CHAPTER 36 External and middle ear
By convention, the ear is subdivided into three parts, the external, middle and inner ear. It is largely, but not entirely, contained within the temporal bone. The ears not only receive, modulate, conduct, amplify and discriminately analyse the complex pressure waves that are sound, but also contain the end organs of balance.
TEMPORAL BONE
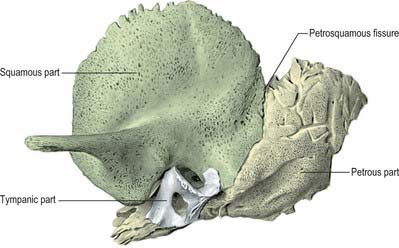
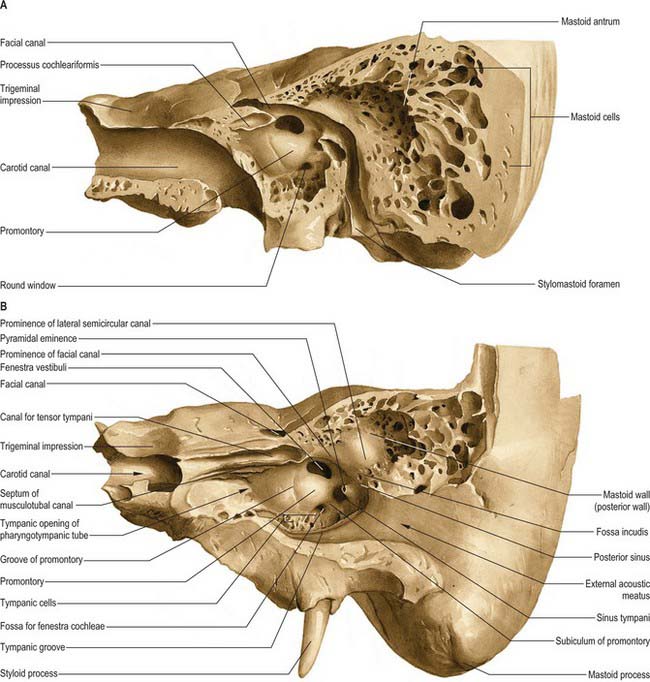
Each temporal bone consists of four components: the squamous, petromastoid and tympanic parts and the styloid process (Fig. 36.1). The squamous part has a shallow mandibular fossa associated with the temporomandibular joint (Ch. 31). The petromastoid part is relatively large: its petrous portion houses the auditory apparatus and is formed of compact bone. In contrast, the mastoid process is trabecular and variably pneumatized. The tympanic part has the form of a thin and incomplete ring whose ends are fused with the squamous part. The styloid process gives attachment to the styloid group of muscles. Two canals are associated with the temporal bone. The external acoustic meatus, visible on the lateral surface, conveys sound waves to the tympanic membrane. The internal acoustic meatus, evident on the medial surface, conveys the facial and vestibulocochlear nerves.
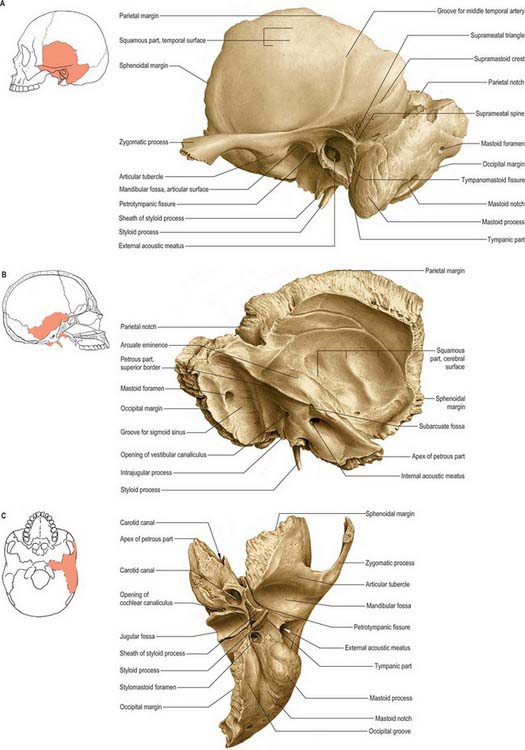
Fig. 36.1 Left temporal bone. A, Lateral aspect. B, Medial aspect. C, Inferior aspect.
(From Sobotta 2006.)
Squamous part
The squamous part has a zygomatic process and a mandibular fossa.
Mandibular fossa
The mandibular fossa is limited in front by the articular eminence of the zygomatic process. It presents an anterior articular area, formed by the squamous part, and a posterior non-articular area, formed by the tympanic element. The articular surface is smooth, oval and concave, and contacts the articular disc of the temporomandibular joint (Ch. 31). Unlike most other synovial joints, it is lined by fibrous tissue rather than hyaline cartilage, reflecting its intramembranous development. The non-articular area sometimes contains part of the parotid gland. A small, conical postglenoid tubercle separates the articular surface laterally from the tympanic plate.
Petromastoid part
Mastoid part
The superior border of the mastoid part is thick and serrated for articulation with the mastoid angle of the parietal bone. The posterior border is also serrated and articulates with the inferior border of the occipital bone between its lateral angle and jugular process. The mastoid element is fused with the descending process of the squamous part: below, it appears in the posterior wall of the tympanic cavity.
Petrous part
The anterior surface contributes to the floor of the middle cranial fossa (Ch. 27) and is continuous with the cerebral surface of the squamous part (although the petrosquamosal suture often persists late in life). The whole surface is adapted to the inferior temporal gyri. Behind the apex is a trigeminal impression for the trigeminal ganglion. Bone anterolateral to this impression roofs the anterior part of the carotid canal, but is often deficient. A ridge separates the trigeminal impression from another hollow behind which partly roofs the internal acoustic meatus and cochlea. This, in turn, is limited behind by the arcuate eminence which is raised by the superior (anterior) semicircular canal. Laterally, the anterior surface roofs the vestibule and, partly, the facial canal. Between the squamous part laterally and the arcuate eminence and the hollows just described medially, the anterior surface is formed by the tegmen tympani, a thin plate of bone which forms the roof of the mastoid antrum, and extends forwards above the tympanic cavity and the canal for tensor tympani. The lateral margin of the tegmen tympani meets the squamous part at the petrosquamosal suture, turning down in front as the lateral wall of the canal for tensor tympani and the osseous part of the pharyngotympanic tube: its lower edge is in the squamotympanic fissure. Anteriorly the tegmen bears a narrow groove related to the greater petrosal nerve (which passes posterolaterally to enter the bone by a hiatus anterior to the arcuate eminence). The groove passes forwards to the foramen lacerum. A smaller and similar hiatus and groove may be found more laterally: they are related to the lesser petrosal nerve (which runs to the foramen ovale). The posterior slope of the arcuate eminence overlies the posterior and lateral semicircular canals. Lateral to the eminence, the posterior part of the tegmen tympani roofs the mastoid antrum.
