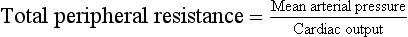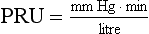EXERCISE AND THE CARDIOVASCULAR SYSTEM
Dynamic (isotonic) exercise
• an increase in stroke volume of the heart
• a modest increase in mean arterial blood pressure
• changes in the distribution of blood flow to support exercising muscles.
Increased extraction of oxygen from arterial blood (see Chapter 1) in exercising muscles will also contribute to the increase in oxygen delivery.
Initiation of the cardiovascular responses to exercise
Identification of the key physiological events at the initiation of dynamic exercise has long been contentious and is still the subject of debate, despite the existence of theoretical models for more than 100 years. Reflex control of the circulation normally relies on sensory information provided from baroreceptors and chemoreceptors (see Chapter 10). These same reflexes function during ongoing exercise, but mean arterial BP, Po2 and Pco2 do not change significantly at the start of exercise. The changes in cardiac function during exercise therefore occur too rapidly to be explained by activation of baroreceptor and chemoreceptor reflexes.
The most popular hypothesis used to explain the initiation of cardiovascular responses to exercise is the ‘feedforward’ or ‘central command’ theory. This suggests that the areas of the brain which drive muscle contraction at the start of exercise also produce the drive to increase heart rate and lung ventilation. This theory was first proposed in 1913 and numerous modifications have been suggested since then. The parts of the brain particularly involved in ‘central command’ are thought to be the motor cortex and the hypothalamus. The cerebellum, which has the major role in the coordination of muscle movement, is also involved in coordinating the cardiovascular response to exercise.
Areas within the medulla control the sympathetic and parasympathetic nerves which normally regulate heart rate and cardiac contractility as part of the baroreceptor reflex (see Chapter 10). At the start of exercise, the influence of central command over the medullary centres is to decrease vagal tone and to increase sympathetic drive, thus increasing heart rate. Part of this response may be an alteration in the operation of the baroreceptor reflex (see Chapter 10). Under normal conditions, this reflex keeps blood pressure relatively constant at a ‘set point’. During exercise, central command induced changes in this set point have the same effect as a blood pressure which is too low, hence leading to an increase in heart rate.
Reflex activation of the sympathetic nervous system also leads to peripheral vasoconstriction (see Chapter 9). This occurs particularly in the splanchnic and kidney circulations and in the vessels of non-contracting muscles. This helps to divert blood flow to active muscles. The central command mechanism is probably complemented by feedback from metabolite concentrations, particularly [K+], detected by chemoreceptors in contracting muscles and information from mechanoreceptors within moving joints and muscles.
Cardiovascular changes in sustained exercise
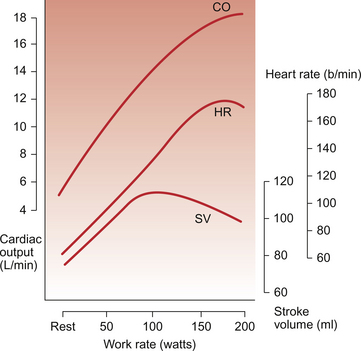
Cardiac output is a function of both heart rate and stroke volume. Figure 13.1 shows the effect of different intensities of dynamic exercise on cardiac output, heart rate and stroke volume in healthy young individuals. Intense exercise in trained athletes may require a fivefold increase in cardiac output. Changes in stroke volume reach maximum levels at a moderate exercise intensity (see below). Beyond this level, further increases in cardiac output are provided solely by increases in heart rate. Increased oxygen delivery to tissues is also achieved by increasing the extraction of oxygen from arterial blood (see Chapter 1). Thus, the arterial–venous oxygen content difference widens with increasing exercise intensity.
