Chapter 47 External submucosal glossectomy
1 INTRODUCTION
The major contributing factors to retrolingual collapse in sleep apnea are macroglossia, hypotonia, retrognathia and lingual tonsillar hyperplasia. Any or all of these may contribute to varying degrees in any given individual. All current low morbidity options have limitations in treating the macroglossia component. The ideal procedure for macroglossia would be low morbidity, allow large tissue volume reduction, have low risk of alteration in tongue function and be single stage. Submucosal glossectomy techniques aim to fulfill these criteria, with preservation of mucosa causing minor pain postoperatively.
2 PATIENT SELECTION
Clinical indicators of macroglossia are most commonly used, but are subjective. Flexible nasendoscopic observation (supine, standardized for phase of respiration) is commonly used to examine the retrolingual airway.1 Variation in muscle tone changes airway size during quiet breathing.If the retrolingual airspace is reduced in mid-respiration (and particularly if reduced on end inspiration when muscle tone is greatest), and there is no co-existing retrognathia (defined as sella-nasion-supramentale (SNB) <76° on cephalostat) or lingual tonsillar hyperplasia, it can be assumed that macroglossia exists.
Tongue size, as visualized orally, has been shown (when considered with tonsillar size) to predict uvulopalatopharyngoplasty outcomes.2 Friedman tongue positions 3 and 4 are potential candidates for submucosal glossectomy techniques. Untreated tongue bulk of the middle third of the tongue (measured antero-posteriorly) may precipitate or exacerbate retropalatal collapse by prolapse onto the oral side of the palate during sleep, which is not readily perceived by nasendoscopic examination (awake or sedated). In addition, a bulky dorsal tongue will reduce the oropalatal airway, which may be symptomatic, particularly if palatal surgery has advanced the free edge of the soft palate anteriorly to treat retropalatal obstruction (with or without scarring of the free palatal edge). Symptoms of a reduced oropalatal airway typically include globus pharyngeus (which can be localized to the free edge of soft palate on examination), reduced exercise tolerance (less oral airway), and sometimes frequent gagging. These symptoms can be expected to resolve with adequate separation of the tongue dorsum and the free edge of soft palate as a result of successful tongue reduction surgery.
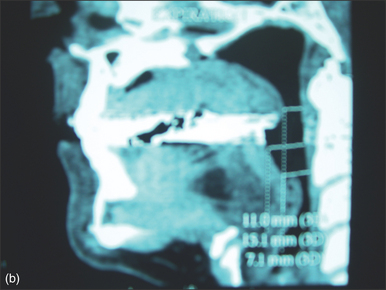
Radiological parameters offer more objective measures of tongue size. Chabolle et al. have published anatomical data (in French) from a midsagittal MRI, demonstrating a lingual area of 25.8 ± 4.6 cm2 in sleep apneic patients, compared to 20.2 ± 3.2 cm2 in normal patients (P<0.001).3I use a midsagittal reconstruction from a spiral multislice CT scan to define the same area and thus quantify tongue size. Chabolle suggested tongue reduction surgery if the lingual area was greater than 26 cm,2,3 which was later revised to >28 cm2 in relation to open tongue base reduction with hyoepiglottoplasty.4 I consider submucosal glossectomy techniques in patients with a lingual area greater than 26 cm2, but the ideal parameters to define which patients with retrolingual collapse need bulk reduction, which need tensioning of the tongue and which need both strategies are, at present, unknown.
3 OUTLINE OF PROCEDURE
The external submucosal glossectomy procedure follows Chabolle’s principles,4 but mucosal preservation significantly reduces pain, delay of oral intake and hospital stay. Hyoid advancement toward the mandible with mylohyoid shortening is incorporated, but epiglottic suture verticalization is omitted, as adequate verticalization is planned to be achieved from hyoepiglottic ligament pull alone.