External acoustic meatus
The temporal bone contains the bony (osseous) part of the external acoustic meatus (see p. 620).
Ossification
The four temporal components ossify independently (Fig. 36.2). The squamous part is ossified in a sheet of condensed mesenchyme from a single centre near the zygomatic roots, which appear in the seventh or eighth week in utero. The petromastoid part has several centres which appear in the cartilaginous otic capsule during the fifth month: as many as 14 have been described. These centres vary in order of appearance. Several are small and inconstant, soon fusing with others. The otic capsule is almost fully ossified by the end of the sixth month. The tympanic part is also ossified in mesenchyme from a centre identifiable about the third month; at birth it is an incomplete tympanic ring, deficient above, its concavity grooved by a tympanic sulcus for the tympanic membrane. The malleolar sulcus for the anterior malleolar process, chorda tympani and anterior tympanic artery inclines obliquely downwards and forwards across the medial aspect of the anterior part of the ring. The styloid process develops from two centres at the cranial end of cartilage in the second visceral or hyoid arch: a proximal centre for the tympanohyal appears before birth, and another, for the distal stylohyal, appears after birth. The tympanic ring unites with the squamous part shortly before birth, and the petromastoid fuses with it and the tympanohyal during the first year. The stylohyal does not unite with the rest of the process until after puberty and may never do so.
Once ossified, the tympanic cavity, mastoid antrum and the posterior end of the pharyngotympanic tube become surrounded by bone. The petrous part forms the roof, floor and medial wall of the cavity, while the squamous and tympanic parts, together with the tympanic membrane, form its lateral wall. At birth the middle and inner ears are adult size, and the tympanic cavity, mastoid antrum, tympanic membrane and auditory ossicles are all almost adult size. The anterior process does not join the malleus until 6 months later. The internal acoustic meatus is approximately 6 mm in horizontal diameter, 4 mm in vertical diameter and 7 mm in length at birth, and the adult diameters are 7.7 mm and 11 mm respectively.
EXTERNAL EAR
AURICLE (PINNA)
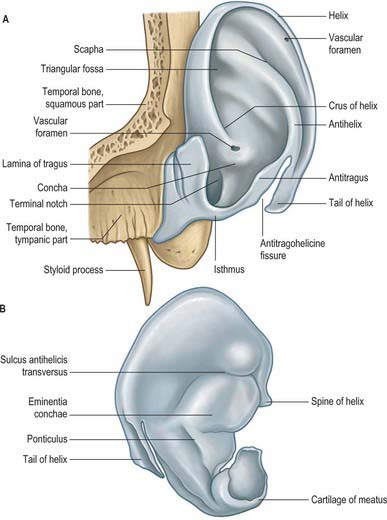
The lateral surface of the auricle is irregularly concave, faces slightly forwards, and displays numerous eminences and depressions (Fig. 36.3). It has a prominent curved rim, the helix. This usually bears a small tubercle posterosuperiorly, Darwin’s tubercle, which is quite pronounced around the sixth month of intrauterine life. The antihelix is a curved prominence, parallel and anterior to the posterior part of the helix: it divides above into two crura which flank a depressed triangular fossa. The curved depression between the helix and antihelix is the scaphoid fossa. The antihelix encircles the deep, capacious concha of the auricle, which is incompletely divided by the crus or anterior end of the helix. The conchal area above this, the cymba conchae, overlies the suprameatal triangle of the temporal bone, which can be felt through it, and which overlies the mastoid antrum. The tragus is a small curved flap below the crus of the helix and in front of the concha: it projects posteriorly, partly overlapping the meatal orifice. The antitragus is a small tubercle opposite the tragus and separated from it by the intertragic incisure or notch. Below it is the lobule, composed of fibrous and adipose tissues. It is soft, unlike the majority of the auricle which is supported by elastic cartilage and is firm. The cranial surface of the auricle presents elevations which correspond to the depressions on its lateral surface, and after which they are named (e.g. eminentia conchae, eminentia fossae triangularis).
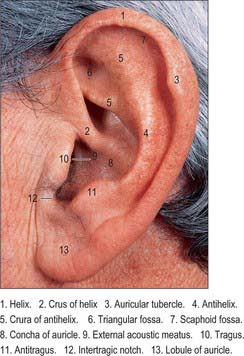
Fig. 36.3 Lateral surface of the left auricle.
(By permission from Berkovitz BKB, Moxham BJ 2002 Head and Neck Anatomy. London: Martin Dunitz.)
A number of common abnormalities have been recognized and carry descriptive names or eponyms (Porter & Tan 2005).
Stahl’s bar (also known as Satiro’s ears) is a common congenital deformity of the auricle where the helix is flattened and the upper crus of the antihelix is duplicated, producing a ridge of cartilage running from the antihelix to the rim of the helix. This causes a pointing of the ear and a reversal of the normal concavity of the scaphoid fossa. Occasionally, the upper part of the pinna flops over to produce an appearance known as ‘lop ear’. A variety of surgical procedures have been designed to correct this deformity using external moulds etc. Attempts at correction are made in the neonatal period while the cartilage is soft and pliable: delay until such time as the cartilage has become stiff may jeopardize a good outcome.
