) for evaluating stenosis of the proximal internal carotid artery. Eur J Vasc Endovasc Surg. 2002;24:43-52.
14 Kennedy J, Quan H, Ghali WA, Feasby TE. Importance of the imaging modality in decision making about carotid endarterectomy. Neurology. 2004;62:901-904.
15 Wardlaw JM, Chappell FM, Best JJ, Wartolowska K, Berry E. Non-invasive imaging compared with intra-arterial angiography in the diagnosis of symptomatic carotid stenosis: a meta-analysis. Lancet. 2006;367:1503-1512.
16 Heijenbrok-Kal MH, Nederkoorn PJ, Buskens E, van der Graaf Y, Hunink MG. Diagnostic performance of duplex ultrasound in patients suspected of carotid artery disease: the ipsilateral versus contralateral artery. Stroke. 2005;36:2105-2109.
17 van Everdingen KJ, van der Grond J, Kappelle LJ. Overestimation of a stenosis in the internal carotid artery by duplex sonography caused by an increase in volume flow. J Vasc Surg. 1998;27:479-485.
18 Koelemay MJW, Nederkoorn PJ, Reitsma JB, Majoie CB. Systematic review of computed tomographic angiography for assessment of carotid artery disease. Stroke. 2004;35:2306-2312.
19 Kaufmann TJ, Kallmes DF. Utility of MRA and CTA in the evaluation of carotid occlusive disease. Sem Vasc Surg. 2005;18:75-82.
20 U-King-Im JM, Trivedi RA, Graves MJ, Higgins NJ, Cross JJ, Tom BD, et al. Contrast-enhanced MR angiography for carotid disease: Diagnostic and potential clinical impact. Neurology. 2004;62:1282-1290.
21 Johnston DCC, Eastwood JD, Nguyen T, Goldstein LB. Contrast-enhanced magnetic resonance angiography of carotid arteries: Utility in routine clinical practice. Stroke. 2002;33:2834-2838.
22 Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491-1502.
23 Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
24 Johnston DCC, Chapman KM, Goldstein LB. Low rate of complications of cerebral angiography in routine clinical practice. 2001;57:2012-2014.
25 Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet. 1999;354:1594-1597.
26 Sauve JS, Thorpe KE, Sackett DL, Taylor W, Barnett HJ, Haynes RB, Fox AJ. Can bruits distinguish high-grade from moderate symptomatic carotid stenosis? The North American Symptomatic Carotid Endarterectomy Trial. Ann Intern Med. 1994;120:633-637.
27 Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569-573.
28 Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915-924.
29 Mitchell P, Wang JJ, Smith W. Risk factors and significance of finding asymptomatic retinal emboli. Clin Experiment Ophthalmol. 2000;28:13-17.
30 Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333-2342.
31 Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157-1166.
32 Eikelboom BC, Riles TR, Mintzer R, Baumann FG, DeFillip G, Lin J, Imparato AM. Inaccuracy of angiography in the diagnosis of carotid ulceration. Stroke. 1983;14:882-885.
33 Widder B, Paulat K, Hackspacher J, Hamann H, Hutschenreiter S, Kreutzer C, et al. Morphological characterization of carotid artery stenoses by ultrasound duplex scanning. Ultrasound Med Biol. 1990;16:349-354.
34 Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107-116.
35 Morgenstern LB, Fox AJ, Sharpe BL, Eliasziw M, Barnett HJ, Grotta JC. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. North American Symptomatic Carotid Endarterectomy Trial (NASCET) group. Neurology. 1997;48:911-915.
36 Ringelstein EB, Zeumer H, Angelou D. The pathogenesis of strokes from internal carotid artery occlusion. Diagnostic and therapeutical implications. Stroke. 1983;14:867-875.
37 Wodarz R. Watershed infarctions and computed tomography. A topographical study in cases with stenosis or occlusion of the carotid artery. Neuroradiology. 1980;19:245-248.
38 Momjian-Mayor I, Baron J-C. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke. 2005;36:567-577.
39 Baquis GD, Pessin MS, Scott RM. Limb shaking—a carotid TIA. Stroke. 1985;16:444-448.
40 Sage JI, Van Uitert RL. Man-in-the-barrel syndrome. Neurology. 1986;36:1102-1103.
41 North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
42 Endarterectomy for moderate symptomatic carotid stenosis: interim results from the MRC European carotid Surgery Trial. Lancet. 1996;347:1591-1593.
43 Executive committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421-1428.
44 Abbott AL, Chambers BR, Stork JL, Levi CR, Bladin CF, Donnan GA. Embolic signals and prediction of ipsilateral stroke or transient ischemic attack in asymptomatic carotid stenosis: a multicenter prospective cohort study. Stroke. 2005;36:1128-1133.
45 Rothwell PM, Coull AJ, Giles MF, Howard SC, Silver LE, Bull LM, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet. 2004;363:1925-1933.
46 Spence JD, Tamayo A, Lownie SP, Ng WP, Ferguson GG. Absence of microemboli on transcranial Doppler identifies low-risk patients with asymptomatic carotid stenosis. Stroke. 2005;36:2373-2378.
47 Tegos TJ, Kalodiki E, Nicolaides AN, Sabetai MM, Stevens JM, Thomas DJ. Brain CT infarction in patients with carotid atheroma. Does it predict a future event? Int Angiol. 2001;20:110-117.
48 Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, Caltagirone C. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122-2127.
49 Olin JW, Fonseca C, Childs MB, Piedmonte MR, Hertzer NR, Young JR. The natural history of asymptomatic moderate internal carotid artery stenosis by duplex ultrasound. Vasc Med. 1998;3:101-108.
50 Rockman CB, Riles TS, Lamparello PJ, Giangola G, Adelman MA, Stone D, et al. Natural history and management of the asymptomatic, moderately stenotic internal carotid artery. J Vasc Surg. 1997;25:423-431.
51 Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
52 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. The Lancet 368:1239–1247.
53 Mas J-L, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin J-P, et althe EVASI. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660-1671.
54 Caplan LR, Wityk RJ, Glass TA, Tapia J, Pazdera L, Chang HM, et al. New England Medical Center Posterior Circulation Registry. Ann Neurol. 2004;56:389-398.
55 de Bray JM, Pasco A, Tranquart F, Papon X, Alecu C, Giraudeau B, et al. Accuracy of color-Doppler in the quantification of proximal vertebral artery stenoses. Cerebrovasc Dis. 2001;11:335-340.
56 Ackerstaff RG, Hoeneveld H, Slowikowski JM, Moll FL, Eikelboom BC, Ludwig JW. Ultrasonic duplex scanning in atherosclerotic disease of the innominate, subclavian and vertebral arteries. A comparative study with angiography. Ultrasound Med Biol. 1984;10:409-418.
57 Farres MT, Grabenwoger F, Magometschnig H, Trattnig S, Heimberger K, Lammer J. Spiral CT angiography: study of stenoses and calcification at the origin of the vertebral artery. Neuroradiology. 1996;38:738-743.
58 Kollias SS, Binkert CA, Ruesch S, Valavanis A. Contrast-enhanced MR angiography of the supra-aortic vessels in 24 seconds: a feasibility study. Neuroradiology. 1999;41:391-400.
59 Khan S, Cloud G, Kerry S, Markus HS. Imaging of vertebral artery stenosis: a systematic review. J Neurol Neurosurg Psychiatry. 2007;78:1218-1225.