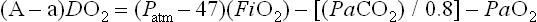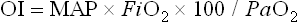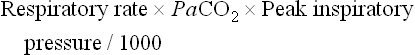Extracorporeal Membrane Oxygenation
Extracorporeal membrane oxygenation (ECMO) is a life-saving technology that employs partial heart/lung bypass for extended periods. It provides gas exchange and perfusion for patients with acute, reversible cardiac or respiratory failure. This affords the patient’s cardiopulmonary system a time to ‘rest,’ during which the patient is spared the deleterious effects of high airway pressure, high FiO2, traumatic mechanical ventilation, and impaired perfusion. As of 2011, the Extracorporeal Life Support Organization (ELSO) has registered approximately 40,000 neonates and children treated with ECMO for a variety of cardiopulmonary disorders. The number of centers providing extracorporeal support and reporting to ELSO continues to increase along with the total number of cases.1
History
The initial effort to develop extracorporeal bypass came from cardiac surgeons. Their goal was to correct intracardiac lesions and, therefore, they needed to arrest the heart, divert and oxygenate the blood, and perfuse the patient so that repair could be performed. The first cardiopulmonary bypass circuits involved cross circulation between the patient and another subject (usually the patient’s mother or father) acting as both the pump and the oxygenator.2
The first attempts at establishing cardiopulmonary bypass and oxygenation by complete artificial circuitry were constructed with disk-and-bubble oxygenators, and were limited because of hemolysis encountered by direct mixing of oxygen and blood. The discovery of heparin and the development of semipermeable membranes (silicone rubber) capable of supporting gas exchange by diffusion were major advancements toward the development of ECMO.3 During the 1960s and early 1970s, this silicone membrane was configured into a number of oxygenator models.4–7
In 1972, the first successful use of prolonged cardiopulmonary bypass was reported.8 The patient had sustained a ruptured aorta following a motorcycle accident. Venoarterial extracorporeal bypass support was maintained for three days. A multicenter prospective randomized trial sponsored by the National Heart, Lung, and Blood Institute (a branch of the National Institutes of Health) studied the efficacy of ECMO for adult respiratory distress syndrome. In 1979, they concluded that the use of ECMO had no advantage over conventional mechanical ventilation, and the trial was stopped before completion.9 However, Bartlett and colleagues noted that all of the patients in the study had irreversible pulmonary fibrosis before the initiation of ECMO. In 1976, they reported the first series of infants with ECMO.10 Six (43%) of 14 babies with respiratory distress syndrome survived. Many of these infants were premature and weighed less than 2 kg. In addition, 22 patients with meconium aspiration syndrome had a 70% survival rate, although these neonates tended to be larger.
Since then, despite study design issues, three randomized controlled trials and a number of retrospective published reports have confirmed the efficacy of ECMO over conventional mechanical ventilation.11–18 By 1996, 113 centers had ECMO programs registered with ELSO.1 Over the next two decades, improvements in technology, a better understanding of the pathophysiology of pulmonary failure, and a greater experience using ECMO have contributed to improved outcomes for infants with respiratory failure. In 2003, the University of Michigan reported an association between ECMO volume and an observed reduction in neonatal mortality seen in that state between 1980 and 1999.19
ELSO, formed in 1989, is a collaboration of health care professionals and scientists with an interest in ECMO. The organization provides the medical community with guidelines, training manuals and courses, and a forum in which interested individuals can meet and discuss the future of extracorporeal life support. The group also provides a registry to investigators for the collection of data from most centers with an ECMO program throughout the world. This database provides valuable information for analysis of this life-saving biotechnology.20,21
Clinical Applications
Neonates are the patients who benefit most from ECMO. Cardiopulmonary failure in this population secondary to meconium aspiration syndrome (MAS), congenital diaphragmatic hernia (CDH), persistent pulmonary hypertension of the newborn (PPHN), and congenital cardiac disease are the most common pathophysiologic processes requiring ECMO. In children, the most common disorders treated with ECMO are viral and bacterial pneumonia, acute respiratory distress syndrome (ARDS), acute respiratory failure (non-ARDS), sepsis, and cardiac disease. Treatment of patients who cannot be weaned from bypass after cardiac surgery and patients with end-stage ventricular failure needing a bridge to heart transplantation are areas where ECMO use is increasing.1,22,23 Some less frequently used indications for ECMO include respiratory failure secondary to smoke inhalation,24 severe asthma,25 rewarming of hypercoagulopathic/hypothermic trauma patients,26 and maintenance of an organ donor pending liver allograft harvest and transplantation.27
Pathophysiology of Newborn Pulmonary Hypertension
Failure of the transition from fetal circulation to newborn circulation is described as PPHN or persistent fetal circulation (PFC).28 Clinically, PPHN is characterized by hypoxemia out of proportion to pulmonary parenchymal or anatomic disease. In hypoxic fetuses and infants, the proliferation of smooth muscle in the arterioles may extend far beyond the terminal bronchioles, resulting in thickened and more reactive vessels. In response to hypoxia, these vessels undergo significant self-perpetuating vasoconstriction. Although sometimes idiopathic, PPHN can occur secondary to a number of disease processes such as MAS, CDH, polycythemia, and sepsis.
Data recommending permissive hypercapnia and spontaneous respirations as principles of treatment for these children have been reported.29 Hyperventilation and neuromuscular blockade are not part of the treatment strategy. This strategy has decreased morbidity, mortality, and the need for ECMO in several centers.
Patient Selection Criteria
Recommended pre-ECMO studies are listed in Box 6-1. The definition of ‘conventional therapy’ is not consistent for each indication. Nevertheless, ECMO is indicated when (1) there is a reversible disease process; (2) the ventilator treatment is causing more harm than good; and (3) tissue oxygenation requirements are not being met. A discussion of generally accepted selection criteria for using neonatal ECMO follows.
Reversible Cardiopulmonary Disorders
The underlying principle of ECMO relies on the premise that the patient has a reversible disease process that can be corrected with either therapy (including the possibility of organ transplantation) or ‘rest’, and that this reversal will occur in a relatively short period of time. Prolonged exposure to high-pressure mechanical ventilation with high concentrations of oxygen can have a traumatic effect on the newborn’s lungs and frequently leads to the development of bronchopulmonary dysplasia (BPD).30 It has been suggested that BPD can result from high levels of ventilatory support for as little as four days or less.31 The pulmonary dysfunction that follows barotrauma and oxygen toxicity associated with mechanical ventilation typically requires weeks to months to resolve. Therefore, patients who have been ventilated for a long time and in whom lung injury has developed are not amenable to a short course of therapy with ECMO. Most ECMO centers will not accept patients who have had more than ten to 14 days of mechanical ventilation, owing to the high probability of established, irreversible pulmonary dysfunction.
Coexisting Anomalies
Every effort should be made to establish a clear diagnosis before the initiation of ECMO. Infants with anomalies incompatible with life do not benefit from ECMO (i.e., trisomy 13 or 18). ECMO is not a resource that is intended to delay an inevitable death. Many lethal pulmonary conditions, such as overwhelming pulmonary hypoplasia, congenital alveolar proteinosis, and alveolar capillary dysplasia, may present as reversible conditions but are considered lethal.32
Gestational Age
The gestational age of an ECMO patient should be at least 34 to 35 weeks. In the early experience with ECMO, significant morbidity and mortality related to intracranial hemorrhage (ICH) was associated with premature infants (<34 weeks’ gestation).33 Despite modifications in the ECMO technique over the past two decades, premature infants continue to be at risk for ICH. In preterm infants, ependymal cells within the brain are not fully developed, thus making these infants susceptible to hemorrhage. Systemic heparinization necessary to maintain a thrombus-free circuit adds to this risk.