Six auricular hillocks, the embryological precursors of the auricle, form round the margins of the dorsal portion of the first pharyngeal cleft, three on the caudal edge of the first pharyngeal arch and three on the cranial edge of the second pharyngeal arch (see Ch. 40). They fuse to form the auricle and surround the dorsal end of the first branchial cleft from which the external acoustic meatus arises. Sinuses and cysts are often found just anterior to the root of the helix, near to the point of fusion of the hillocks derived from the first branchial arch and those derived from the second branchial arch. There is debate as to whether the abnormalities are epithelial inclusions between the hillocks or remnants of the first branchial cleft. The sinuses may be simple pits or complex branching sinuses that occasionally extend deeply towards the external acoustic meatus so that they lie close to the facial nerve. Clinically they may become chronically infected and require surgical excision: this may be technically demanding surgery given the close proximity to the facial nerve.
Skin
The skin of the auricle continues into the external auditory meatus to cover the outer surface of the tympanic membrane. It is thin, has no dermal papillae, and is closely adherent to the cartilaginous and osseous parts of the canal (inflammation of the canal skin is very painful because of this attachment to the underlying structures). The thick subcutaneous tissue of the cartilaginous part of the meatus contains numerous ceruminous glands that secrete wax, or cerumen. Their coiled tubular structure resembles that of sweat glands. The secretory cells are columnar when active, but cuboidal when quiescent; they are covered externally by myoepithelial cells. Ducts open either onto the epithelial surface or into the nearby sebaceous gland of a hair follicle. Cerumen prevents the maceration of meatal skin by trapped water. Antibacterial properties have been attributed to cerumen, but the evidence for this is lacking (Campos et al 2000, Pata et al 2003).
Two types of wax, wet and dry, are recognized. They are genetically determined. Dry wax is common in East Asians, while the wet type is more common in other ethnic groups (Yoshiura et al 2006). Overproduction, accumulation or impaction of wax may completely occlude the meatus, thereby hindering sound from reaching the tympanic membrane and also restricting the natural vibration of the drum. Although ceruminous glands and hair follicles are largely limited to the cartilaginous meatus, a few small glands and fine hairs are also present in the roof of the lateral part of the osseous part of the canal. The warm, humid environment of the relatively enclosed meatal air aids the mechanical responses of the tympanic membrane.
Cartilaginous framework
The auricle is a single thin plate of elastic fibrocartilage covered by skin, its surface moulded by eminences and depressions (Fig. 36.4). It is connected to the surrounding parts by ligaments and muscles, and is continuous with the cartilage of the external acoustic meatus. There is no cartilage in the lobule or between the tragus and the crus of the helix, where the gap is filled by dense fibrous tissue. Anteriorly, where the helix curves upwards, there is a small cartilaginous projection, the spine of the helix. Its other extremity is prolonged inferiorly as the tail of the helix and it is separated from the antihelix by the fissura antitragohelicina. The cranial aspect of the cartilage bears the eminentia conchae and eminentia scaphae, which correspond to the depressions on the lateral surface. The two eminences are separated by a transverse furrow, the sulcus antihelicis transversus, which corresponds to the inferior crus of the antihelix on the lateral surface. The eminentia conchae is crossed by an oblique ridge, the ponticulus, for the attachment of auricularis posterior. There are two fissures in the auricular cartilage, one behind the crus of the helix and another in the tragus.
Auricular muscles
Intrinsic muscles
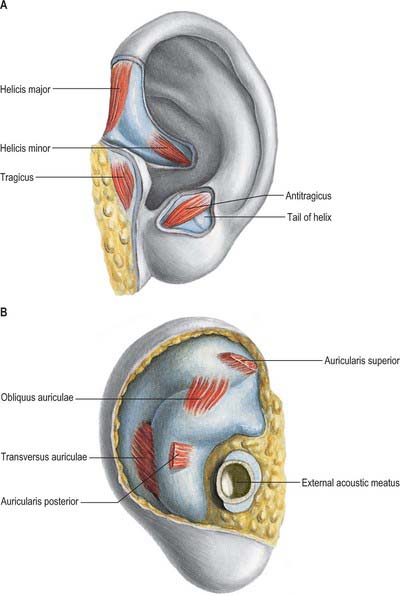
The intrinsic auricular muscles are helicis major and minor, tragicus, antitragicus, transversus auriculae and obliquus auriculae (Fig. 36.5). Helicis major is a narrow vertical band on the anterior margin of the helix, passing from its spine to its anterior border, where the helix is about to curve back. Helicis minor is an oblique fasciculus covering the crus of the helix. Tragicus is a short, flattened, vertical band on the lateral aspect of the tragus. Antitragicus passes from the outer part of the antitragus to the tail of the helix and the antihelix. Transversus auriculae, located on the cranial aspect of the auricle, consists of scattered fibres, partly tendinous, partly muscular, which extend between the eminentia conchae and the eminentia scaphae. Obliquus auriculae, also located on the cranial aspect of the auricle, consists of a few fibres which extend from the upper and posterior parts of the eminentia conchae to the eminentia scaphae.
Vascular supply and lymphatic drainage
The posterior auricular branch of the external carotid artery is the dominant blood supply (Imanishi et al 1997). It supplies three or four branches to the cranial surface of the auricle: twigs from these arteries reach the lateral surface, some through fissures in the cartilage, others round the margin of the helix. The posterior auricular artery ascends between the parotid gland and the styloid process to the groove between the auricular cartilage and mastoid process. The superior auricular artery has a constant course and connects the superior temporal artery and the posterior auricular arterial network: this branch can provide a reliable vascular pedicle for retro-auricular flaps (Moschella et al 2003) The auricle is also supplied by anterior auricular branches of the superficial temporal artery, which are distributed to its lateral surface, and by a branch from the occipital artery.
Innervation
The sensory innervation of the auricle is complex and not fully determined. This is perhaps because the external ear represents an area where skin originally derived from a branchial region meets skin originally derived from a postbranchial region. The sensory nerves involved are the great auricular nerve, which supplies most of the cranial surface and the posterior part of the lateral surface (helix, antihelix, lobule); the lesser occipital nerve, which supplies the upper part of the cranial surface; the auricular branch of the vagus, which supplies the concavity of the concha and posterior part of the eminentia; the auriculotemporal nerve, which supplies the tragus, crus of the helix and the adjacent part of the helix; and the facial nerve, which together with the auricular branch of the vagus probably supplies small areas on both aspects of the auricle, in the depression of the concha, and over its eminence. The details of the cutaneous innervation derived from the facial nerve require further clarification. It is possible that as the auricular branch of the vagus traverses the temporal bone and crosses the facial canal, approximately 4 mm above the stylomastoid foramen, it contributes an ascending branch to the facial nerve, and that in this way fibres of the vagus are carried via the facial nerve to the pinna (see p. 494).
