53 Extracorporeal Life Support for Cardiopulmonary Failure
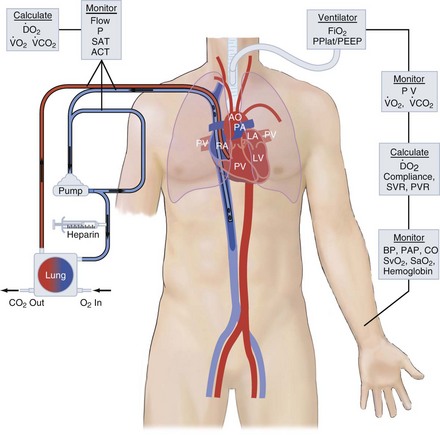
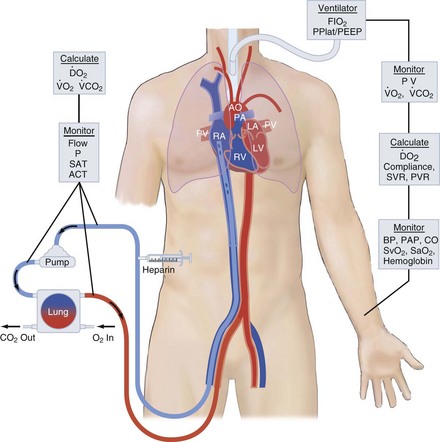
ECMO controls gas exchange and perfusion, stabilizes the patient physiologically, decreases the risk of ongoing ventilator- or vasopressor-induced iatrogenic injury, and allows ample time for diagnosis, treatment, and recovery from the primary injury or disease. Right atrial venous blood is drained through a large cannula, pumped through an artificial lung and back into the patient, either into the aorta (venoarterial [VA]) or into the right atrium (venovenous [VV] mode). VA access puts the artificial lung in parallel with the native lungs and substitutes for both heart and lung function. VV access puts the artificial lung in series with the native lung. These modes of access are shown in Figures 53-1 and 53-2. For respiratory failure, VV access is preferred because normal hemodynamics are maintained, and there is little risk of systemic embolism. For total support in either mode, the blood flow required is 60 to 100 mL/kg/min (the entire cardiac output); large-bore, low-resistance cannulas are required to achieve this amount of flow. The flow is limited by resistance in the venous access catheter. For vascular access, the cannulas are placed via the large vessels in the neck or groin. Cannulas can be placed by direct cutdown access to these vessels or, more commonly, via percutaneous placement over a guidewire. After cannulas are placed, the circuit primed with crystalloid solution is attached, heparin is given for anticoagulation, and extracorporeal flow is established at 50 to 100 mL/kg/min. The membrane lung is ventilated with 100% oxygen.
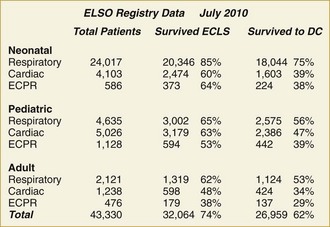
ECMO is used in a variety of clinical circumstances, and results depend on the primary indication. ECMO provides life support, but it is not treatment. The clinical outcome depends on the response to treatment for the primary condition. Because ECMO is a life-support technique, the primary outcome variable is survival. Survival outcome for nine categories of patients is shown in Figure 53-3. Survival ranges from 30% in extracorporeal cardiopulmonary resuscitation (ECPR) to 95% for neonatal meconium aspiration syndrome.
The devices for extracorporeal support used in the past carried a significant risk of blowout, air embolism or thromboembolism, and device failure. The current generation of devices are much simpler and inherently safer. The major change is in the membrane lung. The Kolobow spiral coil membrane lung1 has been reliably used for ECMO for over 30 years. This membrane lung works well for weeks at a time but has an affinity for platelets, causing thrombocytopenia, and has high blood flow resistance, requiring high pressure generated by the pump for high blood flow. When centrifugal pumps are used to generate high pressure, hemolysis and thrombosis can occur, so most of the experience with ECMO has been with modified roller pumps.
The major use of ECLS has gone from neonatal respiratory failure to many causes of cardiorespiratory failure in all age groups. The Extracorporeal Life Support Organization (ELSO; elso@med.umich.edu) is an international consortium of medical centers with major ECMO programs. ELSO maintains a registry of ECMO cases. The types of cases represented in the ELSO Registry are shown in Figure 53-3. The indications, practice management, and outcome are quite different in each of these patient groups.