Bleeding Complications
Infants with ongoing, uncontrollable bleeding or an uncorrectable bleeding diathesis pose a relative contraindication to ECMO.20 Any coagulopathy should be corrected before initiating ECMO because the need for continuous systemic heparinization adds an unacceptable risk of bleeding.
Intracranial Hemorrhage
As a general rule, candidates for ECMO should not have had an ICH. A preexisting ICH may be exacerbated by the use of heparin and the unavoidable alterations in cerebral blood flow while receiving ECMO. Patients with small intraventricular hemorrhages (grade I) or small intraparenchymal hemorrhage can be successfully treated on ECMO by maintaining a lower than optimal activated clotting time in the range of 180–200 seconds. These patients should be closely observed for extension of the intracranial bleeding. Patients posing a particularly high risk for ICH are those with a previous ICH, a cerebral infarct, prematurity, coagulopathy, ischemic central nervous system injury, or sepsis. Consideration of these patients for ECMO should be individualized.32
Failure of Medical Management
With recent innovations in medical management, ECMO use has been obviated in patients who otherwise meet ECMO criteria. These innovations include the use of permissive hypercapnia with spontaneous ventilation, avoidance of muscle paralysis, and the avoidance of chest tubes. In 1978, the Children’s Hospital of New York initiated a nontraditional approach to the management of patients with PPHN, which has been successfully extended to infants with CDH.34 Hyperventilation, hyperoxia, and muscle relaxants were not used, and permissive hypercapnia in conjunction with spontaneous ventilation was emphasized. Low-pressure ventilator settings were used and a persistent PaCO2 of 50–60 mmHg and a PaO2 of 50-70 mmHg were allowed. With careful attention to maintaining a preductal oxygen saturation greater than 90% or PaO2 of 60 mmHg or greater, 15 infants who met ECMO criteria with PPHN and in severe respiratory failure were initially treated with this approach and survived without ECMO.
Risk Assessment
where Patm is the atmospheric pressure and FiO2 is the inspired concentration of oxygen.
where MAP is the mean airway pressure.
Although criteria for ECMO varies from institution and by diagnosis, it is generally accepted that, in the setting of optimal management, an (A–a)DO2 greater than 625 mmHg for more than four hours, or an (A–a)DO2 greater than 600 mmHg for more than 12 hours, or an OI of greater than 40 establishes both a relatively sensitive and specific predictor of mortality. Other criteria used by many institutions include a preductal PaO2 less than 35–50 mmHg for two to 12 hours or a pH of less than 7.25 for at least two hours along with intractable hypotension. These are sustained values measured over a period of time and are not accurate predictors of mortality.14,20,35–37 Patients with CDH are in their own category, and criteria for this disease are discussed later in this chapter.
The combination of a ventilation index greater than 40 and an OI more than 40 correlates with a 77% mortality.38 A mortality of 81% is associated with an (A–a)DO2 greater than 580 mmHg and a peak inspiratory pressure of 40 cmH2O.38 Indications for support in patients with cardiac pathology are based on clinical signs such as hypotension despite the administration of inotropes or volume resuscitation, oliguria (urine output < 0.5 mL/kg/h), and decreased peripheral perfusion.
Congenital Diaphragmatic Hernia
Various other strategies have been tried to manage critically ill newborns with CDH.39 High-frequency oscillation may have its major role in forestalling respiratory failure when used as a ‘front end’ strategy rather than as a ‘rescue therapy’.40 Surfactant plays no more than an anecdotal role. Nitric oxide may be helpful as a vasodilator in the treatment of pulmonary hypertension in these patients. Other pulmonary vasculature vasodilators such as epoprostenol, sildenafil, and iloprost are starting to demonstrate significant efficacy in babies with CDH. The primary indicator for ECMO in the CDH patient occurs when tissue oxygen requirements are not being met, as evidenced by progressive metabolic acidosis, mixed venous oxygen desaturation, and multiple organ failure. The other major indicator is mounting iatrogenic pulmonary injury.
The goal is to maintain preductal oxygen saturations between 90–95%. Spontaneous breathing is preserved by rigorously avoiding muscle relaxants.41,42 Sedation is used only as needed. Meticulous attention to maintaining a clear airway and the well-being of the infant is obvious, but critical. Permissive hypercapnia with spontaneous respiration is initiated with intermittent mandatory ventilation (IMV), 30–40 breaths per minute, equal I/E time, inspiratory gas flow of 5–7 L/min, peak inspiratory pressure (PIP) of 20–22 cmH2O, and positive end-expiratory pressure (PEEP) of 5 cmH2O. The FiO2 is selected to maintain preductal SaO2 greater than 90%. If this method of ventilation is not effective, as demonstrated by severe paradoxical chest movement, severe retractions, tachypnea, inadequate or labile oxygenation (preductal O2 saturations <80%), or PaCO2 greater than 60 mmHg, then a new mode of ventilation is needed.
Before ECMO is initiated for an infant with CDH, the baby should first demonstrate some evidence of adequate lung parenchyma. Some programs use radiographic parameters to determine adequate lung volumes. The lung-to-head ratio (LHR) is measured by prenatal ultrasonography (US).43,44 It is defined as the product of the orthogonal diameters of the non-affected lung divided by the head circumference. Severe pulmonary hypoplasia is considered when the LHR is less than 1.0 and intermediate hypoplasia lies between 1.0–1.4.45 Recent data have shown that an LHR threshold of 0.85 predicted mortality with 95% sensitivity and 64% specificity.45 The LHR is operator dependent and can only be obtained in a narrow gestational window and therefore leads to poor reproducibility across different centers.
Many centers believe the best method to evaluate pulmonary hypoplasia and predict outcome is to evaluate the patient clinically. This is assessed by having a recorded best PaCO2 less than 50 mmHg and a preductal oxygen saturation greater than 90% for a sustained period of at least one hour at any time in the clinical course. With these criteria, successful ECMO should yield an overall survival rate of 75% or better. If patients with lethal anomalies, overwhelming pulmonary hypoplasia, or neurologic complications are not included, survival approaches 85%.41,42,46
Extracorporeal Cardiopulmonary Resuscitation
Studies demonstrate that 1–4% of pediatric intensive care unit (PICU) admissions suffer a cardiac arrest. Survival to discharge for a patient who has an arrest in the PICU ranges from 14–42%. The ELSO data demonstrate that approximately 73% of extracorporeal cardiopulmonary resuscitation (ECPR) has been used for patients with primary cardiac disease. Overall survival to discharge in this population reached 38%.47 The American Heart Association recommends ECPR for in-hospital cardiac arrest refractory to initial resuscitation, secondary to a process that is reversible or amenable to heart transplantation. Conventional cardiopulmonary resuscitation (CPR) must have failed, no more than several minutes should have elapsed, and ECMO must be readily available. Future research needs to analyze long-term neurologic status amongst survivors and which patients will benefit the most with as little morbidity as possible.
Second Course of ECMO
Approximately 3% of patients that are treated with ECMO will require a second course. The survival rates for patients in this cohort are comparable to the first course. Negative prognostic indicators for second course ECMO patients include patients with renal impairment, higher number of first-course complications, age older than 3 years old, or a prolonged second course.48
Methods of Extracorporeal Support
VV and DLVV bypass provide pulmonary support but do not provide cardiac support. VV bypass is established by drainage from the right atrium via the right internal jugular vein with reinfusion into a femoral vein. DLVV is accomplished by means of a double-lumen catheter inserted into the right atrium via the right internal jugular vein. A major limitation of VV or DLVV ECMO is that a fraction of the infused oxygenated blood re-enters the pump and, at high flows, may limit oxygen delivery due to recirculation. A limitation specific to DLVV is catheter size, which confines use of this method of support to larger neonates, infants, and smaller children. VV and DLVV bypass have become the preferred method of extracorporeal support for all appropriate patients who do not require cardiac support.20
Cannulation
Cannulation can be performed with proper monitoring in the neonatal or PICU under adequate sedation and intravenous anesthesia. The infant is positioned supine with the patient’s head at the foot of the bed. The head and neck are hyperextended over a shoulder roll and turned to the left. Local anesthesia is administered in the incision site. A transverse cervical incision is made along the anterior border of the sternomastoid muscle, one finger-breadth above the right clavicle. The platysma muscle is divided, and dissection is carried down with the sternomastoid muscle retracted to expose the carotid sheath. The sheath is opened, and the internal jugular vein, common carotid artery, and vagus nerve are identified (Fig. 6-1A). The vein is exposed first and encircled with proximal and distal ligatures. Occasionally it is necessary to ligate the inferior thyroid vein. The common carotid artery lies medial and posterior, contains no branches, and is mobilized in a similar fashion. The vagus nerve should be identified and protected.