EXTERNAL ACOUSTIC MEATUS
The external acoustic meatus extends from the concha to the tympanic membrane: it is approximately 2.5 cm from the floor of the concha and approximately 4 cm from the tragus. It has two structurally different parts: its lateral third is cartilaginous and its medial two-thirds is osseous (Figs 36.6A, 36.7, 36.8). It forms an S-shaped curve, directed at first medially, anteriorly and slightly up (pars externa), then posteromedially and up (pars media), and lastly anteromedially and slightly down (pars interna). It is oval in section, its greatest diameter is obliquely inclined posteroinferiorly at the external orifice, but is nearly horizontal at its medial end. There are two constrictions, one near the medial end of the cartilaginous part, the other, the isthmus, in the osseous part about 2 cm from the bottom of the concha. The tympanic membrane, which closes its medial end, is obliquely set, which means that the floor and the anterior wall of the meatus are longer than its roof and posterior wall.
The osseous part is approximately 16 mm long, and is narrower than the cartilaginous part. In sagittal section it is oval or elliptical and it is directed anteromedially and slightly downwards, with a slight posterosuperior convexity. Its medial end is smaller than the lateral end and it terminates obliquely. The anterior wall projects medially approximately 4 mm beyond the posterior and is marked, except above, by a narrow tympanic sulcus or anulus, to which the perimeter of the tympanic membrane is attached. Its lateral end is dilated and mostly rough for the attachment of the meatal cartilage. The anterior, inferior, and most of the posterior, parts of the osseous meatus are formed by the tympanic plate of the temporal bone, which in the fetus is only a tympanic ring. The posterosuperior region is formed by the squamous part of the temporal bone. The outer wall of the meatus is bounded above by the posterior zygomatic root, below which there may be a suprameatal spine.
The sensory innervation of the external acoustic meatus is derived from the auriculotemporal branch of the mandibular nerve, which supplies the anterior and superior walls, and the auricular branch of the vagus, which supplies the posterior and inferior walls. The facial nerve may also contribute via its communication with the vagus nerve.
EXTERNAL SURGICAL APPROACHES TO THE MIDDLE EAR
More extensive resections of the temporal bone are undertaken using extended pre- or postauricular incisions into the temporal region and neck. The blood supply of the pinna is sufficient to maintain viability despite significant elevation and undermining.
MIDDLE EAR
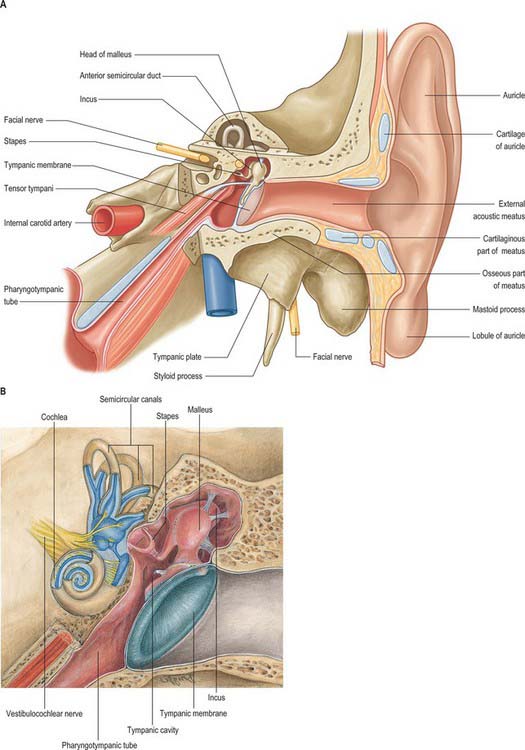
The middle ear is an irregular, laterally compressed space in the petrous part of the temporal bone. It is lined with mucous membrane and filled with air, which reaches it from the nasopharynx via the pharyngotympanic tube (Figs 36.6, 36.7, 36.9). The middle ear contains three small bones, the malleus, incus and stapes, collectively called the auditory ossicles, which form an articulated chain connecting the lateral and medial walls of the cavity, and which transmit the vibrations of the tympanic membrane across the cavity to the cochlea.
The space within the middle ear can be subdivided into three parts. These are the mesotympanum or tympanic cavity proper, which is opposite the tympanic membrane; the epitympanum or attic, which is above the level of the membrane, and contains the head of the malleus and the body and short process of the incus; and the hypotympanum, which is in the floor of the cavity between the jugular bulb and the lower margin of the tympanic membrane. The vertical and anteroposterior diameters of the mesotympanum and hypotympanum are each approximately 15 mm; the transverse diameter is 6 mm superiorly and 4 mm inferiorly, narrowing to 2 mm opposite the umbo. The cavity is bounded laterally by the tympanic membrane and medially by the lateral wall of the internal ear, the promontory. It communicates posteriorly with the mastoid antrum and the mastoid air cells, and anteriorly with the nasopharynx via the pharyngotympanic tube (Figs 36.6, 36.7).
BOUNDARIES OF THE TYMPANIC CAVITY
The tympanic cavity has a roof and a floor, and lateral, medial, posterior and anterior walls.