Maximum heart rate in intense exercise in a physically fit 20 year old ‘textbook person’ is 180–195 bpm. This figure changes with age so that maximum heart rate in a 10-year-old may be 210 bpm (see Chapter 12), whereas in a person aged 65 it is only about 165 bpm. Central command dominates heart rate control in mild exercise but, in more intense forms of exercise, autonomic reflexes (see Chapter 10) and the indirect effects of metabolites (see Chapter 9) generated in contracting muscles become increasingly important. Secretion of adrenaline (epinephrine) and noradrenaline (norepinephrine) from the adrenal medulla, and their effect on the sinoatrial node, also contributes to the increase in heart rate.
Stroke volume of the heart increases during dynamic exercise but reaches a maximum level at an exercise intensity of about half maximum O2 consumption (Fig. 13.1). The extent of changes in stroke volume appears to vary with the type of dynamic exercise and the physical fitness of the individual. In trained individuals there may be relatively greater changes in stroke volume at lower workloads. In Chapter 4 the factors determining the stroke volume of the heart are identified as:
Increased filling of the right ventricle (increased preload) occurs as a result of increased contraction of limb muscles and therefore increased venous return to the heart. A simple, but often neglected, concept is that increases in cardiac output can only be achieved if there is an equivalent increase in venous return of blood to the heart, you can’t get out what you don’t put in! Sympathetic nervous system activation during dynamic exercise will lead to venoconstriction which also increases preload on the heart. When resting in a supine position, because there is less effect of gravity on venous return from the lower part of the body, stroke volume is already higher than in an upright posture. The cardiac output increases which occur during exercise in a supine position (e.g. swimming) are achieved almost entirely as a result of an increase in heart rate because stroke volume is already approaching maximum levels at the start of exercise.
Contractility increases in the heart are associated with an increase in myocyte intracellular [Ca++] and lead to an increased force of ventricular contraction (see Chapter 4). Sympathetic nervous system activation in exercise and the release of adrenal medulla catecholamines will lead to increased contractility via activation of β-adrenoceptors. The modest increase in mean arterial blood pressure (Fig. 13.2) which normally occurs in dynamic exercise will impose an increased afterload on the heart and hence tend to moderate the preload and contractility induced increases in stroke volume outlined above.
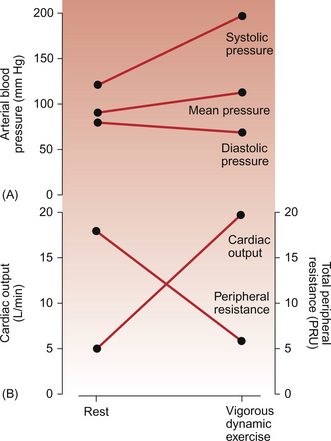
Compare the responses to dynamic exercise shown in this figure with those for static exercise (Fig. 13.5).
Changes in arterial blood pressure during dynamic exercise are summarized in Figure 13.2. The increase in systolic pressure reflects the increased force of ventricular contraction during exercise. Diastolic pressure reflects a combination of changes in resistance to blood flow, particularly through the dilated blood vessels in exercising muscles, and the increase in heart rate. This point needs some further explanation. Metabolite-induced vasodilatation in exercising muscles leads to a decrease in the resistance to blood flow (Fig. 13.2). During diastole, therefore, blood will flow out of the aorta more easily and so the phase of the arterial pressure curve which corresponds to diastole will become steeper. Diastole is terminated at the next time the aortic valve opens. As heart rate increases in exercise this point will be reached more quickly and the pressure fall during diastole will be ended when the next heart beat starts. Overall, mean arterial blood pressure is still mainly regulated by the baroreceptor reflex.
