CHAPTER 7 EXECUTIVE FUNCTION AND ITS ASSESSMENT
EXECUTIVE FUNCTION
The dysexecutive syndrome has long been exemplified by the classic case of Phineas Gage, whose prefrontal damage from a railway construction accident in 1848 completely altered his personality and work performance.1 A more contemporary exemplar, with the advantage of modern neuroimaging and neuropsychological assessment, is that of E.V.R., reported by Eslinger and Damasio.2 E.V.R. was a successful young accountant who underwent surgical removal of a large orbitofrontal meningioma. His postoperative course illustrates the potentially far-reaching and devastating consequences of executive function impairment:
Characterization of Executive Function
Executive function can be understood as the skills that allow humans to solve problems; adapt effectively and flexibly to their environment; and plan, perform, and evaluate goal-directed intentions, such as making a timely financial investment. Correspondingly, executive dysfunction can lead to deficits in the generation and initiation of appropriate behavior, limitations in cognitive flexibility and reasoning skills, and impairments in social judgement.3–10 The breadth of coverage in these descriptions immediately signals the complexity of the processes involved and, not surprisingly, the difficulty that researchers have encountered in formulating adequate and comprehensive explanatory models of these behaviors.
Notwithstanding this challenge, executive function continues to be a focus of interest for both researchers and clinicians, because the concept provides a description of humans’ adaptability to their environment and assistance in developing expectations and predictions of relevance to (1) differential diagnosis in clinical evaluations (for example, in delineating the syndromes of the frontotemporal dementias; see Chapter 74); (2) anticipating dysfunctional behavior of patients in everyday settings11; and (3) estimating decision-making capacity in everyday roles (for example, capacity for handling financial responsibilities12). Understandably, assessment of executive function has become a core component of most neuropsychological assessments.13
Neuropsychological Models of Executive Function
Neuropsychological approaches to conceptualizing a model of executive function are varied. Banich4 provided a helpful roadmap to several of the approaches, including working memory, the supervisory attentional system, script knowledge, and goal-directed behavior. A brief description of two of the major theories follows.
Working Memory
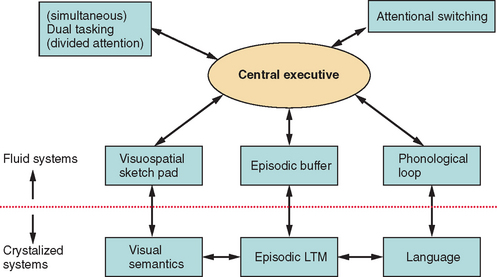
Baddeley’s working memory model14 (Fig. 7-1) consists of multiple specialized and interlinked components of cognition that allow humans to (1) mentally represent their immediate environment, (2) retain information online (available in consciousness in an ongoing manner) to enable acquisition of new knowledge, and (3) formulate and act on current goals, through both an attentional control system—the central executive—and several specialized temporary storage systems, which are slave systems to the central executive, such as the articulatory loop and the visuospatial scratch pad.15 Working memory provides online cognition (manipulation of data in consciousness) that allows a reasoned response to complex tasks.
Research since the 1990s has consistently supported the proposition that the central executive can be fractionated, or, as Baddeley persuasively commented, skills can be governed by an “executive committee” rather than a “homunculus.”16 There have been various attempts at defining the breadth and nature of executive functions.16–18 However, most taxonomies include focusing attention and inhibiting distraction, coordinating and dividing attention, switching attention, planning, and activating and generating representations drawn from long-term memory via an episodic buffer. Impairments in working memory can result in many of the core features of executive dysfunction, such as inability to maintain task focus as a result of susceptibility to distraction, or inability to perform two or more tasks simultaneously (multitasking). (An example of such multitasking would be simultaneously monitoring young children playing in a swimming pool and cooking a meal, while speaking on a mobile phone.)