Lateral wall
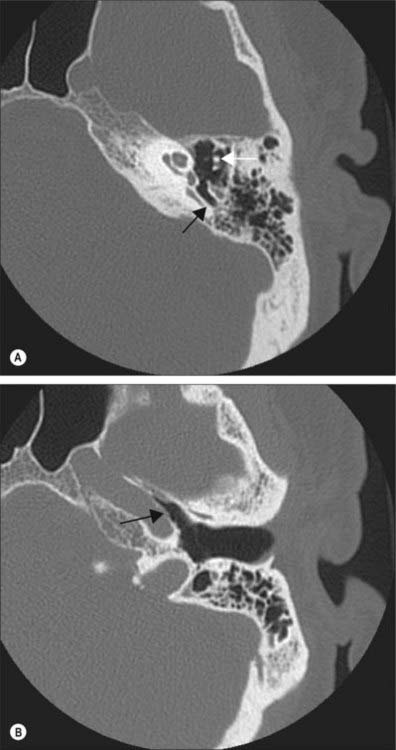
The lateral wall consists mainly of the tympanic membrane, but also contains the ring of bone to which the membrane is attached (see Figs 36.6B, 36.14A),. The lateral epitympanic bony wall is wedge-shaped in section and its sharp inferior portion is known as the outer attic wall or scutum. This part is easily eroded or blunted by cholesteatoma, a feature easily detected on CT scans (see Fig. 36.8). There is a deficiency or notch in the upper part of this ring, close to which are the small openings of the anterior and posterior canaliculi for the chorda tympani and the petrotympanic fissure. The posterior canaliculus for the chorda tympani is situated in the angle between the posterior and lateral walls of the tympanic cavity just behind the tympanic membrane, at a variable position approximately level with the upper end of the handle of the malleus. It leads into a minute canal that descends in front of the facial canal and ends in it about 6 mm above the stylomastoid foramen. The canaliculus transmits the chorda tympani and a branch of the stylomastoid artery to the tympanic cavity. The chorda tympani leaves the tympanic cavity through the anterior canaliculus, which opens at the medial end of the petrotympanic fissure.
Tympanic membrane
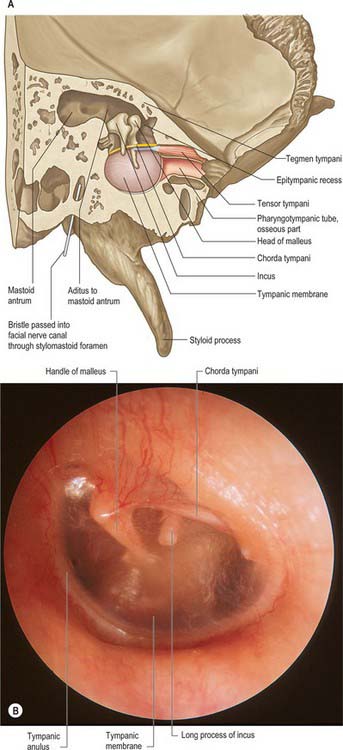
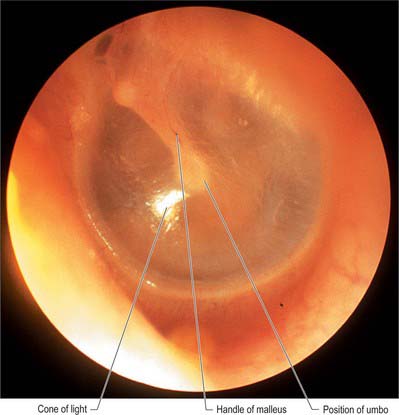
The tympanic membrane separates the tympanic cavity from the external acoustic meatus (Fig. 36.10; see also Figs 38.6, 36.14). It is thin, semi-transparent, and almost oval, though somewhat broader above than below. It lies obliquely, at an angle of approximately 55° with the meatal floor. Its longest, anteroinferior diameter is 9 to 10 mm, and its shortest is 8 to 9 mm. Most of its circumference is a thickened fibrocartilaginous ring or anulus which is attached to the tympanic sulcus at the medial end of the meatus. The anulus contains radially orientated smooth muscle cells in several locations that possibly play a role in controlling blood flow or maintaining tension (Henson et al 2005). The sulcus is deficient superiorly, i.e. it is notched. Two bands, the anterior and posterior malleolar folds, pass from the ends of this notch to the lateral process of the malleus. The small triangular part of the membrane, the pars flaccida, lies above these folds and is lax and thin. The major part of the tympanic membrane, the pars tensa, is taut. The handle of the malleus is firmly attached to the internal surface of the tympanic membrane as far as its centre, which projects towards the tympanic cavity. The inner surface of the membrane is thus convex and the point of greatest convexity is termed the umbo. Although the membrane as a whole is convex on its inner surface, its radiating fibres are curved with their concavities directed inwards.
Medial wall
The medial wall of the tympanic cavity is also the lateral boundary of the internal ear. Its features are the promontory, fenestra vestibuli (fenestra ovalis, oval window), fenestra cochleae (fenestra rotunda, round window) and the facial prominence (Fig. 36.11).
The promontory is a rounded prominence furrowed by small grooves which lodge the nerves of the tympanic plexus. It lies over the lateral projection of the basal turn of the cochlea. A minute spicule of bone frequently connects the promontory to the pyramidal eminence of the posterior wall. The apex of the cochlea lies near the medial wall of the tympanic cavity, anterior to the promontory. A depression behind the promontory is known as the sinus tympani.
Posterior wall
The posterior wall of the tympanic cavity is wider above than below. Its main features are the aditus to the mastoid antrum, the pyramid, and the fossa incudis (Fig. 36.11).
The pyramidal eminence is situated just behind the fenestra vestibuli and in front of the vertical part of the facial nerve canal. It is hollow and contains the stapedius muscle. Its summit projects towards the fenestra vestibuli and is pierced by a small aperture which transits the tendon of stapedius. The cavity in the pyramidal eminence is prolonged down and back in front of the facial nerve canal; it communicates with the canal by an aperture through which a small branch of the facial nerve passes to stapedius.