Distribution of blood flow in exercise
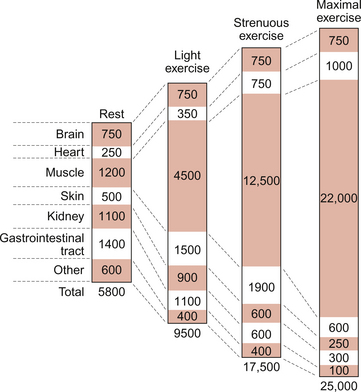
The distribution of blood flow during dynamic exercise is summarized in Figure 13.3. At rest, 20–25% of resting cardiac output is distributed to the muscles, a flow rate of the order of 1 L/min. During maximal exercise about 90% of the increased cardiac output goes to the muscles, a muscle blood flow rate of perhaps 22 L/min. The changes in cardiac output during exercise are discussed above but it must also be remembered that the total peripheral resistance during maximum dynamic exercise is of the order of one third of the resting resistance (Fig. 13.2). Two opposing mechanisms produce a redistribution of blood flow during exercise. In exercising muscles the increased metabolic rate leads to metabolite-induced vasodilatation (see Chapter 9). There is also activation of the sympathetic nervous system during exercise which causes α-receptor-mediated vasoconstriction. In exercising muscles the metabolite vasodilatation overwhelms the effect of the sympathetic vasoconstriction response leading overall to vasodilatation. In circuits such as the kidneys and splanchnic circulation, where there is no exercise-induced metabolite vasodilatation, sympathetically driven vasoconstriction dominates and blood flow is lower than resting levels (Fig. 13.3).
Plasma renin activity, and therefore generation of the vasoconstrictor angiotensin II (see Chapter 9), also increases during exercise and is thought to contribute to the changes in blood flow in the abdominal viscera. However, studies using angiotensin converting enzyme inhibitor drugs, which block the formation of angiotensin II, have shown no effect on maximum oxygen uptake or on exercise performance, suggesting that activation of the renin-angiotensin system is not a critical factor in exercise responses.
Blood vessels in the brain have little α-adrenoceptor-linked vasoconstrictor tone and so brain blood flow does not change during exercise. The myocardium also has little neural vasoconstrictor tone and so metabolite-induced vasodilatation predominates in the heart. Coronary blood flow therefore relates directly to work done by the cardiac muscle. Redistribution of blood flow during exercise throughout the body is also aided by the vasodilator effects of nitric oxide produced by vascular endothelial cells (see Chapter 9). Increased blood flow along a blood vessel leads to increased shear stress between the blood and the endothelial cells. This is a trigger for the release of the vasodilator nitric oxide produced by constitutive enzymes in the endothelium.
The skin has a particular role to play in thermoregulation. Heat generated in exercise is dissipated through increased skin blood flow (see Chapter 9). In moderate exercise, skin blood flow increases three to four fold. In intense exercise, cutaneous vasoconstriction occurs (Fig. 13.3). This has the effect of diverting more blood to exercising muscles but the corollary is that body temperature must rise.
The net effect of all these changes is that muscle blood flow can increase 20 fold during intense exercise (Fig. 13.3).
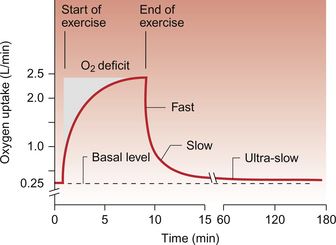
Oxygen debt and the recovery from exercise
As there is a short lag period between the onset of exercise and the development of the full cardiorespiratory response to that level of exercise, an ‘oxygen debt’ occurs (Fig. 13.4). This oxygen debt has three chemically identifiable components:
1. A decrease in cellular [ATP] as it is used to drive muscle movement.
2. Depletion of creatine-phosphate stored within cells as an immediate mechanism for rephosphorylating ADP to ATP (muscle cells contain about four to five times as much creatine-phosphate as ATP).
3. Accumulation of lactic acid in muscles. It has been produced anaerobically and cannot be converted back to pyruvate so that it can enter mitochondria and be metabolized aerobically until sufficient supplies of oxygen are available.