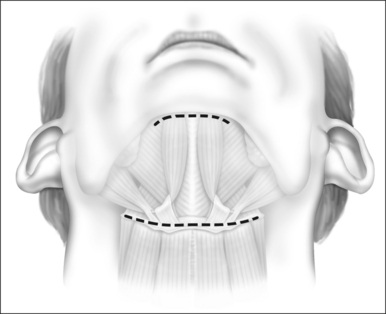
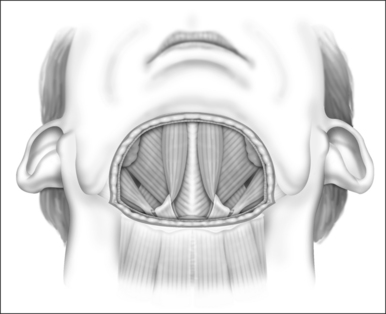
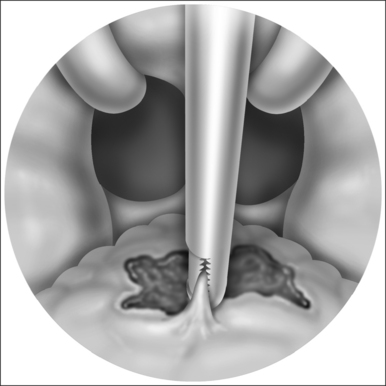
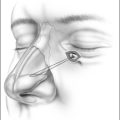
General anesthesia is induced, and the airway secured with nasotracheal intubation. An arterial line is routinely used to measure intraoperative and postoperative blood pressure. Broad-spectrum antibiotics and 8 mg dexametasone are administered IV. The neck is extended with arolled sheet beneath the shoulders. Skin crease incisions over the hyoid and in the submental crease are marked (Fig. 47.1). The incisions are injected with 1% lidocaine+1:100,000 adrenaline, with a 10-minute delay for vasoconstriction to take effect, during which time routine preparation of a sterile field is performed. The incision over the hyoid is extended from the medial edge of one sternomastoid muscle to the other, and a subplatysmal planeis developed to expose the suprahyoid and strap muscles (Fig. 47.2).
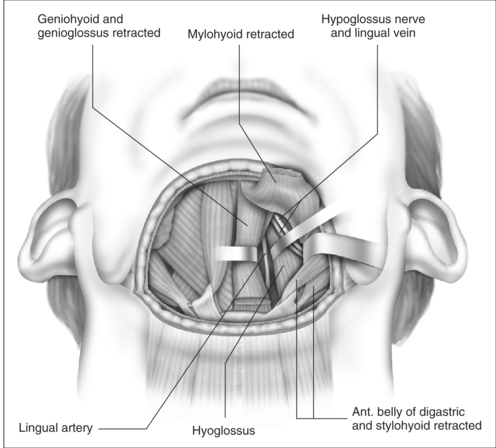
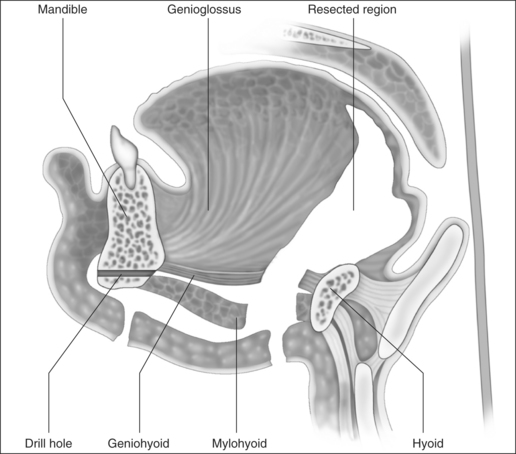
The cut edge of mylohyoid is shortened by 8–10 mm and retracted anteriorly. The anterior belly of digastric and stylohyoid are retracted laterally with Lagenbeck retractors (Fig. 47.3). This exposes the key anatomical landmark to the dissection, which is the hyoglossus muscle. The surgeon will see first the rounded anterolateral edge of hyoglossus, as the muscle is approached obliquely from the anterior direction. Running superficial to hyoglossus is the hypoglossal nerve, with accompanying lingual vein. These structures are gently mobilized with a curved clamp (e.g. a Gemini), and a colored loop of vascular tape is placed around the nerve and vein together, and clipped. Any bleeding is controlled with careful bipolar diathermy. Magnification of the operative field with loupes is helpful, but is not essential. Lagenbeck retractors are now used to retract the hyoglossus laterally, and geniohyoid medially to expose the lingual artery lying deep to hyoglossus (Fig. 47.4). If difficulty is encountered in finding the artery, digital palpation in this area for pulsations can be helpful, as can use of a Doppler or ultrasound machine. Another loop of vascular tape is placed around the artery.