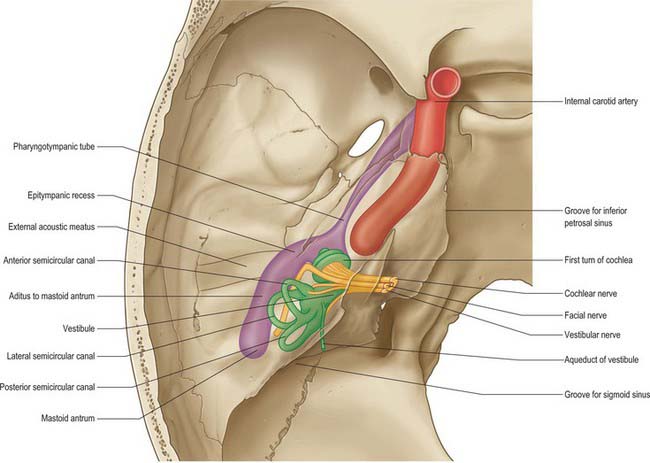
Mastoid antrum
The mastoid antrum is an air sinus in the petrous part of the temporal bone. Its topographical relations are of considerable surgical importance. The aditus to the mastoid antrum, which leads back from the epitympanic recess, opens in the upper part of its anterior wall. The lateral semicircular canal lies medial to the aditus. The descending part of the facial nerve canal is anteroinferior. The medial wall is related to the posterior semicircular canal (see Ch. 37). The sigmoid sinus lies some distance posteriorly: the distance can be extremely variable and is dependent on the degree of pneumatization of the mastoid. The roof is formed by the tegmen tympani, and so the antrum lies below the middle cranial fossa and the temporal lobe of the brain. The floor has several openings which communicate with the mastoid air cells. The lateral wall, which offers the usual surgical approach to the cavity, is formed by the postmeatal process of the squamous part of the temporal bone. This is only 2 mm thick at birth but increases at an average rate of 1 mm a year, attaining a final thickness of 12–15 mm. In adults, the lateral wall of the antrum corresponds to the suprameatal triangle (Macewen’s triangle) on the outer surface of the skull. This is palpable through the cymba conchae: the superior side of the triangle, the supramastoid crest, is level with the floor of the middle cranial fossa; the anteroinferior side, which forms the posterosuperior margin of the external acoustic meatus, indicates approximately the position of the descending part of the facial nerve canal; the posterior side, formed by a posterior vertical tangent to the posterior margin of the external acoustic meatus, is anterior to the sigmoid sinus.
Mastoid air cells
Though the mastoid process antrum is well developed at birth, the mastoid air cells are merely minute antral diverticula at this stage. As the mastoid develops in the second year, the air cells gradually extend into it and by the fourth year they are well formed, although their greatest growth occurs at puberty. They vary considerably in number, form and size. Usually, they interconnect and are lined by a mucosa with squamous non-ciliated epithelium, continuous with that in the mastoid antrum and tympanic cavity. They may fill the mastoid process, even to its tip, and some may be separated from the sigmoid sinus and posterior cranial fossa only by extremely thin bone, which is occasionally deficient (Fig. 36.11). Some may lie superficial to, or even behind, the sigmoid sinus, and others may be present in the posterior wall of the descending part of the facial nerve canal. Those in the squamous part of the temporal bone may be separated from deeper cells in the petrous part by a plate of bone in the line of the squamomastoid suture (Körner’s septum). Sometimes they extend only minimally into the mastoid process, in which case the process consists largely of dense bone or trabecular bone containing bone marrow. Varieties of the mastoid process are recognized. The three types most commonly described are pneumatized (with many air cells); sclerotic or diploic (with few or no air cells); and mixed (contain both air cells and bone marrow).
Anterior wall
The inferior, larger area of the anterior wall of the tympanic cavity is narrowed by the approximation of the medial and lateral walls of the cavity (Fig. 36.6). It is a thin lamina and forms the posterior wall of the carotid canal. It is perforated by the superior and inferior caroticotympanic nerves and the tympanic branch or branches of the internal carotid. The canals for tensor tympani and the osseous part of the pharyngotympanic tube open above it, the canal for tensor tympani being superior to that for the pharyngotympanic tube. Both canals incline downwards and anteromedially, to open in the angle between the squamous and petrous parts of the temporal bone, and are separated by a thin, osseous septum. The canal for tensor tympani and the bony septum runs posterolaterally on the medial tympanic wall, and ends immediately above the fenestra vestibuli. Here, the posterior end of the septum is curved laterally to form a pulley, the processus trochleariformis (cochleariform process), which is a surgical landmark for the identification of the geniculate ganglion of the facial nerve. The tendon of tensor tympani turns laterally over the pulley before attaching to the upper part of the handle of the malleus.
Pharyngotympanic tube blockage in children
Otitis media
It is assumed that acute otitis media usually arises as a result of ascending infection from the nasopharynx via the pharyngotympanic tube to the middle ear cleft. From there it may extend to the mastoid aditus and antrum. Swelling secondary to the infection may result in the closure of both exits from the middle ear, i.e. the pharyngotympanic tube and the aditus, with subsequent accumulation of pus under pressure, which causes lateral bulging and inflammation of the tympanic membrane. The latter may burst, releasing mucopurulent discharge into the external acoustic meatus, which results in a release of the pressure in the middle ear and a diminution in the levels of pain. After a brief period the discharge dries up, and for the most part the resultant perforation of the tympanic membrane heals. Normal ventilation and drainage of mucus from the middle ear is restored once the swelling in the pharyngotympanic tube resolves. On occasion the process will fail to produce a perforation of the tympanic membrane and the inflammatory exudates will not drain. The immune defence system sterilizes the exudates of organisms, resulting in a sterile mucoid effusion, otitis media with effusion (see above).
AUDITORY OSSICLES
Malleus
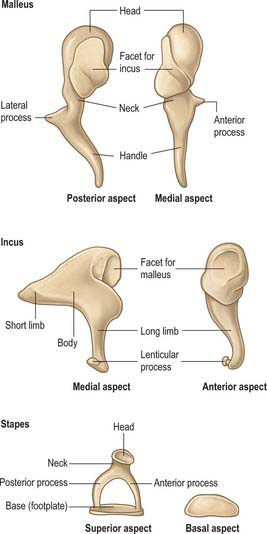
The malleus is the largest of the ossicles, and is shaped somewhat like a mallet (Fig. 36.12). It is 8–9 mm long and has a head, neck, handle (manubrium) and anterior and lateral processes. The head is the large upper end of the bone and is situated in the epitympanic recess. It is ovoid in shape, articulates posteriorly with the incus, and is covered elsewhere by mucosa. The cartilaginous articular facet for the incus is narrowed near its middle and consists of a larger upper part and a smaller lower part, orientated almost at right angles to each other. Opposite the constriction, the lower margin of the facet projects in the form of a process, the spur of the malleus. The neck is the narrowed part below the head, and inferior to this is an enlargement from which the anterior and lateral processes project.