Recovery from exercise has three phases. The ‘fast’ phase (Fig. 13.4) represents the rephosphorylation of ADP and creatine stores within the muscle. Following vigorous exercise the half-life for this phase is about 30 seconds. During the ‘slow’ phase, which has a half-life of about 15 min, lactate is resynthesized into glucose and then glycogen. The ultra-slow phase of recovery from exercise lasts for several hours. It is caused by a rise in body temperature during exercise which produces an increased metabolic rate and hence an increase in oxygen consumption. The extent of the oxygen debt in any given individual will of course reflect the intensity of exercise and how well the cardiovascular and respiratory systems function.
Cardiovascular responses to static exercise
Static exercise involves the sustained development of muscle tension. In maximum static exercise the energy expenditure (oxygen consumption) is relatively small compared to maximum dynamic exercise. This is illustrated by the fact that a weightlifter, unlike a runner, is not very obviously breathless after completing a lift. There are however considerable effects of static exercise on the cardiovascular system. The changes in heart rate are less marked than in dynamic exercise but there is a reflexly induced increase in diastolic as well as systolic blood pressure. An increase in cardiac output, driven by ‘central command’, is not matched by the extensive metabolite induced vasodilatation found in dynamic exercise so diastolic blood pressure increases (Fig. 13.5).
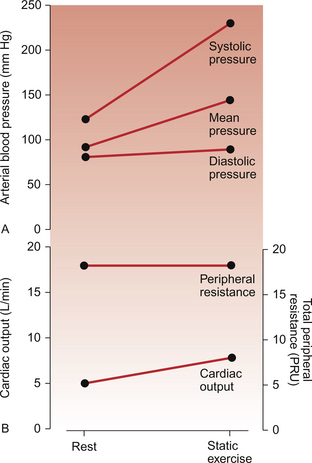
Fig. 13.5 Effects of vigorous static exercise. Typical effect of vigorous static exercise on cardiac output, total peripheral resistance and arterial blood pressure shown as systolic, diastolic and mean pressures. The units for total peripheral resistance (PRU) are defined in the legend to Figure 13.2. Compare the responses to dynamic exercise (Fig. 13.2) to those for static exercise shown in this figure.
Two factors limit the increase in stroke volume during static exercise. Any whole body straining exercise associated with breath holding and closure of the glottis will result in an increase in intrathoracic pressure. This will tend to compress the inferior vena cava and reduce venous return to the heart. Such a series of changes in the cardiovascular system are known as the Valsalva manoeuvre (described on p. 157). Flow of blood through exercising muscles is also limited by the sustained muscle contraction. The second factor limiting stroke volume in static exercise is the increase in arterial blood pressure (Fig. 13.5) which will impose an afterload on the heart (see Chapter 4). Very substantial increases in mean arterial pressure occur during maximal isometric exercise. These occur particularly with arm exercise and so are not just related to changes in venous return. Chemoreceptor reflexes originating within the arm muscles are thought to be important in this event.
Valsalva manoeuvre
Phase I: a sudden rise in arterial pressure associated with the increased intrathoracic pressure. A small decrease in heart rate occurs.
Phase II: the sustained high intrathoracic pressure compresses the vena cava hence leading to reduced venous return and a fall in cardiac output. Arterial pressure starts to fall. The baroreceptor reflex therefore produces a tachycardia.
Phase III: when the straining response ends and intrathoracic pressure decreases there is, transiently, a further small rise in heart rate.
Phase IV: as venous return is no longer impeded, venous blood, which has been pooled in the lower part of the body, surges back to the heart resulting in a rise in cardiac output and arterial blood pressure. The consequence is a reflex bradycardia before the circulatory control mechanisms return to their normal resting state.
Training effects of exercise
The nature of training effects achieved depends on the type of training undertaken. This might be based on a dynamic exercise programme (also called isotonic, aerobic or endurance training) or a static exercise schedule (isometric, strength or power training). With dynamic exercise training there is an increase in the size of the chambers of the heart and an increase in both resting stroke volume and ejection fraction (see Chapter 4). Static exercise training leads to an increase in the thickness of the left ventricle wall and, as a consequence, a decrease in stroke volume and ejection fraction. This form of training therefore brings few benefits in cardiovascular performance.