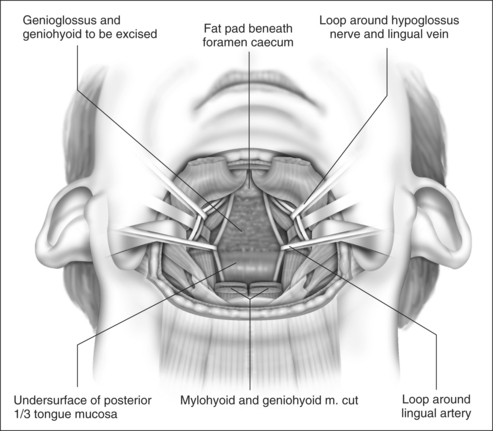
With the neurovascular structures identified and gently retracted laterally, geniohyoid, genioglossus and intrinsic tongue muscles are sectioned until the undersurface of tongue mucosa is reached, beveling approximately 30° superior to the axial plane from hyoid, to avoid dissection of the pre-epiglottic space and hyoepiglottic ligament (Figs 47.4 and 47.5). The extent of dissection is tailored to the patient’s anatomy, but at a minimum includes muscle between the neurovascular bundles from above hyoid to the level of foramen cecum superiorly. The foramen cecum is identified by palpation from the undersurface of mucosa and a ‘sentinel’ fat pad beneath it. Lateral extension of the dissection between the neurovascular bundles and mucosa is frequently desirable, particularly in patients with a bulky lateral tongue base. Extension of the dissection anterior to the foramen cecum is also frequently desirable (into the middle third of the tongue antero-posteriorly), which is the area of bulk assessed by oral examination (Friedman tongue size).2 Enough genioglossus must be left anteriorly for normal tongue function, which is most easily decided by palpation, and is roughly defined by a coronal plane through the foramen cecum. A surgeon starting this technique should err on the side of caution, and it is usual for the extent of muscle excision to increase with increased experience. Therefore, if viewed in a sagittal plane, the cavity dissected is submucosal and crescenteric (Fig. 47.5).
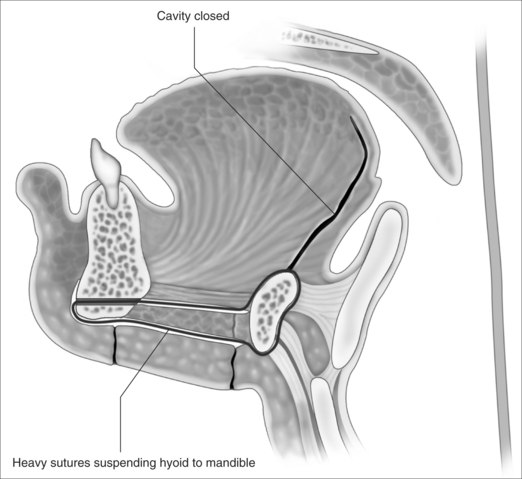
Hemostasis with the mean arterial blood pressure at 120 mm Hg, and a Valsalva maneuver with hand ventilation are advised at this point while wide access to the muscle bed is available. Small arterial branches should be ligated rather than diathermied (see below). Cavity closure of the undersurface of mucosa to remnant tongue muscle is performed with 4×2-0 Vicryl. Two 0 Vicryl sutures are looped around the hyoid, passed through the subplatysmal tunnel while grasped in a clamp, passed backward through the mandibular drill hole, and returned through the subplatysmal tunnel to be clipped and cut. The cut edges of mylohyoid are stitched with 4×2-0 Vicryl (all clipped and cut). A 15-gauge suction drain is placed between mucosa and genioglossus remnant, but should also be placed so that some holes drain the subcutaneous space superficial to mylohyoid. This is removed the morning after surgery (unless there is ongoing drainage). The heavy 0 Vicryl sutures are tied to suspend the hyoid from mandible anteriorly, then the2-0 Vicryl is tied to close mylohyoid (Fig. 47.6). 3-0 Vicryl is used to platysma and staples to skin.
