Exposure of the Superior Mesenteric Artery and Celiac Axis
Surgical Anatomy
The celiac axis refers to a short arterial trunk originating from the anterior surface of the proximal abdominal aorta as it passes between the diaphragmatic crura at the level of the 12th thoracic vertebra (T12). The artery divides most often into three major branches within 2 cm of its origin: the common hepatic, splenic, and left gastric arteries (Fig. 34-1, A and B). These arterial branches and their tributaries provide the blood supply for the stomach, liver, spleen, portions of the pancreas, and proximal duodenum. The common hepatic artery gives rise to the superior pancreaticoduodenal arteries, cystic artery, and right gastric artery in addition to its left and right hepatic arteries. In approximately 18% of cases, the right hepatic artery is “replaced” and originates from the superior mesenteric artery. The splenic artery gives off the dorsal pancreatic artery, left gastroepiploic artery, and short gastric arteries before completing its tortuous course toward the spleen. The left gastric artery supplies the gastric cardia and fundus before anastomosing with the right gastric artery. A “replaced” left hepatic artery originates from the left gastric artery in approximately 12% of cases.
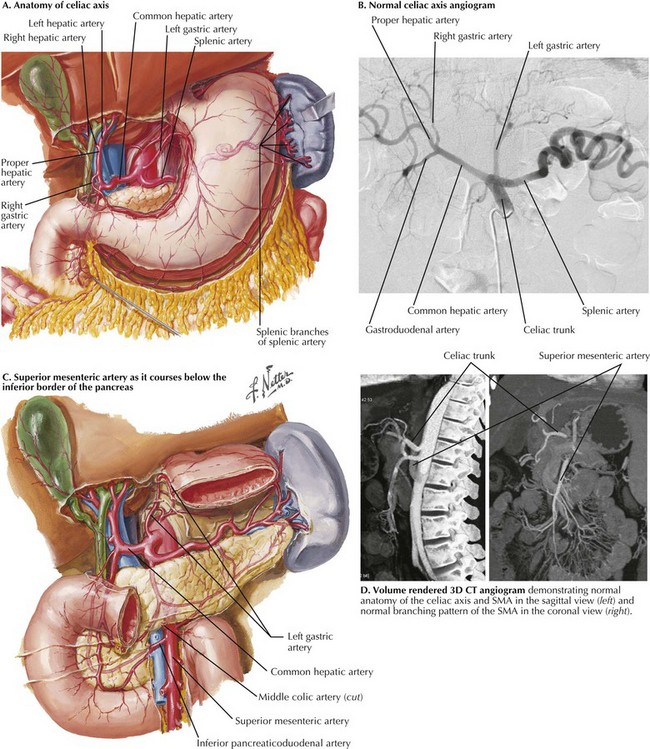
FIGURE 34–1 Celiac axis and superior mesenteric artery (SMA). (D from Horton KM, Fishman EK. Multidetector CT angiography in the diagnosis of mesenteric ischemia. Radiol Clin North Am 2007;45:275-88.)
Atherosclerotic pathology of the SMA most likely involves its origin, whereas emboli may lodge more distally in its course proximal or distal to the origin of the middle colic artery (Fig. 34-1, C and D).
Anterior Transperitoneal Exposure
For antegrade bypass of the SMA, attention is first directed to exposing the supraceliac aorta. This portion of the aorta is often the last to be involved in patients with extensive atherosclerosis and is the preferred site of proximal anastomosis for a bypass graft. The esophagus and lesser curvature of the stomach are identified and retracted to the patient’s left after division of the gastrohepatic ligament. The triangular ligament of the left lobe of the liver is divided (Fig. 34-2, A), and the left lateral segment of the liver is gently retracted to the right.
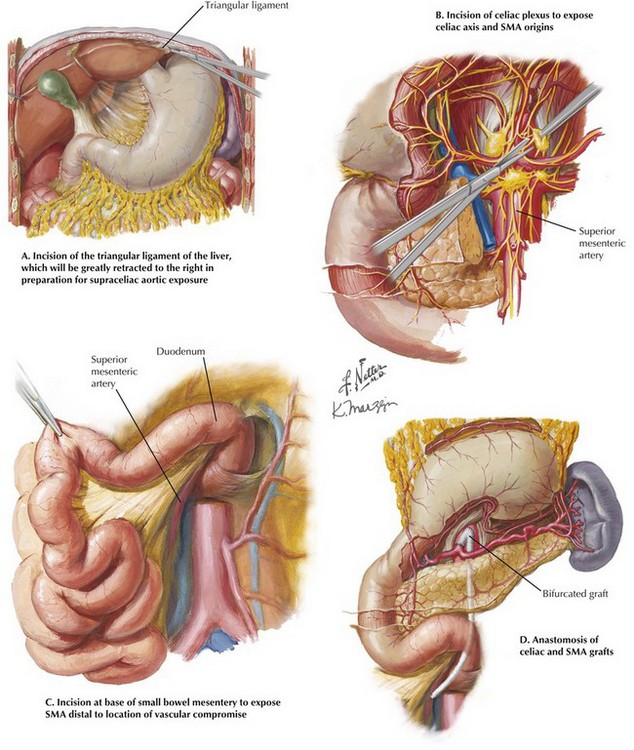
FIGURE 34–2 Anterior transperitoneal exposures and configuration of graft.