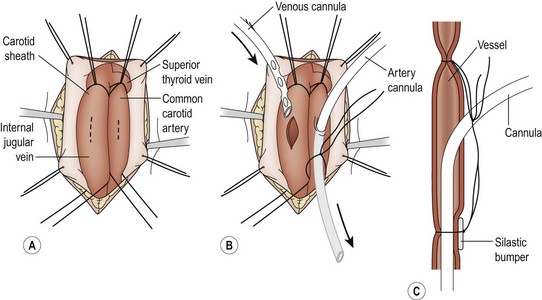
FIGURE 6-1 The cannulation procedure. (A) The carotid sheath is exposed, the sternocleidomastoid muscle is retracted laterally, and the common carotid artery and the internal jugular vein are dissected free. (B) The patient is anticoagulated after the vessels are dissected and ligated cephalad. The arterial cannula is passed into the junction of the innominate artery and the aorta. The venous catheter is passed into the right atrium. (C) A polymeric silicone (Silastic) bumper is used to facilitate ligation of the cannulas. The two ligatures on each vessel are then tied together.
The arterial cannula (usually 10 French for newborns) is measured so that the tip will lie in the ascending aorta. This is approximately one-third the distance between the sternal notch and the xiphoid. The venous cannula (usually 12–14 French for neonates) is measured so that its tip lies in the distal right atrium which is approximately half the distance between the suprasternal notch and the xiphoid process. If time permits, an activated clotting time (ACT) should be checked before heparinization. The patient is then systemically heparinized with100 U/kg of heparin, which is allowed to circulate for two to three minutes, which should produce an ACT of more than 300 seconds. The arterial cannula is usually inserted first with VA bypass. The carotid artery is ligated cephalad. Proximal control is obtained, and a transverse arteriotomy is made near the cephalad ligature (Fig. 6-1B). To help prevent intimal dissection, fine Prolene sutures can be placed around the arteriotomy and used for retraction when introducing the arterial cannula. The saline-filled cannula is inserted to its premeasured position and secured with two silk ligatures (2-0 or 3-0). A small piece of vessel loop (bumper) may be placed under the ligatures on the anterior aspect of the carotid to protect the vessel from injury during decannulation (Fig. 6-1C).
In preparation for the venous cannulation, the patient is given succinylcholine to prevent spontaneous respiration. The proximal internal jugular vein is then ligated cephalad to the site selected for the venotomy. Gentle cephalad traction on this ligature helps during insertion the venous catheter. A venotomy is made close to the ligature. The saline-filled venous catheter is inserted into the right atrium and secured in a manner similar to that used for the arterial catheter. Any air bubbles are removed from the cannulas as they are connected to the ECMO circuit. Bypass is initiated. The cannulas are then secured to the skin above the incision. The incision is closed in layers, ensuring that hemostasis is meticulous.
The cannula positions for VA ECMO are confirmed by chest radiograph and/or transthoracic echocardiogram. The venous catheter should be located in the inferior aspect of the right atrium, and the arterial catheter in the ascending aorta about 1–2 cm above the aortic valve. With a double-lumen venous catheter, the tip should be in the mid-right atrium with oxygenated blood flow directed toward the tricuspid valve.49
A challenging situation arises when one attempts to cannulate a newborn with a right-sided CDH. Anatomic distortion of the mediastinum can lead to cannulation of the azygos vein, which will then fail to provide adequate ECMO support. This is usually detected by poor pump function and echocardiography, which will not demonstrate the cannula in the superior vena cava or right atrium. In these patients, attempted manipulation of the cannula is often wrought with failure, and one should consider other avenues for venous drainage, including central cannulation.50
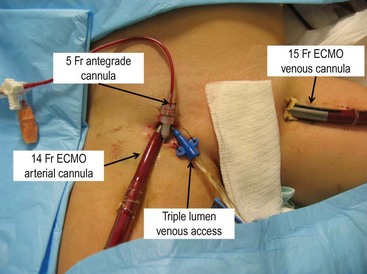
The small pediatric population (ages 2–12 years of age) presents a difficult and controversial scenario with regard to cannulation. Some centers continue to perform arterial cannulation via the carotid artery. The long-term neurologic outcome is unknown in this population. Due to this concern, some centers will cannulate these patients via femoral access. However, the arterial cannula is large and can either partially or completely obstruct antegrade arterial flow. This can result in distal limb ischemia which can lead to sensory or motor deficits, tissue loss, or even limb loss. One potential way to avoid this problem is to provide antegrade flow via a percutaneously placed distal perfusion catheter (Fig. 6-2).51
ECMO Circuit
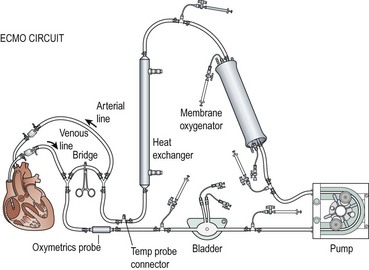
Venous blood is drained into a small reservoir or bladder through the cannula that is in the right atrium via the right internal jugular vein (Fig. 6-3). The bladder is a 30–50 mL reservoir that acts as a safety valve. If the venous drainage does not keep up with arterial outflow from the pump, the bladder volume will be depleted, sounding an alarm, and automatically shutting off the pump. Sensors can be placed into the circuit to measure arterial oxygen saturation, mixed venous saturation, hematocrit, and pump flow. Hypovolemia is one of the most common causes of decreased venous inflow into the circuit, but kinking and occlusion of the venous line should be suspected first. An algorithm for managing pump failure due to inadequate venous return is shown in Figure 6-4.
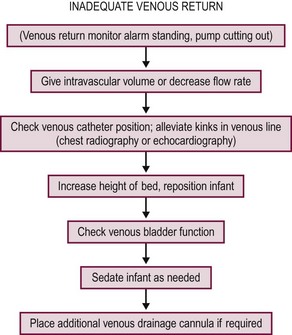
FIGURE 6-4 Suggested algorithm for the management of inadequate venous return during extracorporeal membrane oxygenation. (Adapted from DeBerry BB, Lynch J, Chung DH, Zwischenberger JB. Emergencies during ECLS and their management. In: Van Meurs K, Lally KP, Peek G, Zwischenberger JB, editors. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. 3rd ed. Ann Arbor, MI: Extracorporeal Life Support Organization; 2005. p. 133–56.)
Two types of ECMO pumps, centrifugal and roller head, are used to pump blood through the membrane oxygenator. Centrifugal pumps are dependent on adequate preload and afterload, and have continuous flow. The revolutions per minute (RPM) are adjusted to maintain the desired flow rate. A low preload or high afterload will lead to lower flow despite a fixed RPM. Alternatively, roller pumps operate by displacing a fixed volume of blood per revolution and are afterload independent. The roller pumps are designed with microprocessors that allow for calculation of the blood flow based on the roller-head speed and tubing diameter of the circuit. The pumps are connected to continuous pressure monitoring throughout the circuit and are servoregulated if pressures within the circuit exceed preset parameters. Another safety device, the bubble detector (not depicted in Figure 6-3), is interposed between the pump and the membrane oxygenator that halts perfusion to the patient if air is detected in the circuit.