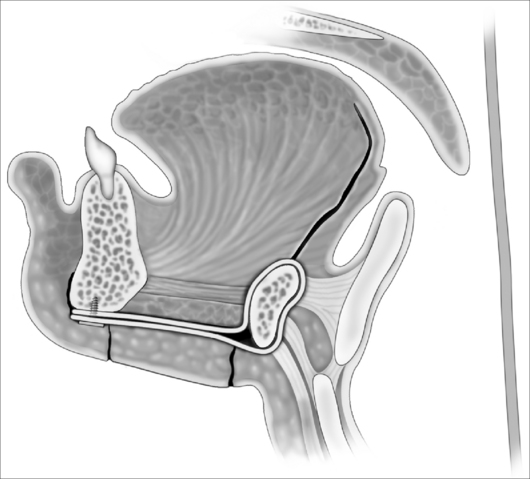
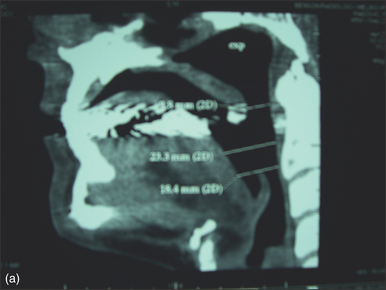
In contrast to Chabolle’s experience when all muscle and mucosa of the posterior third of the tongue was also excised,4 the anteriorly advanced hyoid position was not maintained over time with absorbable sutures in this operation. In view of this, a variation with suspension of the hyoid from mandible with two fascia lata strips, secured to the inferior mandible with a four-hole miniplate, is suggested (Fig. 47.7). The ability of fascia lata to resist stretching when used for this purpose has not yet been demonstrated conclusively, but in a large series of hyoid suspension with fascia lata to mandible done at Stanford University in the 1980s and early 1990s,5 the hyoid position anecdotally appeared stable in most cases over years (N. Powell, personal communication, 2006). Some authorities have found autologous gracilis tendon or cadaveric semitendinosis tendon more reliable than fascia lata for hyomandibular suspension (K. Moore, personal communication, 2006). An example of changes in airway size is demonstrated by pre- and postoperative spiral CT scan sagittal reconstructions (Fig. 47.8).
4 POSTOPERATIVE MANAGEMENT
Tongue base surgery for sleep apnea has three major considerations for postoperative care, which have been previously well described by Riley et al.6
4.2 HYPERTENSION WITH HEMATOMA
In the large series of upper airway surgeries reported from Stanford, around 40% of patients had baseline hypertension, but 70% needed postoperative antihypertensives.6It is essential to keep the mean arterial pressure<100 mm Hg postoperatively (after securing hemostasis during surgery at a MAP of 110–120 mm Hg with Valsalva). Our initial experience was with beta-blocker and clonidine intraoperatively, and postoperative hydralazine boluses, as required. Although many strategies for blood pressure control exist, our current preference is for a dexmeditomidene infusion intraoperatively, which is continued postoperatively, with dose titration to control blood pressure. The characteristics of antihypertension, analgesia and sedation without respiratory depression make this an ideal drug for use in any tongue base surgery for sleep apnea. The danger time for postoperative surges in blood pressure is in the first 6 hours postoperatively which, if not avoided, may result in sudden tongue base hematoma and rapid airway obstruction requiring emergency reintubation or cricothyrotomy/tracheotomy.
4.3 POSTOPERATIVE EDEMA
Dexametasone (8 mg), with broad-spectrum antibiotics, is administered on induction of anesthesia, and both are continued for 24–48 hours. Any concurrent palatal surgery is advised to be performed first, with re-scrub and re-preparation of a sterile field prior to commencing the external submucosal glossectomy procedure, as prolonged pressure from the tongue blade of a mouth gag on a dissected tongue will increase the risk of edema. Due to the risk of tongue edema with glossectomy techniques, it is advisable toobserve the patient while still nasotracheally intubated on ICU for the first 2 hours postoperatively, then extubate the patient if there is no significant tongue swelling. If there is any doubt regarding tongue size, it is preferable to leave the patient intubated overnight while steroids diminish edema. If the patient is able to use nasal continuous positive airway pressure (CPAP) (but wishes to discontinue in the longer term), CPAP should be worn whenever asleep for at least 2 weeks postoperatively (and preferably until just before the postoperative sleep study at 3–6 months) to minimize edema.