 Neonatal Respiratory Failure
Neonatal Respiratory Failure
The major application of ECMO began with neonatal respiratory failure. The first successful case was reported in 1975, and ECMO became standard treatment in major neonatal centers.2 In retrospect, the reason for this success was that regardless of primary diagnosis, the major pathophysiology in neonatal respiratory failure is persistent fetal circulation (PFC), a condition that is almost always reversible in a few days. In the early 1980s, PFC was treated by hyperventilation to induce alkalosis, which is damaging to the neonatal lung. ECMO eliminated this iatrogenic injury and allowed time for PFC to resolve. Neonatal ECMO was proven effective and beneficial in four prospective randomized trials, an effect that was confirmed in a Cochrane meta-analysis.3–7 A major lesson learned from the neonatal experience was the advantage of resting the native lungs by extracorporeal support.
Inhaled nitric oxide administered with high-frequency oscillation was shown to be effective treatment for PFC in the 1990s.8,9 The need for extracorporeal support thereafter decreased significantly. The exception is PFC combined with lung hypoplasia in congenital diaphragmatic hernia patients. This condition is now the primary indication for ECMO in newborn infants. Vascular access in neonates is always gained via the neck vessels, usually by placement of a double-lumen catheter into the right atrium via the jugular vein.
 Pediatric Respiratory Failure
Pediatric Respiratory Failure
Severe respiratory failure in children arises from a wide range of conditions including viral infections in infants and trauma in 18-year-olds. The indication for ECMO is failure to respond to optimal ventilator and supportive care. Vascular access is venovenous, usually with a double-lumen catheter placed via the jugular vein. Green demonstrated the efficacy of ECMO in pediatric respiratory failure in a matched-pairs analysis using a large multicenter database.10 In that analysis, survival with ECMO was 75% compared to 50% with conventional management.10
 Adult Respiratory Failure
Adult Respiratory Failure
Hill reported the first successful ECMO case in an adult with respiratory failure in 1972.11 This led to a prospective randomized trial of ECMO in acute respiratory distress syndrome (ARDS) in 1975-1978. After 90 patients, the trial was stopped for futility.12 In retrospect, this study was undertaken prematurely in inexperienced centers using conventional and ECMO management methodologies that would not be used today. Nonetheless, the report of this trial essentially stopped research on ECMO for ARDS for many years. Over the ensuing decades, a few centers reported 50% survival in severe ARDS.13 A second prospective randomized trail was conducted in the United Kingdom from 2004-2007, within which the best conventional care in many intensive care units (ICUs) was compared to protocolized care including ECMO in a single center.14 Twenty-eight day survival was 76% in protocolized care compared to 50% with conventional care. Six-month survival free of disability was 63% versus 47%.
The H1N1 worldwide flu epidemic in 2009 renewed interest in ECMO for ARDS. Investigators in Australia and New Zealand reported 78% survival in 68 H1N1 patients managed with ECMO.15 In the recent studies suggesting benefit with ECMO, new ECMO devices were used, emphasizing the safety and simplicity of the second generation of ECMO. At present, vascular access is gained by a large double-lumen catheter placed via the right internal jugular vein, or by drainage from the inferior vena cava via the femoral vein and reinfusion into the right atrium via the jugular vein. On ECMO, the ventilator is set at rest settings to avoid ongoing iatrogenic injury. Native lung function usually becomes even worse before it gets better and, ECLS support is usually required for 10 to 20 days.
Another approach to extracorporeal gas exchange is selective CO2 removal (ECCOR).16 In ECCOR, a membrane lung is used with low blood flow to remove CO2, so mechanical ventilation is not necessary, and oxygen is supplied by insufflation of the native lungs. Gattinoni reported 56% survival with ECCOR in ARDS in 1986.17 Morris later reported a small randomized trial that showed no survival difference between patients treated with ECCOR and conventional therapy.18
ECCOR has been studied and refined by Zwischenberger and others.19 ECCOR using a low-resistance membrane lung perfused by a femoral arterial venous shunt or pumped VV access is being studied in the management of ARDS in Europe. ECCOR is ideal for CO2 retention syndromes like status asthmaticus but does not provide sufficient oxygenation for full respiratory support.