Patient Management on ECMO
Once the cannulas are connected to the circuit, bypass is initiated, and the flow is slowly increased to 100–150 mL/kg/min. Continuous in-line monitoring of the (pre-pump) SvO2 and arterial (post-pump) PaO2 as well as pulse oximetry is vital. The goal of VA ECMO is to maintain an SvO2 of 37–40 mmHg and saturation of 65–70%. VV ECMO is more difficult to monitor because of recirculation, which may produce a falsely elevated SvO2. Inadequate oxygenation and perfusion are indicated by metabolic acidosis, oliguria, hypotension, elevated liver function studies, and seizures. Arterial blood gases should be monitored closely with PaO2 and PaCO2 maintained as close to normal levels as possible. The oxygen level of the blood returning to the patient should be fully saturated. To increase a patient’s oxygen delivery on ECMO, one can either increase the ECMO flow rate (~ cardiac output) or the hemoglobin can be increased to maintain hemoglobin at 15 g/dL (~ oxygen content). CO2 elimination is extremely efficient, and it is important to adjust the sweep (gas mixing) to maintain a PaCO2 in the range of 40–45 mmHg. This is important, especially during weaning, because a low PaCO2 inhibits the infant’s spontaneous respiratory drive. Serial monitoring allows timely adjustments. The arterial blood gas is measured hourly. As soon as these parameters are met, all vasoactive drugs are weaned and ventilator levels are adjusted to ‘rest’ settings. Gastrointestinal prophylaxis (H2 antagonists or proton pump inhibitors) is initiated, and mild sedation and analgesia are provided, usually with fentanyl and midazolam. Paralyzing agents are avoided. Cefazolin is used for antimicrobial prophylaxis. Routine blood cultures should be obtained.20,49 A daily chest radiograph is performed. Opacification or ‘white out’ is often noted during the early ECMO course. The reasons for this are multifactorial and include decreased ventilatory pressures (both PIP and PEEP), reperfusion of the injured lung, and exposure of the blood to a foreign surface, causing an inflammatory response with the release of cytokines. A list of typical diagnostic tests is shown in Table 6-1.
TABLE 6-1
General Studies Obtained During ECMO
| Laboratory Study | General Frequency and Comments |
| Chest radiography | Daily |
| Cranial ultrasonography | Only for neonates, the first three days and then as needed |
| Activated clotting time | Every hour, more often if outside of parameters |
| Preoxygenator blood gas | Every four hours |
| Postoxygenator blood gas | Every four hours |
| Patient blood gas | Every six hours |
| Glucose monitoring test | Every four hours |
| Complete blood cell count with platelets | Every six hours; include a differential daily |
| Chem-7 | Every six hours, including magnesium, calcium, and phosphorus daily |
| Fibrinogen | Daily and after infusion of cryoprecipitate and fresh frozen plasma; may also include prothrombin time and D-dimer |
Heparin is administered (30–60 mg/kg/h) throughout the ECMO course to preserve a thrombus-free circuit. ACTs should be monitored hourly and maintained at 180–220 seconds. A complete blood cell count should be obtained every six hours and coagulation profiles obtained daily. To prevent thrombocytopenia, platelets are transfused to maintain a platelet count greater than 100,000/mm3. The use of fibrinogen and other clotting factors is controversial. Fresh frozen plasma should be considered in infants with international normalized ratio (INR) levels >1.5 in order to replete coagulation cascade factors and allow for adequate anticoagulation. In cases of heparin resistance, anti-thrombin 3 levels should be checked and repleted as necessary. The hematocrit should remain above 40% by using red blood cell transfusions so that oxygen delivery is maximized.20
The volume management in patients on ECMO is very important and difficult. It is imperative that all inputs and outputs be diligently recorded and electrolytes monitored every six hours. Fluid losses should be repleted and electrolyte abnormalities corrected. Patients should receive maintenance fluids as well as adequate nutrition by using parenteral hyperalimentation. The first 48 to 72 hours on ECMO typically involve fluid extravasation into the soft tissues. The patient becomes edematous and often requires volume replacement (crystalloid, colloid, or blood products) to maintain adequate intravascular and bypass flows, appropriate hemodynamics, and urine output greater than 1 mL/kg/h. By the third day of bypass, diuresis of the excess extracellular fluid begins and can be facilitated with the use of diuretics and, if necessary, an in-line hemofilter.20,49
Selective hypothermia for cerebral ischemia/hypoxia may improve neurologic outcome. It is not yet clear if whole body or cap cooling provides significant improvement in ECMO outcomes. It is possible to maintain temperature of 34°C for 45 hours on ECMO without increasing morbidity.52 The largest study to date is underway in the UK. The Neonatal ECMO Study of Temperature (NEST) is a multicenter prospective randomized control trial of mild hypothermia versus normothermia in neonates on ECMO.53
Operative Procedure on ECMO
Operations, such as CDH repair, can be performed while the child remains on bypass, but one must account for the continued postoperative anticoagulation. Hemorrhagic complications are a frequent morbidity associated with an operation on ECMO, and these complications increase mortality. To try to avoid these problems, the platelet count should be greater than 150,000/mm3, the ACT can be reduced to 180–200 seconds, and ECMO flow is increased to full support. Moreover, it is imperative that meticulous hemostasis is obtained throughout the operation. The fibrinolysis inhibitor aminocaproic acid (100 mg/kg) is administered just prior to incision, and is infused continuously (30 mg/kg/h) until there is no evidence of bleeding.20,49
Weaning and Decannulation
As the patient improves, less blood flow is required to pass through the ECMO circuit and the flow can be weaned at a rate of 10–20 mL/h as long as the patient maintains good oxygenation and perfusion. The most important guide to VA ECMO weaning is the SvO2. For VV ECMO, it is the SaO2. Regardless of the cannulation format, successful weaning is marked by stable acid–base balance and good urine output. Flows should be decreased to 30–50 mL/kg/min, and the ACT should be at a higher level (200–240 seconds) to prevent thrombosis. Newer oxygenators have higher limits of allowable flow, which may limit full weaning. Adjustable shunts placed across the oxygenator allow higher overall flow to the oxygenator with a lower flow being delivered to the patient. Also, flow probes placed on the arterial cannula can be used to accurately guide weaning. Moderate conventional ventilator settings are used, but higher settings can be used if the patient needs to be weaned from ECMO urgently. If the child tolerates the low flow, all medications and fluids should be switched to vascular access on the patient. The cannulas are flushed and clamped, with the circuit bypassing the patient via the bridge. If it is possible that the child may need to be returned to bypass, then the cannulas should be flushed with heparin (2 U/mL). The patient is then observed for two to four hours. If this is tolerated, decannulation can be accomplished.
Decannulation is performed under sterile conditions with the patient in the Trendelenburg position. With the use of a short acting muscle relaxant to prevent air aspiration into the vein, ventilator settings should be increased. The venous catheter is typically removed first, and the jugular vein is ligated. Repair of the carotid artery is controversial. Short-term results demonstrate acceptable patency rates and equivalent short-term neurodevelopmental outcomes when compared with children undergoing carotid artery ligation.54,55 Another study of neonates who underwent arterial repair found a 72% incidence of an occluded or highly stenotic right common carotid artery at two years of age.56 Similar to other studies, there was no significant difference in neurologic development when compared to controls. The incision should be irrigated and closed over a small drain, which is removed 24 hours later.20,49
Complications
Mechanical Complications
Membrane Failure
Failure of the membrane oxygenator is seen with a decrease in oxygenation or retention of CO2. The cause of such complications include a fibrin clot or water condensation, both of which diminish the oxygenator’s ability to transfer oxygen and CO2. Oxygenator failure has been reported in 21.6% of respiratory ECMO runs in the neonatal and pediatric population.1 The oxygenator should not be subject to high pressures, which should be continuously monitored. Pressure limits are specific for different manufacturers and for the size of the membrane. Clots in the oxygenator can be seen but the extent of the clot cannot be determined. The progressive consumption of coagulation factors, such as platelets and fibrinogen, also indicates that the membrane may be progressively building clot, and the need to change the oxygenator should be considered. Another sign of impending membrane failure is rising CO2 levels in the post-oxygenator blood.