A lower peripheral resistance in the trained athlete will tend to reduce arterial blood pressure and hence decrease cardiac afterload. A sustained reduction in arterial blood pressure means that there has been a resetting of the baroreceptor reflex (see Chapter 10). The site where this change occurs is thought to be the cardiovascular control centres in the caudal hypothalamus.
Cardiovascular health benefits of exercise
The mechanisms behind these beneficial effects of exercise are not entirely clear but they probably include a decrease in atherogenesis (see Chapter 8) or an improvement in the heart’s ability to tolerate the consequences of ischaemia. This may be manifested by a decrease in thrombotic or arrhythmic events in the heart. After a period of exercise, blood pressure in hypertensive subjects is reduced for several hours. This appears to be associated with a resetting of the baroreceptor reflex.
Clinical uses of exercise testing
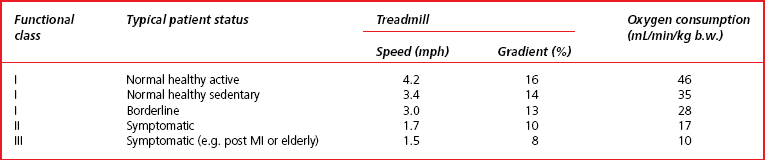
The patient exercises on either a treadmill or stationary exercise bicycle. The exercise level is progressively increased through a series of standardized stages until a level is reached at which the test is terminated. One widely used protocol based on a treadmill test is the Bruce test. The workload is increased every 3 minutes. Table 13.1 summarizes the different stages of the test. The lowest level of exercise (functional class III) is set at a level of oxygen consumption which is about three times resting levels whereas the highest level of the test is about 13 times normal resting oxygen consumption. A patient with a maximum work capacity of only 17 mL O2/min/kg b.w. (functional class II) would have a reduced exercise tolerance for normal daily activities. During the test the patient is continually observed and questioned about developing symptoms of angina and breathlessness. Arterial blood pressure is regularly recorded together with ECG and heart rate.
• duration and extent of exercise capability
• extent of upward or downward deflection of the ST segment of the ECG—these changes are usually myocardial ischaemia related
As exercise testing is a potentially hazardous procedure detailed guidelines (Box 13.1) have been published which stipulate contraindications for starting an exercise test and for terminating a test. The examples shown in these two tables have been selected from the much more extensive guidelines published in 2002 by an American College of Cardiology and American Heart Association (ACC/AHA) task force.
Astrand, P. -O., Rodahl, K., Dahl, H. A., Strømme, S. B. Textbook of Work Physiology, fourth ed. Champaign, IL: Human Kinetics; 2003.
Gibbons, R. J., Balady, G. J., Bricker, J. T., et al. ACC/AHA 2002. Guideline update for exercise testing: summary article. Circulation. 2002; 106:1883–1892.
Herd, J. A. Cardiovascular response to stress. Physiol. Rev.. 1991; 71:305–326.
Jones, J. H., Linstedt, S. L. Limits to maximal performance. Annu. Rev. Physiol.. 1993; 55:547–569.
McArdle, W. D., Katch, F. I., Katch, V. L. Exercise Physiology: Energy, Nutrition and Human Performance, sixth ed. Baltimore: Lippincott/Williams and Wilkins; 2006.
Robergs, R. A., Keteyian, S. J. Exercise Physiology, second ed. New York: McGraw-Hill; 2003.
Waldrop, T. G., Eldridge, F. L., Iwamoto, G. A., Mitchell, J. H. Central Neural Control of Respiration and Circulation During Exercise, American Handbook of Physiology Section 12: Exercise: Regulation and Integration of Multiple Systems. Bethesda, MD: American Physiological Society; 1996.
Wilmore, J. H., Costill, D. L., Kenny, W. L. Physiology of Sport and Exercise, fourth ed. Champaign, IL: Human Kinetics; 2007.