The handle of the malleus is connected by its lateral margin to the tympanic membrane (Figs 36.6, 36.10; see also Fig. 36.14A). It is directed downwards, medially and backwards. It decreases in size towards its free end, which is curved slightly forwards and is flattened transversely. Near the upper end of its medial surface there is a slight projection to which the tendon of tensor tympani is attached. The anterior process is a delicate bony spicule, directed forwards from the enlargement below the neck, and connected to the petrotympanic fissure by ligamentous fibres. In fetal life it is the longest process of the malleus and is continuous in front with Meckel’s cartilage. The lateral process is a conical projection from the root of the handle of the malleus. It is directed laterally and is attached to the upper part of the tympanic membrane and, via the anterior and posterior malleolar folds, to the sides of the notch in the upper part of the tympanic sulcus.
Incus
The incus is shaped less like an anvil, from which it is named, than a premolar tooth with its two diverging roots. It has a body and two processes (Fig. 36.12). The body is somewhat cubical but laterally compressed. On its anterior surface it has a saddle-shaped facet for articulation with the head of the malleus. The long process, rather more than half the length of the handle of the malleus, descends almost vertically, behind and parallel to the handle. Its lower end bends medially and ends in a rounded lenticular process, the medial surface of which is covered with cartilage and articulates with the head of the stapes. The short process, somewhat conical, projects backwards and is attached by ligamentous fibres to the fossa incudis in the lower and posterior part of the epitympanic recess.
Stapes
The stapes is also known as the stirrup. It has a head, neck, two limbs (processes or crura) and a base (footplate) (Fig. 36.12). The head (caput) is directed laterally and has a small cartilaginous facet for articulation with the lenticular process of the incus. The neck is the constricted part supporting the head, and the tendon of stapedius is attached to its posterior surface. The processes diverge from the neck and are connected at their ends by a flattened oval plate, the base, which forms the footplate of the stapes. The footplate is attached to the margin of the fenestra vestibuli by a ring of fibres (the anular ligament). The anterior process is shorter, thinner and less curved than the posterior.
Ossicular ligaments
The ossicles are connected to the tympanic walls by ligaments (Fig. 36.6B): three for the malleus and one each for the incus and stapes. Some are mere mucosal folds which carry blood vessels and nerves to and from the ossicles and their articulations, and others contain a central, strong band of collagen fibres.
The anterior ligament of the malleus stretches from the neck of the malleus, just above the anterior process, to the anterior wall of the tympanic cavity near the petrotympanic fissure. Some of its collagen fibres traverse this fissure to reach the spine of the sphenoid, and others continue into the sphenomandibular ligament. The latter, like the anterior malleolar ligament, is derived from the perichondrial sheath of Meckel’s cartilage. The anterior malleolar ligament may contain muscle fibres, called laxator tympani or musculus externus mallei. The lateral ligament of the malleus is a triangular band which stretches from the posterior part of the border of the tympanic incisure to the head of the malleus. The superior ligament of the malleus connects the head of the malleus to the roof of the epitympanic recess.
MUSCLES
There are two intratympanic muscles, tensor tympani and stapedius.
Tensor tympani
Tensor tympani is a long slender muscle which occupies the bony canal above the osseous part of the pharyngotympanic tube, from which it is separated by a thin bony septum (Fig. 36.6). It arises from the cartilaginous part of the pharyngotympanic tube and the adjoining region of the greater wing of the sphenoid, as well as from its own canal. It passes back within its canal, and ends in a slim tendon which bends laterally round the pulley-like processus cochleariformis and attaches to the handle of the malleus, near its root.
VASCULAR SUPPLY AND LYMPHATIC DRAINAGE
The anterior tympanic branch of the first part of the maxillary artery ascends behind the temporomandibular joint and enters the tympanic cavity through the petrotympanic fissure. It ramifies on the interior of the tympanic membrane, and forms a vascular circle around it with the posterior tympanic branch of the stylomastoid artery. It also anastomoses with twigs of the artery of the pterygoid canal and caroticotympanic branches of the internal carotid artery in the mucosa of the tympanic cavity.
In early fetal life, a stapedial artery traverses the stapes.
INNERVATION
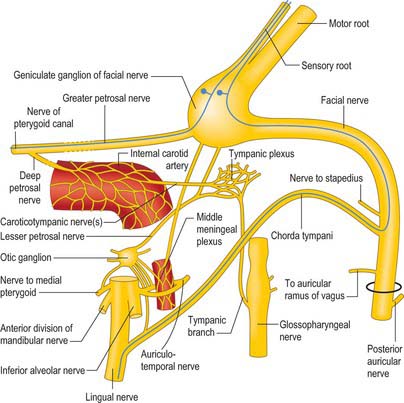
Tympanic plexus
The nerves that constitute the tympanic plexus ramify on the surface of the promontory on the medial wall of the tympanic cavity. They are derived from the tympanic branch of the glossopharyngeal nerve and the caroticotympanic nerves (Fig. 36.13). The former arises from the inferior ganglion of the glossopharyngeal nerve, and reaches the tympanic cavity via the tympanic canaliculus for the tympanic nerve. The superior and inferior caroticotympanic nerves are postganglionic sympathetic fibres which are derived from the carotid sympathetic plexus and traverse the wall of the carotid canal to join the plexus.
Facial nerve
The facial nerve enters the temporal bone through the internal acoustic meatus accompanied by the vestibulocochlear nerve (Fig. 36.7). At this point, the motor root, which supplies the muscles of the face, and the nervus intermedius, which contains sensory fibres concerned with the perception of taste and parasympathetic (secretomotor) fibres to various glands, are separate components. They merge within the meatus. At the end of the meatus, the facial nerve enters its own canal, the facial canal, which runs across the medial wall and down the posterior wall of the tympanic cavity to the stylomastoid foramen. As the nerve enters the facial canal, there is a bend (genu) which houses the geniculate ganglion (Figs 36.7, 36.13).
The chorda tympani (Figs 36.13, 36.14) leaves the facial nerve some 6 mm above the stylomastoid foramen and runs anterosuperiorly in a canal to enter the tympanic cavity via the posterior canaliculus. It then curves anteriorly in the substance of the tympanic membrane between its mucous and fibrous layers (Fig. 36.14A), and crosses medial to the upper part of the handle of the malleus to the anterior wall of the tympanic cavity, where it enters the anterior canaliculus. It exits the skull at the petrotympanic fissure, and its further course is described on page 543. The chorda tympani contains parasympathetic fibres which supply the submandibular and sublingual salivary glands via the submandibular ganglion and taste fibres from the anterior two-thirds of the tongue.
The geniculate ganglion also communicates with the lesser petrosal nerve.