Patient Complications
Neurologic Complications
Neurologic complications develop in 25% of infants and children on ECMO.1 ICH, infarct, and seizure carry significant mortality when encountered in ECMO patients. Frequent neurologic examinations should be performed and the use of paralytic agents avoided. The examination should include evaluation of alertness and interaction, spontaneous movements, eye exams, the presence of seizures, fullness of the fontanelles, tone, and reflexes. Electroencephalography (EEG) may also be helpful in the neurologic evaluation. Cranial ultrasound should be performed on all neonates before initiating ECMO to identify those patients in whom significant ICH already exists. A retrospective analysis revealed that birth weight and gestational age were the most significant correlating factors with ICH in neonates on ECMO.33 Once the patient is placed on ECMO, ultrasound is repeated during the first three days when indicated by the clinical condition. If the examination reveals a new moderate (grade II) hemorrhage or an expanding ICH, ECMO is usually discontinued.
In the event that an ICH is suspected, or detected on cranial ultrasound, and deemed to be small in size, it is reasonable to maintain a low ACT (180–200 seconds) with a platelet count greater than 125,000–150,000/mm3. Serial head ultrasound should be performed to monitor the progression of the hemorrhage.20
Cannula Site or Bleeding at Other Sites
The ECMO registry reports an 8.4% incidence of cannulation site bleeding and a 13% incidence of other surgical site bleeding.1 Contact of blood with the foreign surface of the circuit activates the coagulation cascade. The number of platelets and their function are also affected. With anticoagulation, the risk of bleeding while undergoing an operation on ECMO is considerable. To reduce this risk, meticulous hemostasis needs to be maintained during the procedure and before closure. If necessary, the surgeon should employ topical hemostatic agents. Lowering the ACT parameters to 180–200 seconds and maintaining a platelet count of at least 125,000/mm3 can assist with hemostasis. If bleeding from the cervical incision is greater than 10 mL/h for two hours despite conservative treatment strategies, exploration may be needed.20
Bleeding into previous operative sites occurs frequently and must be handled aggressively. A decreasing hematocrit, an increasing heart rate, a decline in the blood pressure, or inadequate venous return are signs of ongoing hemorrhage. Treatment includes replenishing blood products, including coagulation factors, if necessary. ACT parameters should be decreased to 180 to 200 seconds and the platelet count maintained greater than 125,000/mm3. Agents that inhibit fibrinolysis, such as aminocaproic acid, also can help prevent bleeding. The use of recombinant activated factor VII (NovoSeven, Novo Nordisk, Inc., Princeton, NJ) has been described in the management of bleeding unresponsive to conventional methods.57 This is an off-label use, and thrombosis is a significant concern. Often, one must evacuate the hematoma and explore for the cause as is often the case in the postcardiac surgery patient with an open chest and central cannulation. If bleeding is not quickly controlled, decannulation and stopping the anticoagulation may need to be strongly considered.
Coagulation Abnormalities
ECMO patients have a coagulopathy secondary to consumption by the circuit. Removal of the source and a circuit change is a logical approach. Disseminated intravascular coagulation (DIC) occurs in approximately 2% of ECMO cases.1 DIC is characterized by the consumption of plasma clotting factors and platelets, resulting in deposition of fibrin thrombi in the microvasculature. Once the factor levels and platelet count decrease below certain levels, bleeding will occur. Sepsis, acidosis, hypoxia, and hypotension are the most common causes which is why ECMO patients are at risk for developing DIC. The most common cause of a coagulopathy is consumption of clotting factors by the circuit and rarely is it due to sepsis or DIC.
Renal Failure
Oliguria is common in ECMO patients and is often seen during the first 24 to 48 hours. The capillary leak that occurs after placing a child on ECMO may cause decreased renal perfusion. Alternatively, it may result from the nonpulsatile blood flow that occurs with VA ECMO. Once the patient is adequately volume resuscitated, and fluid shifts have stabilized, the use of furosemide (1–2 mg/kg) can improve urine output. If the creatinine continues to rise, then renal ultrasound is recommended. The use of continuous hemofiltration, which can be added in-line to the ECMO circuit, is another mechanism to assist in managing the fluid shifts, hyperkalemia, and azotemia. Hemofiltration removes plasma water and dissolved solutes while retaining proteins and the cellular components of the intravascular space.20
Hypertension
The incidence of hypertension on ECMO varies from 28% to as high as 92%.58 According to the ELSO registry, 13% of ECMO patients require pharmacologic intervention.1 One group reported that detectable ICH occurred in 44% of their hypertensive patients and clinically significant ICH developed in 27%.59 The patient should be assessed initially for reversible causes of hypertension, such as pain, hypercarbia, and hypoxia. Embolic renal infarction is another cause of hypertension. Medical management includes the use of hydralazine, nitroglycerin, and captopril.
Infection
The incidence of nosocomial infections during ECMO has been reported as high as 30%.1 Associated risk factors include the duration of the ECMO course, the length of hospitalization, and procedures performed before the initiation of ECMO or during the run.60 The ELSO registry data from July 2011 describes an 8% culture-proven infection rate in ECMO neonates and pediatric patients.1 This is remarkably low, considering the large surface area of the circuit, the duration of bypass, and the frequency of access to the circuit. Fungal infections carry a significantly higher hospital mortality rate, and sepsis carries a higher morbidity and mortality rate in neonates.61,62 Access to the circuit should be minimized and meticulous sterile techniques are important.
Results
ECMO is a prime example of the evolution from an experimental technique to a commonly used therapeutic approach. The ELSO registry has accumulated data since the early 1980s from all registered centers throughout the world. The number of registered centers continues to rise, as does the number of ECMO cases. In 1992, over 1500 ECMO cannulations for neonatal respiratory disease were performed. In 2010, only 747 cases were reported. The decline in case volume is likely due to improvements in ventilation management and the addition of new agents including inhaled nitric oxide, smooth muscle relaxants, exogenous surfactant, and high-frequency oscillation.1,20
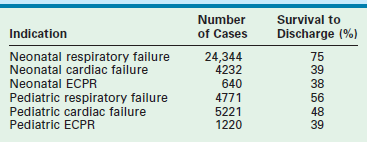
Overall survival to discharge for neonates and children is 63% for all diagnoses.1 Higher survival rates are seen in neonates with respiratory diseases (75%) versus children with respiratory failure (56%), but older patients (48%) fair better than neonates (39%) with cardiac failure as the reason for ECMO (Table 6-2).1 According to the 2011 ELSO Registry data, newborns with MAS who require ECMO have the best survival rate at 94%, whereas ECMO survival for infants with CDH is only 51% (Table 6-3).1
TABLE 6-3
ELSO Registry Data Comparing Outcomes in Neonates Requiring ECMO
| Indication | Number of Cases | Survival to Discharge (%) |
| Meconium aspiration syndrome | 7743 | 94 |
| Respiratory distress syndrome | 1496 | 84 |
| PPHN/PFC | 4043 | 78 |
| Sepsis | 2635 | 75 |
| Congenital diaphragmatic hernia | 6147 | 51 |
The pediatric population of ECMO patients represents a diverse group with regard to patient age as well as diagnoses. Almost an equal number of respiratory cases (n = 3854) and cardiac cases (n = 4181) have been reported.1 This is in contrast to the neonatal population in which there is an almost 3 : 1 ratio of a primary respiratory to a primary cardiac diagnosis.1 A higher complication rate is found in children, reflecting the longer duration of bypass required for reversal of the respiratory failure.