SMA, Superior mesenteric artery.
Care is taken to avoid excessive force when using self-retaining retraction systems, to prevent damage to liver parenchyma as the left lobe is folded toward the right. The right crus of the diaphragm is divided by electrocautery and the underlying median arcuate ligament incised, often through dense lymphatic and neural tissue. The posterior peritoneum may then be incised and the supraceliac aorta visualized and evaluated for its suitability for proximal anastomosis. Dissection in an inferior direction will expose the origin of the celiac axis and its primary branches. If the pancreas can be anteriorly retracted, the celiac origin and a limited portion of the SMA origin can be accessed (Fig. 34-2, B). If bypass to the celiac axis is planned, adequate exposure will be available for graft anastomosis to either the common hepatic artery or the cut end of the main celiac trunk.
Most patients with atherosclerotic disease will require a bypass to a more distal portion of the SMA. Through the main peritoneal cavity, elevating and superiorly displacing the transverse colon allows palpation of its mesentery and identification of the middle colic artery and the SMA. The main vessel is exposed by incising the peritoneum directly above it at the root of the mesentery (Fig. 34-2, C). The vessel usually is easily identified, but care must be taken to avoid injury to parallel veins and often-sizable arterial branches. A retropancreatic tunnel is formed by blunt finger dissection to allow passage of a graft from the supraceliac aorta to the exposed portion of the SMA (Fig. 34-2, D).
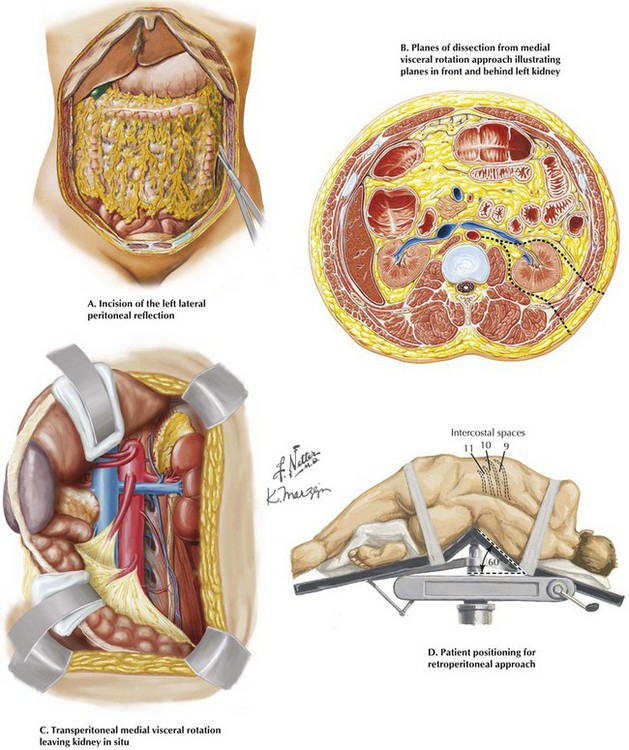
A most useful alternative to the transcrural approach to the SMA origin is medial visceral rotation. This approach is preferred for more extensive proximal revascularization, such as transaortic endarterectomy of the celiac artery, SMA, and renal vessels or repair of suprarenal aortic aneurysms. Incising the left lateral peritoneal reflection from the diaphragm to the pelvis allows mobilization of the descending colon (Fig. 34-3, A).
Next, the splenorenal and phrenocolic ligaments are carefully divided. With extension of the surgeon’s hand under the descending colon, stomach, pancreas, and spleen, the visceral bundle is rotated anteriorly and medially. The plane of the dissection can be anterior or posterior to the left kidney (Fig.34-3, B). The posterior approach provides continuous exposure of the visceral aorta as well as the proximal SMA and celiac axis (Fig. 34-3, C). As discussed next, rotating the patient to a lateral position with the right side down facilitates this exposure.
Retroperitoneal Exposure
The patient is positioned with the left side up, rotated about 60 degrees from the supine position. Care is taken to position the “break” of the operating room table equidistant between the rib cage and pelvis and properly pad both the torso and the left arm, which is secured on a special arm holder and positioned crossing the chest (Fig. 34-3, D). Depending on how high an exposure is needed, the left flank incision is made along the 9th, 10th, or 11th rib. The selected rib is carefully dissected free from the intercostal neurovascular bundle on its underside. The OR table is flexed upward in its midpoint, enlarging the musculoskeletal portal. The peritoneal envelop is identified laterally and posteriorly and the dissection carried out as previously described for transperitoneal medial visceral rotation.
Klempnauer, J, Grothues, F, Bektas, H, Pichlmayr, R. Long-term results after surgery for acute mesenteric ischemia. Surgery. 1997 Mar;121(3):239–243.
Moawad, J, McKinsey, J, Wyble, CW. Current results of surgical therapy for chronic mesenteric ischemia. Arch Surg. 1997;132:613–646.
Schlidt, SA, Desai, TR, Gewertz, BL. Anatomy and physiology of the mesenteric circulation. In: Gewertz BL, ed. Surgery of the aorta and its branches. Philadelphia: Saunders; 2000:329–334.