5 COMPLICATIONS
The morbidity of the procedure in terms of pain and return to oral intake was markedly lower than mucosal sacrificing glossectomy techniques.4,7 Analgesia was achieved with regular paracetamol (from the day of surgery) and ibuprofen (commencing on the first postoperative day). Oxycodone 5–10 mg 4-hourly as required was given as a breakthrough pain control medication, and was often needed very little, or not at all (average number of 5 mg tablets used was six, range 0–20). The length of stay was 1–2 days (except one patient with a complication who stayed 6 days). No infections or subjective significant alteration to swallowing, speech or taste were noted at 3 months.
6 OUTCOMES
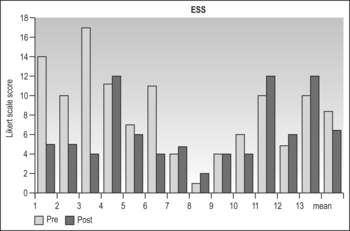
Snoring resolved in all patients. The Epworth Sleepiness Scale fell (non-significantly) from a mean of 8.5 to 6.2 (P=0.3) (Table 47.1). The Apnea Index (AI) fell from 27.3 ± 19.2 to 9.2 ± 15.2 (P=0.08). ‘Clinical success’ by AI criteria of AI < 10 and fall > 50% was 10/13=77%.
 |
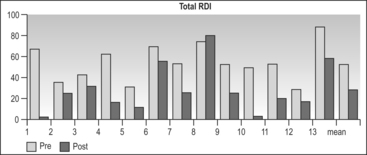
The total Respiratory Disturbance Index (RDI) fell from 54+16.8 to 28.7 ± 22.9 (P=0.004) (Table 47.2). A total of 69% of patients achieved RDI < 30 measuring hypo-pneas with pressure transducers, which corresponds to the same target of RDI < 20 when hypopneas are measured with thermistors.8 This corresponds to mild disease, and is slightly less, but in a similar range to the outcomes of around 80% success with mucosal sacrificing glossectomy techniques, by either endoscopic7 or external4 approaches.
Table 47.2 Total Respiratory Disturbance Index
 |
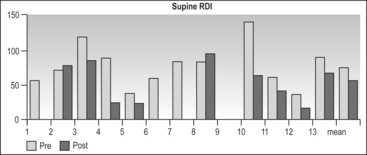
The lateral RDI fell from 40.6 ± 28.2 to18.5 ± 24.1 (P=0.014) (Table 47.3). A total of 82% of patients achieved a lateral RDI < 30. The results for supine sleep were far inferior, with supine RDI falling from 75.3 ± 29.5 to 57.9 ± 29.4 (P=0.2) (Table 47.4). Only 33% of patients achieved a supine RDI < 30. The fact that most patients achieved a satisfactory RDI overall is due to the majority of sleep time being in the lateral position (which was similar pre- and postoperatively). The lowest oxygen saturation rose from 80% ± 8.6 to 84.7 ± 6.5 (P=0.16). The Body Mass Index (BMI) fell slightly from 30.9 ± 3.7 to 29.6 ± 2.9 (P<0.001).