Feeding and Growth Sequelae
Approximately one-third of ECMO-treated infants have feeding problems.63–65 The possible causes for the poor feeding are numerous and include tachypnea, generalized central nervous system depression, poor hunger drive, soreness in the neck from the operation, manipulation or compression of the vagus nerve during the cannulation, sore throat from prolonged intubation, and poor oral motor coordination.66,67 Newborns with CDH have a higher incidence of feeding difficulties when compared to those with MAS. CDH children often have foregut dysmotility, which leads to significant gastroesophageal reflux, delayed gastric emptying, and feeding difficulties. Respiratory compromise and chronic lung disease add to the problem.66–70
Although normal growth is commonly reported in ECMO-treated patients, these children are more likely to experience problems with growth when compared to normal controls. Head circumference below the fifth percentile occurs in 10% of ECMO-treated children.70 Growth problems are most commonly associated with ECMO patients who had CDH or have residual lung disease.67
Respiratory Sequelae
Respiratory morbidity is more likely to be iatrogenic than a consequence of congenital lung disease. Nevertheless, approximately 15% of infants require supplemental oxygen at 4 weeks of age in some series. At age 5 years, ECMO children are twice as likely to have reported cases of pneumonia as compared with controls (25% vs 13%).70,71 These children with pneumonia are more likely to require hospitalization, and the pneumonia occurs at a younger age (half of the pneumonias were diagnosed before 1 year of life). CDH infants often have severe lung disease after ECMO and often require supplemental oxygen at the time of discharge.67,72–75
Neurodevelopmental Sequelae
Probably the most serious post-ECMO morbidity is neuromotor injury. The total rate of neurologic injury from 540 patients at 12 institutions was 6%, with a range from 2–18%.70,76–89 ECMO survivors have significant developmental delay, ranging from none to 21%.71,73 This is comparable to other critically ill, non-ECMO-treated neonates.90–92 A single-center study using multivariate analysis identified ventilator time as the only independent predictor of motor problems at age 1 in CDH patients.90 Auditory defects are reported in more than one-quarter of ECMO neonates at discharge.93 These deficits are detected by brain stem auditory evoked response (BAER) testing, are considered mild to moderate, and generally resolve over time. The auditory defects may be iatrogenic, or caused by induced alkalosis, diuretics, or gentamicin ototoxicity. As a result, all patients should have a hearing screening at the time of discharge. Visual deficits are uncommon in ECMO neonates who weigh more than 2 kg.94
Seizures are widely reported among ECMO neonates, ranging from 20% to 70%.95–99 However, by age 5 years, only 2% had a diagnosis of epilepsy. Seizures in the neonatal population are associated with neurologic disease and worse outcomes, including cerebral palsy and epilepsy.99 Severe nonambulatory cerebral palsy has an incidence of less than 5% and is usually accompanied by significant developmental delay.70,76,82 Milder cases of cerebral palsy are seen in up to 20% of ECMO survivors. Overall, ECMO-treated neonates function within the normal range and the rate of handicap appears to be stable across studies with an average of 11%, ranging from 2% to 18%.70,76,79–89 This morbidity reflects how desperately ill these children are and is not a direct effect of ECMO.
References
1. Extracorporeal Life Support Organization. International Registry Report of the Extracorporeal Life Support Organization. Ann Arbor: University of Michigan Medical Center; 2011.
2. Lillehei, CW, Cohen, M, Warden, HE, et al. The direct-vision intracardiac correction of congenital anomalies by controlled cross circulation. Surgery. 1955; 38:11–29.
3. Clowes, GHA, Jr., Hopkins, AL, Neville, WE. An artificial lung dependent upon diffusion of oxygen and carbon dioxide through plastic membranes. J Thorac Surg. 1956; 32:630–637.
4. Kolobow, T, Zapol, W, Pierce, JE, et al. Partial extracorporeal gas exchange in alert new born lambs with a membrane artificial lung perfused via an AV shunt for periods up to 96 hours. Trans Am Soc Artif Intern Organs. 1968; 14:328–334.
5. Osborn, JJ, Bramson, ML, Main, FB, et al. Clinical experience with a disposable membrane oxygenator. Bull Soc Int Chir. 1966; 25:346–353.
6. Peirce, EC, 2d., Thebaut, AL, Kent, BB, et al. Techniques of extended perfusion using a membrane lung. Ann Thorac Surg. 1971; 12:451–470.
7. Lande, AJ, Edwards, L, Block, JH, et al. Prolonged cardiopulmonary support with a practical membrane oxygenator. Trans Am Soc Artif Intern Organs. 1970; 16:352–356.
8. Hill, D, O’Brien, TG, Murray, JJ, et al. Extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome): Use of the Bramson Membrane Lung. N Engl J Med. 1972; 286:629–634.
9. Zapol, WM, Snider, MT, Hill, JD, et al. Extracorporeal membrane oxygenation in severe respiratory failure. JAMA. 1979; 242:2193–2196.
10. Bartlett, RH, Gazzaniga, AB, Jefferies, MR, et al. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infants. Trans Am Soc Artif Intern Organs. 1976; 22:80–93.
11. Bartlett, RH, Roloff, DW, Cornell, RG, et al. Extracorporeal circulation in neonatal respiratory failure: A prospective randomized study. Pediatrics. 1985; 76:479–487.
12. O’Rourke, PP, Crone, RK, Vacanti, JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: A prospective randomized study. Pediatrics. 1989; 84:957–963.
13. Firmin R. United Kingdom Neonatal ECMO Study. Presented at the 7th International ELSO Conference, Dearborn, Michigan, 1995.
14. Krummel, TM, Greenfield, LJ, Kirkpatrick, BU, et al. Extracorporeal membrane oxygenation in neonatal pulmonary failure. Pediatr Ann. 1982; 11:905–908.
15. Toomasion, JM, Snedecor, SM, Cornell, RG, et al. National experience with extracorporeal membrane oxygenation for newborn respiratory failure: Data from 715 cases. Trans Am Soc Artif Intern Organs. 1988; 34:140–147.
16. Stolar, CJH, Snedecor, SM, Bartlett, RH. Extracorporeal membrane oxygenation and neonatal respiratory failure: Experience from the extracorporeal life support organization. J Pediatr Surg. 1991; 26:563–571.
17. O’Rourke, PP, Stolar, CJ, Zwischenberger, JB, et al. Extracorporeal membrane oxygenation: Support for overwhelming pulmonary failure in the pediatric population: Collective experience from the extracorporeal life support organization. J Pediatr Surg. 1993; 28:523–528.
18. Galantowicz, ME, Stolar, CJ. Extracorporeal membrane oxygenation for perioperative support in pediatric heart transplantation. J Thorac Cardiovasc Surg. 1991; 102:148–151.
19. Campbell, BT, Braun, TM, Schumacher, RE, et al. Impact of ECMO on neonatal mortality in Michigan (1980–1999). J Pediatr Surg. 2003; 38:290–295.
20. Van Meurs K, Lally KP, Peek G, et al, eds. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care, 3rd ed, Ann Arbor, MI: Extracorporeal Life Support Organization, 2005.
21. Van Meurs K, ed. ECMO Specialist Training Manual, 3nd ed, Ann Arbor, MI: Extracorporeal Life Support Organization, 2010.