Lung transplantation has been very successful in the management of end-stage lung disease but is rarely considered in intubated ventilated patients because nosocomial pneumonia and multiple organ failure usually occur before a donor is found. ECMO is rarely used as a bridge to transplant for the same reason.20 In addition, many patients with respiratory failure awaiting lung transplant have right ventricular failure requiring venoarterial access.21 Recently, some centers have reported success with ECMO as a bridge to lung transplantation, using an implantable (paracorporeal) membrane lung allowing extubation, ambulation, and rehabilitation while bridging to lung transplantation.22
 Cardiac Failure in Children
Cardiac Failure in Children
The major application of ECMO today is to support children with profound cardiac failure.23 Most of these patients are infants who show cardiovascular deterioration immediately after operations for congenital heart disease. Support with VA ECLS is used as a bridge to recovery from myocardial stunning, and if recovery does not occur, as a bridge to a cardiac-assist device and perhaps transplantation.24 Other applications for ECMO include myocarditis and myocardiopathy. Venoarterial access is required via the neck vessels or using direct cardiac cannulas if the chest is already open.
 Cardiac Failure in Adults
Cardiac Failure in Adults
Unlike the pediatric population, the major application of ECLS in adult cardiac failure is cardiogenic shock following myocardial infarction, myocardiopathy, myocarditis, or inability to come off cardiopulmonary bypass following cardiac operation.25–27 Venoarterial access is required in these settings. The femoral vessels are used in almost all cases because of a 10% to 15% incidence of stroke when the carotid artery is used in patients with profound shock or cardiac arrest. Vascular access is usually percutaneous, although direct cutdown access is the most reliable in patients with profound cardiogenic shock. Centers in Paris28 and Taiwan29 have reported a large experience in cardiogenic shock using ECMO to stabilize hemodynamics while proceeding to cardiac catheterization and revascularization of the myocardium if needed, followed by cardiac recovery or bridging to a ventricular assist device and perhaps transplantation.
 Extracorporeal Support During Cardiopulmonary Resuscitation
Extracorporeal Support During Cardiopulmonary Resuscitation
Extracorporeal support during cardiopulmonary resuscitation (ECPR) is the extension of VA support in cardiogenic shock to patients in overt cardiac arrest.30,31 Venoarterial access is used, usually by direct vessel exposure. The neck vessels are used for children up to the age of 5 or 6, and the femoral vessels for older children and adults. The use of ECPR requires having a primed circuit and a cannulation team immediately available. With the new simplified devices, and with appropriate training of emergency room physicians, the use of ECPR is increasing in major academic hospitals.
 Other Applications of ECMO
Other Applications of ECMO
ECMO is being investigated for applications to other conditions where perfusion and gas-exchange support is needed. Controlled warming after accidental hypothermia has been reported with ECMO.32 A major advantage for ECMO compared to conventional techniques is to avoid or treat the cardiac arrhythmias that often occur during rewarming. Maclaren and others from Australia have reported the use of ECMO in profound septic shock in children.33 They found that very high blood flow achieved with direct cardiac vascular access led to 75% survival in profound septic shock. When the team and circuit can be quickly assembled in the setting of massive pulmonary embolism, the results are very good.34 The management of prematurity using ECMO as an artificial placenta to avoid intubation and mechanical ventilation is being studied in the laboratory.
The major limitation to organ transplantation is availability of donors. The largest potential source of donors is donation after cardiac death (DCD). However, this technique is rarely used because of poor organ function and long periods of lung ischemia. Several centers are using VA ECMO after cardiac death to resuscitate abdominal organs35 and lungs to transplantable status.36 Organs resuscitated in this fashion function as well or better than those obtained from conventional brain-dead donors.
Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286(12):629-634.
The first successful case of ECLS in adult respiratory failure.
Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80-93.
The first successful cases of cardiac and neonatal ECLS.
Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76(4):479-487.
The prospective randomized trial of ECMO in neonatal respiratory failure.
Gattinoni L, Pesenti A, Mascheroni D, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(7):881-886.
The first case series of ECCOR in ARDS.
Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-1363.
The randomized trial of protocol care including ECMO in ARDS.
Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404-1411.
1 Kolobow T, Zapol WM, Sigman RL, Pierce J. Partial cardiopulmonary bypass lasting up to seven days in alert lambs with membrane lung blood oxygenation. J Thorac Cardiovasc Surg. 1970;60(6):781-788.
2 Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80-93.
3 Mugford M, Elbourne D, Field D. Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev 2008;(3):CD001340.
4 O’Rourke PP, Crone RK, Vacanti JP, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84(6):957-963.
5 Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76(4):479-487.
6 Bennett CC, Johnson A, Field DJ, Elbourne D. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet. 2001;357(9262):1094-1096.
7 Bifano EM, Hakanson DO, Hingre RV, Gross SJ. Prospective randomized controlled trial of conventional treatment or transport for ECMO in infants with persistent pulmonary hypertension (PPHN). Pediatr Res. 1992;31:196A.
8 Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131(1 Pt 1):55-62.