Working memory, therefore, can provide a comprehensive account of many features of executive dysfunction. Furthermore, the executive aspects of working memory have been closely associated with the prefrontal regions, especially the dorsolateral prefrontal cortex.19
Supervisory Attentional System
Within this model, the emphasis is on the role of attentional control (executive function) in everyday actions. Shallice20,21 provided a two-layer model of an attention system that influences behavior: contention scheduling (automatic processing) and the supervisory attentional system (controlled processing). Contention scheduling allows fast automatic execution of well-learned action sequences. This may be sufficient for many everyday tasks, but it is also prone to error as it operates under minimal conscious supervision. For example, making a cup of coffee can become relatively automatic, but sometimes when a person is tired or distracted, he or she may unintentionally pour milk into the coffee pot instead of into the cup. In contrast, the supervisory attentional system is activated when conscious effort is required: for example, in situations of novelty or crisis or when new skills are learned. An impaired supervisory attentional system and an unmonitored contention scheduling system can account for many of the qualitative features of executive dysfunction, such as perseveration (failing to cease an ongoing behavior when it is no longer appropriate).
Contention scheduling and the supervisory attentional system have been proposed to be operated by distinct neural substrates. Specifically, the supervisory attentional system is associated with the prefrontal cortex.22 Initially, the supervisory attentional system was conceptualized as a unitary construct, but more recently, Stuss and Alexander argued for a multicomponent supervisory attentional system within which specific processes interlink with specific neural substrates of the frontal cortical-subcortical neural network.10 Furthermore, Stuss and Alexander cautioned against a simple conceptualization of supervisory attentional control. They emphasized that there are many types and levels of attentional control of behavior (e.g., Fig. 7-2) and that the concept of a simple frontal/posterior dissociation related to control/automatic processes would not adequately capture the complexity of linkage of the system to particular neural substrates. They thereby concurred with other authorities that executive function fractionates into various subordinate roles important for goal-directed behavior.
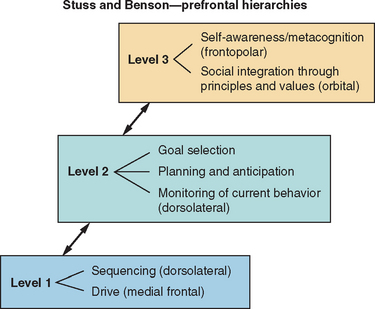
Figure 7-2 Diagrammatic representation of model of executive function.
(From: Stuss DT, Alexander MP, Benson DF. Frontal Lobe Functions. In Trimble MR, Cummings JL, eds: Contemporary Behavioral Neurology. Boston: Butterworth Heinemann, 1996, pp 169-187.)
Although varied, existing theories of executive function are not necessarily mutually exclusive. Within most models, there is recognition of a multicomponent executive function system subserving multiple roles. Furthermore, although executive function disorders are more commonly observed with frontal system dysfunction, most researchers argue that it would be overly simplistic to reduce executive function to a concept of frontal lobe disorder; instead, executive skills undoubtedly rely on networks of interactive systems.10
Neuroanatomical Substrates of Executive Functioning
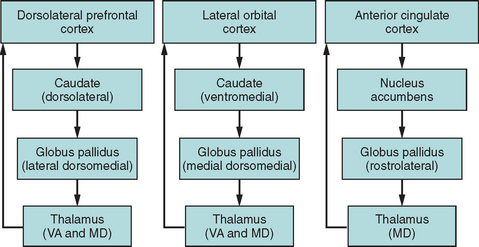
Functional imaging studies have confirmed that complex behaviors such as executive functions are subserved by networks of interconnected brain regions rather than by discrete cortical areas (Fig. 7-3), and a series of parallel frontal-subcortical circuits that link specific regions of the frontal cortex to the striatum, globus pallidus, and thalamus have been described.23,24 Miller and Cummings provided an extensive model of five defined frontal-subcortical circuits,25 of which the dorsolateral prefrontal, orbital frontal, and anterior cingulate circuits are purported to subserve cognitive and behavioral aspects of executive function.26 A corollary of this network structure is that interruption of such circuits outside the frontal cortex leads to many of the classic features of “frontal” dysfunction. For example, infarcts in the caudate head may disturb planning and sequencing, with disinhibition or apathy, depending on which part of the caudate—and therefore which circuit—is disrupted (e.g., see Mendez et al27). Progressive supranuclear palsy is another example of a disorder whose pathological effect is subcortical rather than on the prefrontal cortex and yet that is characterized by a prominent dysexecutive syndrome on the basis of disruption of these circuits.28
Orbitofrontal Circuit
The orbitofrontal circuit is important for inhibition, and changes in this control mechanism can, in a variety of ways, affect the behavioral response to environmental and social demands.24,26 Affected patients have been described as acting impulsively, exhibiting emotional and socially inappropriate behavior, and being liable to increased distractibility.29 In severe instances, dysfunction can result in the phenomenon of utilization behavior, in which patients become devastatingly stimulus-bound to environmental cues.30 The orbitofrontal network serves as a braking mechanism to stop automatic responding and allows for a flexible approach to environmental manipulation.