22. Gajarski, RJ, Mosca, RS, Ohye, RG, et al. Use of extracorporeal life support as a bridge to pediatric cardiac transplantation. J Heart Lung Transplant. 2003; 22:28–34.
23. Bae, J, Frischer, J, Waich, M, et al. Extracorporeal membrane oxygenation in pediatric cardiac transplantation. J Pediatr Surg. 2005; 40:1051–1057.
24. Lessin, JS, el-Eid, SE, Klein, MD, et al. Extracorporeal membrane oxygenation in pediatric respiratory failure secondary to smoke inhalation injury. J Pediatr Surg. 1996; 31:1285–1287.
25. Tobias, JD, Garrett, JS. Therapeutic options for severe, refractory status asthmaticus: Inhalational anesthetic agents, extracorporeal membrane oxygenation and helium/oxygen ventilation. Paediatr Anesth. 1997; 7:47–57.
26. Travis JA, Pranikoff T, Chang MC, et al. Extracorporeal rewarming in trauma patients. Presented at the 13th Annual ELSO Conference, Scottsdale, Arizona, 2002.
27. Johnson, LB, Plotkin, JS, Howell, CD, et al. Successful emergency transplantation of a liver allograft from a donor maintained on extracorporeal membrane oxygenation. Transplantation. 1997; 63:910–911.
28. Gersony, WM, Duc, GV, Sinclair, JC. ‘PFC’ syndrome (persistence of the fetal circulation). Circulation. 1969; 40(Suppl. 111):87.
29. Gupta, A, Shantanu, R, Rakesh, S, et al. Inhaled nitric oxide and gentle ventilation in the treatment of pulmonary hypertension of the newborn—a single-center 5-year experience. J Perinatol. 2002; 22:435–441.
30. Northway, WH, Rosan, RC, Porter, DY. Pulmonary disease following respiratory therapy of hyaline membrane disease. N Engl J Med. 1967; 276:357–368.
31. Kornhauser, MS, Cullen, JA, Baumgart, S, et al. Risk factors for bronchopulmonary dysplasia after extracorporeal membrane oxygenation. Arch Pediatr Adolesc Med. 1994; 148:820–825.
32. Kim, ES, Stolar, CJ. ECMO in the newborn. Am J Perinatol. 2000; 17:345–356.
33. Cilley, RE, Zwischenberger, JB, Andrews, AF, et al. Intracranial hemorrhage during extracorporeal membrane oxygenation in neonates. Pediatrics. 1986; 78:699–704.
34. Wung, JT, James, LS, Kilchevsky, E, et al. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985; 76:488–494.
35. Beck, R, Anderson, KD, Pearson, GD, et al. Criteria for extracorporeal membrane oxygenation in a population of infants with persistent pulmonary hypertension of the newborn. J Pediatr Surg. 1986; 21:297–302.
36. Marsh, TD, Wilkerson, SA, Cook, LN. Extracorporeal membrane oxygenation selection criteria: Partial pressure of arterial oxygen versus alveolar-arterial oxygen gradient. Pediatrics. 1988; 82:162–166.
37. Ortiz, RM, Cilley, RE, Bartlett, RH. Extracorporeal membrane oxygenation in pediatric respiratory failure. Pediatr Clin North Am. 1987; 34:39–46.
38. Rivera, RA, Butt, W, Shann, F. Predictors of mortality in children with respiratory failure possible indications for ECMO. Anaesth Intensive Care. 1990; 18:385–389.
39. Garcia, AV, Stolar, CJH. Congenital diaphragmatic hernia and protective ventilation strategies in pediatric surgery. Surg Clin N Am. 2012; 92:659–668.
40. Azarow, K, Messineo, A, Pearl, R, et al. Congenital diaphragmatic hernia—a tale of two cities: The Toronto experience. J Pediatr Surg. 1997; 32:395–400.
41. Wung, JT, Sahni, R, Moffitt, ST, et al. Congenital diaphragmatic hernia: Survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg. 1995; 30:406–409.
42. Boloker, J, Bateman, DA, Wung, JT, et al. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnia/spontaneous respiration/elective repair. J Pediatr Surg. 2002; 37:357–366.
43. Metkus, AP, Filly, RA, Stringer, MD, et al. Sonographic predictors of survival in fetal diaphragmatic hernia. J Pediatr Surg. 1996; 31:148–151.
44. Aspelund, G, Fisher, JC, Simpson, LL, et al. Prenatal lung-head ratio: Threshold to predict outcome for congenital diaphragmatic hernia. J Matern Fetal Neona. 2012; 25:1011–1016.
45. Graham, G, Devine, PC. Antenatal diagnosis of congenital diaphragmatic hernia. Semin Perinatol. 2005; 29:69–76.
46. Stolar, CJH, Dillon, PW, Reyes, C, et al. Selective use of extracorporeal membrane oxygenation in the management of congenital diaphragmatic hernia. J Pediatr Surg. 1988; 23:207–211.
47. Fiser, RT, Morris, MC. Extracorporeal cardiopulmonary resuscitation in refractory pediatric cardiac arrest. Pediatr Clin North Am. 2008; 55:929–941.
48. Fisher, JC, Stolar, CJH, Cowles, RA. Extracorporeal membrane oxygenation of cardiopulmonary failure in pediatric patients: Is a second course justified? J Surg Res. 2008; 148:100–108.
49. Frischer, JS, Stolar, CJH. Extracorporeal membrane oxygenation. In: Puri P, Hollwarth M, eds. Operative Pediatric Surgery. Heidelberg: Springer, 2006.
50. Fisher, JC, Jefferson, RA, Kuenzler, KA, et al. Challenges to cannulation for extracorporeal support in neonates with right-sided congenital diaphragmatic hernia. J Pediatr Surg. 2007; 42:2123–2128.
51. Haley, MJ, Fisher, JC, Ruiz-Elizalde, AR, et al. Percutaneous distal perfusion of the lower extremity following femoral cannulation for venoarterial ECMO in a small child. J Pediatr Surg. 2009; 44:437–440.
52. Horan, M, Ichiba, S, Firmin, RK, et al. A pilot investigation of mild hypothermia in neonates receiving extracorporeal membrane oxygenation (ECMO). J Pediatr. 2004; 144:301–308.
53. Field, DJ, Firmin, R, Azzopardi, DV, et al. Neonatal ECMO Study of Temperature (NEST)- a randomized controlled trial. BMC Pediatr. 2010; 10:24.
54. Levy, MS, Share, JC, Fauza, DO, et al. Fate of the reconstructed carotid artery after extracorporeal membrane oxygenation. J Pediatr Surg. 1995; 30:1046–1049.
55. Cheung, PY, Vickar, DB, Hallgren, RA, et al. Carotid artery reconstruction in neonates receiving extracorporeal membrane oxygenation: A 4-year follow-up study. J Pediatr Surg. 1997; 32:560–564.
56. Buesing, KA, Kilian, AK, Schaible, T, et al. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: Follow-up MRI evaluating carotid artery reocclusion and neurologic outcome. Am J Roentgenol. 2007; 188:1636–1642.
57. Preston, TJ, Olshove, VF, Ayad, O, et al. NovoSeven use in a non-cardiac pediatric ECMO patient with uncontrolled bleeding. J Extra Corpor Technol. 2008; 40:123–126.
58. Boedy, RF, Goldberg, AK, Howell, CG, et al. Incidence of hypertension in infants on extracorporeal membrane oxygenation. J Pediatr Surg. 1990; 25:258–261.
59. Sell, LL, Cullen, ML, Lerner, GR, et al. Hypertension during extracorporeal membrane oxygenation: Cause, effect, and management. Surgery. 1987; 102:724–730.
60. Coffin, SE, Bell, LM, Manning, M, et al. Nosocomial infections in neonates receiving extracorporeal membrane oxygenation. Infect Control Hosp Epidemiol. 1997; 18:93–96.