9 Ford JW. Neonatal ECMO: Current controversies and trends. Neonatal Netw. 2006;25(4):229-238.
10 Green TP, Timmons OD, Fackler JC, Moler FW, Thompson AE, Sweeney MF. The impact of extracorporeal membrane oxygenation on survival in pediatric patients with acute respiratory failure. Pediatric Critical Care Study Group. Crit Care Med. 1996;24(2):323-329.
11 Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286(12):629-634.
12 Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242(20):2193-2196.
13 Lewandowski K. Extracorporeal membrane oxygenation for severe acute respiratory failure. Crit Care. 2000;4(3):156-168.
14 Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-1363.
15 Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-1895.
16 Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet. 1980;2(8189):292-294.
17 Gattinoni L, Pesenti A, Mascheroni D, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(7):881-886.
18 Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):295-305.
19 Brunston RLJr, Zwischenberger JB, Tao W, Cardenas VJJr, Traber DL, Bidani A. Total arteriovenous CO2 removal: simplifying extracorporeal support for respiratory failure. Ann Thorac Surg. 1997;64(6):1599-1604.
20 Fumagalli R, Bombino M, Borelli M, et al. Percutaneous bridge to heart transplantation by venoarterial ECMO and transaortic left ventricular venting. Int J Artif Organs. 2004;27(5):410-413.
21 Scherer M, Moritz A, Martens S. The use of extracorporeal membrane oxygenation in patients with therapy refractory cardiogenic shock as a bridge to implantable left ventricular assist device and perioperative right heart support. J Artif Organs. 2009;12(3):160-165.
22 Puehler T, Philipp A, Schmid C. Paracorporeal artificial lung circuit as a possibility for bridge to lung transplantation. Ann Thorac Surg. 2009;88(1):352-353.
23 Loforte A, Delmo Walter EM, Stiller B, et al. Extracorporeal membrane oxygenation for intraoperative cardiac support in children with congenital heart disease. Interact Cardiovasc Thorac Surg. 2010.
24 Imamura M, Dossey AM, Prodhan P, et al. Bridge to cardiac transplant in children: Berlin Heart versus extracorporeal membrane oxygenation. Ann Thorac Surg. 2009;87(6):1894-1901.
25 Rastan AJ, Dege A, Mohr M, et al. Early and late outcomes of 517 consecutive adult patients treated with extracorporeal membrane oxygenation for refractory postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 2010;139(2):302-311. 311
26 Formica F, Avalli L, Colagrande L, et al. Extracorporeal membrane oxygenation to support adult patients with cardiac failure: predictive factors of 30-day mortality. Interact Cardiovasc Thorac Surg. 2010.
27 Cooper DS, Jacobs JP, Moore L, et al. Cardiac extracorporeal life support: state of the art in 2007. Cardiol Young. 2007;17(Suppl 2):104-115.
28 Combes A, Leprince P, Luyt CE, et al. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit Care Med. 2008;36(5):1404-1411.
29 Hsu PS, Chen JL, Hong GJ, et al. Extracorporeal membrane oxygenation for refractory cardiogenic shock after cardiac surgery: predictors of early mortality and outcome from 51 adult patients. Eur J Cardiothorac Surg. 2010;37(2):328-333.
30 Raymond TT, Cunnyngham CB, Thompson MT, Thomas JA, Dalton HJ, Nadkarni VM. Outcomes among neonates, infants, and children after extracorporeal cardiopulmonary resuscitation for refractory in-hospital pediatric cardiac arrest: A report from the National Registry of CardioPulmonary Resuscitation. Pediatr Crit Care Med. 2010;11(3):362-371.
31 Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87(3):778-785.
32 Eich C, Brauer A, Kettler D. Recovery of a hypothermic drowned child after resuscitation with cardiopulmonary bypass followed by prolonged extracorporeal membrane oxygenation. Resuscitation. 2005;67(1):145-148.
33 Maclaren G, Butt W, Best D, Donath S, Taylor A. Extracorporeal membrane oxygenation for refractory septic shock in children: one institution’s experience. Pediatr Crit Care Med. 2007;8(5):447-451.
34 Maggio P, Hemmila M, Haft J, Bartlett RH. Extracorporeal life support for massive pulmonary embolism. J Trauma. 2007;62(3):570-576.
35 Magliocca JF, Magee JC, Rowe SA, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58(6):1095-1101. discussion 1101-2
36 Broome M, Palmer K, Schersten H, Frenckner B, Nilsson F. Prolonged extracorporeal membrane oxygenation and circulatory support as bridge to lung transplant. Ann Thorac Surg. 2008;86(4):1357-1360.