Dehiscences of the facial nerve canal
The facial nerve may be somewhat variable in its anatomical course through the temporal bone (Proctor & Nager 1982). It may split into two or three strands, starting at the geniculate ganglion and then make its way across the promontory to the stylomastoid foramen, or pass a few millimetres posteriorly to its second genu, before it turns inferiorly posterior to the fossa incudis, a position where it is particularly vulnerable during surgical exploration of the mastoid antrum. The more proximal the division into strands, the more bizarre is the subsequent course. More distal bifurcations pass either side of the fenestra vestibuli. It may be dehiscent, particularly in its second part, when it occasionally overhangs the stapes, or run inferior to the stapedial superstructure, a position which renders it vulnerable during surgery to the stapes (Barnes et al 2001). The motor fibres to the face may be carried through the chorda tympani, which is then enlarged. When this is the case, the distal facial nerve dwindles to a fibrous strand in a narrowed stylomastoid foramen. In chronic bone disease in the tympanic cavity, the facial nerve may be exposed in its canal. Inflammation may lead to facial paralysis of the infranuclear or lower motor neurone type.
TYMPANIC MUCOSA
The mucosa of the tympanic cavity is pale, thin and slightly vascular. It is continuous with that of the pharynx, via the pharyngotympanic tube, and covers the ossicles, muscles and nerves in the cavity to form the inner layer of the tympanic membrane and the outer layer of the secondary tympanic membrane. It also spreads into the mastoid antrum and air cells. The middle ear mucosa is a mucus-secreting respiratory mucosa bearing cilia on its surface. The precise distribution of the mucociliary epithelium varies in normal middle ears, being more widespread in the young. Three distinct mucociliary pathways have been identified, epitympanic, promontorial and hypotympanic, the latter being the largest. Each of these pathways coalesces at the tympanic orifice of the pharyngotympanic tube (Gleeson et al 1991).
The mucosa forms several vascular folds which extend from the tympanic walls to the ossicles: one descends from the roof of the cavity to the head of the malleus and the upper margin of the body of the incus, and a second surrounds stapedius. Other folds invest the chorda tympani nerve and tensor tympani. The folds separate off saccular recesses which give the interior of the tympanic cavity a somewhat honeycombed appearance: these mucosal folds have described in greater detail by Proctor (1964). Of note, the superior recess of the tympanic membrane, Prussak’s space, lies between the neck of the malleus and the pars flaccida, bounded by the lateral malleolar fold. This space can play an important role in the retention of keratin and subsequent development of cholesteatoma.
Anderson SD. The intratympanic muscles. In: Hinchcliffe R, editor. Scientific Foundations of Otolaryngology. London: Heinemann; 1976:257-280.
Anson BJ, Donaldson JA. The Surgical Anatomy of the Temporal Bone and Ear. Philadelphia: Saunders, 1976.
Barnes G, Liang J, Hall S, Michaels L, Wright A, Gleeson MJ. Development of the Fallopian canal in humans – a morphologic and radiologic study. Otol Neurotol. 2001;22:931-937.
Bluestone C, Klein J. Otitis media, atelectasis and Eustachian tube dysfunction. Bluestone CD, Stool SE, editors. Pediatric Otolaryngology, 4th edn., vol 1. Philadelphia: Saunders, 2002;474-486.
Campos A, Betancor L, Arias A, et al. Influence of human wet cerumen on the growth of common and pathogenic bacteria of the ear. J Laryngol Otol. 2000;114:925-929.
Couter RT. A Colour Atlas of Temporal Bone Surgical Anatomy. London: Wolfe Medical, 1980.
Glassock MEIII, Shambaugh GE. Surgery of the Ear, 4th edn. Philadelphia: Saunders, 1990.
Gleeson M, Felix H, Neivergelt J. Quantitative and qualitative analysis of the human middle ear mucosa. In: Sade J, editor. The Eustachian Tube, Basic Aspects. Amsterdam: Kugler & Ghedini; 1991:125-131.
Grey P. The clinical significance of the communicating branches of the somatic sensory supply of the middle and external ear. J Laryngol Otol. 1995;109:1141-1145.
Henson MM, Madden VJ, Rask-Andersen H, Henson OW. Smooth muscle in the annulus fibrosus of the tympanic membrane in bats, rodents, insectivores and humans. Hear Res. 2005;200:29-37.
Honjo I. Eustachian Tube and Middle Ear Diseases. Berlin: Springer-Verlag, 1988.
Imanishi N, Nakajima H, Aiso S. Arterial anatomy of the ear. Okajimas Folia Anat Jpn. 1997;73:313-323.
Moschella F, Cordova A, Pirrello R, De Leo A. The supra-auricular arterial network: anatomical bases for the use of superior pedicle retro-auricular skin flaps. Surg Radiol Anat. 2003;25:3-4.
O’Flynn P, Bailey M. Anatomy of the skull base and infratemporal fossa. Gleeson MJ, editor. Scott-Brown’s Otolaryngology, Head and Neck Surgery, 7th edn., vol 3. London: Hodder Arnold, 2008;3897-3910.
Pata YS, Ozturk C, Akbas Y, Gorur K, Unal M, Ozcan C. Has cerumen a protective role in recurrent external otitis? Am J Otolaryngol. 2003;24:209-212.
Phelps PD, Lloyd GAS. Diagnostic Imaging of the Ear, 2nd edn. Berlin: Springer-Verlag, 1990.
Porter CJW, Tan ST. Congenital auricular anomalies: topographic anatomy, embryology, classification and treatment strategies. Plast Reconstr Surg. 2005;115:1701-1712.
Proctor B. The development of the middle ear spaces and their surgical significance. J Otolaryngol. 1964;78:631-649.
Proctor B, Nager GT. The facial canal: normal anatomy, variations and anomalies. Ann Otol Rhinol Laryngol. 1982;91(suppl 93):33-61.
Wright A. Anatomy and ultrastructure of the human ear. Kerr GA, editor. 6th edn. Scott Brown’s Otolaryngology. vol 1. London: Butterworth Heinemann; 1997:1-50.
Yoshiura K, Kinoshita A, Ishida T, et al. A SNP in the ABCC11 gene is the determinant of human earwax type. Nat Genet. 2006;38:324-330.