Table 47.3 Lateral Respiratory Disturbance Index
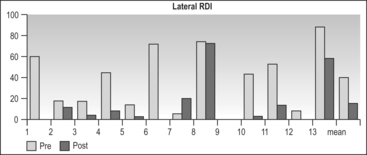 |
Table 47.4 Supine Respiratory Disturbance Index
 |
Anatomically, the posterior airspace (supine, end expiration) increased from 6.7 mm ± 3.7 to10.8 ± 4.3 (P=0.008). The mandibular plane to hyoid distance (MP-H) increased slightly from 22.1 mm ± 7.4 to 22.8 mm ± 7.2 (P<0.001), which is an adverse anatomical change. The hyoid advancement towards mandible component of the surgery aimed to shorten this distance, thus shortening the airway length, advancing the lower tongue base forwards and verticalizing the epiglottis via tension on the hyoepiglottic ligament. Although the hyoid was repositioned anterosuperiorly during the operation, it was concluded that the 0 Vicryl hyomandibular suspension sutures with mylohyoid shortening were not adequate to maintain this new position over time. As noted in the operative description section, instead of the 0 Vicryl sutures, it is now recommended to use fascia lata to resuspend the hyoid to mandible (Fig. 47.7).
7 SALVAGE OPTIONS
No patients failed at the palate with a combination of palatal advancement and conservative UPPP. Palatal advancement +/− UPPP has been shown to be 5.8 times more powerful than UPPP alone when controlled for severity of sleep apnea, age, BMI and tongue base pathology,9 and is therefore my standard palate intervention in patients with absent, 1+ or 2+ sized tonsils. The failures were analyzed, and most were thought to be due to insufficient tongue bulk reduction, particularly early in the series when minimal lateral extension beyond the neurovascular bundles and minimal anterior extension beyond the foramen cecum were performed. Increasing experience allowed more aggressive tongue reduction. Another cause of failure was hypotonicity, particularly in the supine position when the tongue tends to retrodisplace under the influence of gravity. It is postulated that a more durable hyoid advancement with fascia lata may improve this factor, and an additional fascia lata strip anchored from the mandible to submucosally loop around the genioglossus remnant at the level of foramen cecum (as a sling to counter hypotonicity during sleep) may also be helpful, but is yet to be evaluated.
If posterior epiglottic tilt position precipitates supraglottic obstruction during sleep, despite a stable and normal size tongue base, further hyoid advancement towards mandible could be considered. Although hyothyroidopexy10 would also seem a reasonable alternative, the anterior vector required to verticalize the epiglottis appears unlikely to be sustained in the longer term due to remodeling of the underlying superior thyroid cartilage, which I have noted on all patients (6 of 6) having reversal or revision of hyothroidopexy (unreported data). I prefer to avoid partial epiglottectomy for fear of aspiration, and have found endoscopic glossoepiglottopexy unreliable.
1. Woodson BT, Naganuma H. Comparison of methods of airway evaluation in obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. 1999;120(4):460-463.
2. Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127:13-21.
3. Chabolle F, Lachiver X, Fleury B, et al. Intérêt physio-pathologique d’une etude céphalométrique par téléradiographis et IRM dans le syndrome d’apnée du sommeil; déductions thérapeutiques. Ann Oto Laryng (Paris). 1990;107:159-166.
4. Chabolle F, Wagner I, Blumen, et al. Tongue base reduction with hyoepiglottoplasty: a treatment for severe obstructive sleep apnea. Laryngoscope. 1999;109:1273-1280.
5. Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg. 1993;108(2):117-125.
6. Riley R, Powell N, Guilleminault C, et al. Obstructive sleep apnea surgery: risk management and complications. Otolaryngol Head Neck Surg. 1997;117(6):648-652.
7. Woodson BT, Fujita S. Clinical experience with lingualplasty as part of the treatment of severe obstructive sleep apnea. Otolaryngol Head Neck Surg. 1992;107(1):40-48.
8. Aguirregomoscorta JI, Altube L, Menendez I, et al. Comparacion entre las normativas de la SEPAR de 1993 y 2002 en la lectura de los eventos respiratorios de las mismas polisomnografias (Comparison between the 1993 and 2002 Guidelines of the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) for identifying respiratory events in polysomnography tests). Arch Bronconeumol (Spain). 2005;41(12):649-653.
9. Woodson BT, Robinson S, Lim HJ. Transpalatal advancement pharyngoplasty outcomes compared with uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 2005;133:211-217.
10. Riley RW, Powell N, Guilleminault C. Obstructive sleep apnea and the hyoid: a revised surgical procedure. Otolaryngol Head Neck Surg. 1994;111:717-721.