61. Douglass, BH, Keenan, AL, Purohit, DM. Bacterial and fungal infection in neonates undergoing venoarterial extracorporeal membrane oxygenation: An analysis of the registry data of the Extracorporeal Life Support Organization. Artif Organs. 1996; 20:202–208.
62. Meyer, DM, Jessen, ME, Eberhart, RC. Neonatal extracorporeal membrane oxygenation complicated by sepsis. Extracorporeal Life Support Organization. Ann Thorac Surg. 1995; 59:975–980.
63. Grimm, P. Feeding difficulties in infants treated with ECMO. CNMC ECMO Symposium. 25, 1993.
64. Nield, T, Hallaway, M, Fodera, C, et al. Outcome in problem feeders post-ECMO. CNMC ECMO Symposium. 79, 1990.
65. Glass, P. Patient neurodevelopmental outcomes after neonatal ECMO. In: Arensman R, Cornish J, eds. Extracorporeal Life Support. Boston: Blackwell Scientific Publications; 1993:241–251.
66. Tarby, T, Waggoner, J. Are the common neurologic problems following ECMO related to jugular bulb thrombosis? CNMC ECMO Symposium. 110, 1994.
67. Van Meurs, K, Robbins, S, Reed, V, et al. Congenital diaphragmatic hernia: Long-term outcome of neonates treated with ECMO. CNMC ECMO Symposium. 25, 1991.
68. Pace, MRD, Caruso, AM, Farina, F, et al. Evaluation of esophageal motility and reflux in children treated for congenital diaphragmatic hernia with the use of combined multichannel intraluminal impedance and pH monitoring. J Pediatr Surg. 2011; 46:1881–1886.
69. Rajasingham, S, Reed, V, Glass, P, et al. Congenital diaphragmatic hernia: Outcome post-ECMO at 5 years. CNMC ECMO Symposium. 35, 1994.
70. Glass, P, Wagner, A, Papero, P, et al. Neurodevelopmental status at age five years of neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1995; 127:447–457.
71. Gershan, L, Gershan, W, Day, S. Airway anomalies after ECMO: Bronchoscopic findings. CNMC ECMO Symposium. 65, 1992.
72. Wagner, A, Glass, P, Papero, P, et al. Neuropsychological outcome of neonatal ECMO survivors at age 5. CNMC ECMO Symposium. 31, 1994.
73. D’Agostino, J, Bernbaum, J, Gerdes, M, et al. Outcome for infants with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation: The first year. J Pediatr Surg. 1995; 30:10–15.
74. Van Meurs, K, Robbins, S, Reed, V, et al. Congenital diaphragmatic hernia: Long-term outcome in neonates treated with extracorporeal membrane oxygenation. J Pediatr. 1993; 122:893–899.
75. Atkinson, J, Poon, M. ECMO and the management of congenital diaphragmatic hernia with large diaphragmatic defects requiring a prosthetic patch. J Pediatr Surg. 1992; 27:754–756.
76. Adolph, V, Ekelund, C, Smith, C, et al. Developmental outcome of neonates treated with ECMO. J Pediatr Surg. 1990; 25:43–46.
77. Andrews, A, Nixon, C, Cilley, R, et al. One-to-three year outcome for 14 neonatal survivors of extracorporeal membrane oxygenation. Pediatrics. 1986; 78:692–698.
78. Flusser, H, Dodge, N, Engle, W, et al. Neurodevelopmental outcome and respiratory morbidity for ECMO survivors at 1 year of age. J Perinatol. 1993; 13:266–271.
79. Glass, P, Miller, M, Short, BL. Morbidity for survivors of extracorporeal membrane oxygenation: Neurodevelopmental outcome at 12 years of age. Pediatrics. 1989; 83:72–78.
80. Griffin, M, Minifee, P, Landry, S, et al. Neurodevelopmental outcome in neonates after ECMO: Cranial magnetic resonance imaging and ultrasonography correlation. J Pediatr Surg. 1992; 27:33–35.
81. Hofkosh, D, Thompson, A, Nozza, R, et al. Ten years of ECMO: Neurodevelopmental outcome. Pediatrics. 1991; 87:549–555.
82. Krummel, T, Greenfield, L, Kirkpatrick, B, et al. The early evaluation of survivors after ECMO for neonatal pulmonary failure. J Pediatr Surg. 1984; 19:585–590.
83. Schumacher, R, Palmer, T, Roloff, D, et al. Follow-up of infants treated with ECMO for newborn respiratory failure. Pediatrics. 1991; 87:451–457.
84. Towne, B, Lott, I, Hicks, D, et al. Long-term follow-up of infants and children treated with ECMO: A preliminary report. J Pediatr Surg. 1985; 20:410–414.
85. Wildin, S, Landry, S, Zwischenberger, J. Prospective, controlled study of developmental outcome in survivors of ECMO: The first 24 months. Pediatrics. 1994; 93:404–408.
86. Stolar, CJ, Crisafi, MA, Driscoll, YT. Neurocognitive outcome for neonates treated with extracorporeal membrane oxygenation: Are infants with congenital diaphragmatic hernia different? J Pediatr Surg. 1995; 30:366–372.
87. Davis, D, Wilkerson, S, Stewart, D. Neurodevelopmental follow-up of ECMO survivors at 7 years. CNMC ECMO Symposium. 34, 1995.
88. Stanley, C, Brodsky, K, McKee, L, et al. Developmental profile of ECMO survivors at early school age and relationship to neonatal EEG status. CNMC ECMO Symposium. 33, 1995.
89. Hack, M, Taylor, H, Klein, N, et al. School-age outcomes in children with birthweights under 750 g. N Engl J Med. 1994; 331:753–759.
90. Friedman, S, Chen, C, Chapman, JS, et al. Neurodevelopmental outcomes of congenital diaphragmatic hernia survivors followed in a multidisciplinary clinic at ages 1 and 3. J Pediatr Surg. 2008; 43:1035–1043.
91. Leavitt, AM, Watchko, JF, Bennett, FC, et al. Neurodevelopmental outcome following persistent pulmonary hypertension of the neonate. J Perinatol. 1987; 7:288–291.
92. Marron, MJ, Crisafi, MA, Driscoll, JM, Jr., et al. Hearing and neurodevelopmental outcome in survivors of persistent pulmonary hypertension of the newborn. Pediatrics. 1992; 90:392–396.
93. Desai, S, Stanley, C, Graziani, L, et al. Brainstem auditory evoked potential screening (BAEP) unreliable for detecting sensorineural hearing loss in ECMO survivors: A comparison of neonatal BAEP and follow-up behavioral audiometry. CNMC ECMO Symposium. 62, 1994.
94. Haney, B, Thibeault, D, Sward-Comunelli, S, et al. Ocular findings in infants treated with ECMO. CNMC ECMO Symposium. 63, 1994.
95. Hahn, J, Vaucher, Y, Bejar, R, et al. Electroencephalographic and neuroimaging findings in neonates undergoing extracorporeal membrane oxygenation. Neuropediatrics. 1993; 24:19–24.
96. Graziani, L, Streletz, L, Baumgart, S, et al. Predictive value of neonatal electroencephalograms before and during extracorporeal membrane oxygenation. J Pediatr. 1994; 125:969–975.
97. Campbell, L, Bunyapen, C, Gangarosa, M, et al. The significance of seizures associated with ECMO. CNMC ECMO Symposium. 26, 1991.
98. Kumar, P, Bedard, M, Delaney-Black, V, et al. Post-ECMO electroencephalogram (EEG) as a predictor of neurological outcome. CNMC ECMO Symposium. 65, 1994.
99. Scher, M, Kosaburo, A, Beggerly, M, et al. Electrographic seizures in preterm and full-term neonates: Clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics. 1993; 91:128–134.