Anterior Cingulate Circuit
The anterior cingulate circuit is medial in location and has been implicated in resistance to interference (including cognitive inhibition of automatic responses) and in response initiation.31 Cummings24 described the characteristic syndrome as that of apathy, the most extreme form being of akinetic mutism: a profound indifference to the environment in which patients lack any internally generated activity or behavior (see Chapter 9).
Dorsolateral Prefrontal Circuit
Impairment of the dorsolateral prefrontal circuit is argued to contribute to many of the observed cognitive features of executive dysfunction that affect cognitive flexibility or attentional switching and the formulation of novel ideas and responses. Deficits include impairments in self-generated behavioral (motor or cognitive) planning, maintenance of cognitive set and set switching,9 and manipulation of working memory information online.32 Patients with dorsolateral lesions are frequently described as displaying impaired mental flexibility and poor reasoning.
Information Flow to the Frontal Cortex
A complementary view of executive functioning and the prefrontal cortex is that the dorsolateral prefrontal cortex sits at the apex of information flow from the external milieu (the subject’s interaction with the external environment), after that information has been processed through unimodal and then multimodal association cortices, and is able to modulate and select responses to this information. The orbitofrontal and mesial prefrontal cortices, in contrast, receive information on the subject’s internal milieu, including needs and drives, and modulate and select responses to these stimuli.33,34
Therefore, although there is continuing debate on a comprehensive taxonomy of executive processes, most researchers agree that executive function is a fractionated functional system that relies on differentiated underlying neural regions and pathways.
FORMAL ASSESSMENT OF EXECUTIVE FUNCTION
Common Problems Affecting Executive Function Tests
Most clinicians are familiar with the frustrating situation in which patients with executive dysfunction have a significant discrepancy between the reasonably average results of formal testing procedures and major difficulties in real-life behavior.6 Sometimes this may relate to the following aspects of the testing process.
Repeated Testing Can Lead to Marked Practice Effects
All executive function tests suffer from practice effects. Although parallel test versions may be available to obviate direct learning effects of the test material (for example, alternate letters in verbal fluency), procedural learning (implicit learning that can improve performance simply by performance of a task more than once) is common in executive function tests. This may reflect the fact that the tasks are designed to be novel and cognitively engaging and the fact that the participant will actively attempt to rehearse strategies to make the task easier. With repeated exposure, a previously novel task therefore becomes automatic.35 This represents a major difficulty in longitudinal studies.
Testing in the Office Is Not the Same as Real-Life Situations
In formal testing procedures, most typically conducted within a quiet office environment, distractions are minimized, and tasks are often carefully structured to increase the reliability of the test. However, these artificial constraints may substitute for the patient’s defective executive system.36 Furthermore, test procedures can be relatively brief episodes, so that persistent and sustained attention to a task is rarely assessed thoroughly, and demand for multitasking is low.6,13,37 Unfortunately, these constraints of office testing can prevent the demonstration of the essential features of executive dysfunction. To improve the ecological validity of the assessment, formalized versions of real-world activities in which patients are assessed in naturalistic settings (e.g., Shallice and Burgess’s shopping center task9) have been devised; although these approaches have yielded highly informative results for guiding rehabilitation,38 they are generally impractical for a busy hospital-based assessment clinic.
Assessment of the Affective, Social, and Judgmental Changes of Executive Dysfunction Are Not Well Covered by Existing Tests
Alterations in emotional and social behavior are important components of the executive syndrome, to the extent that Stuss and Alexander argued that social behavioral changes represent the most disabling aspect of the disorder.10 Performance on many tests can be undermined by apathy, disinhibition, or other features of lack of emotional control. However, formalized approaches to the documentation of these key characteristics are limited, although research measures of social cognition, such as the faux pas test,39 or of judgment, such as the gambling game,40 show promise.
Level of Premorbid Ability Is Important when Executive Function Is Assessed
It is important to evaluate executive function within the context of the estimated overall premorbid ability of the individual; that is, a below-average score on a test of executive function may be significant if the patient was of above-average premorbid ability, but it would be less suspect if previous general ability was estimated to lie in the below-average range. Furthermore, researchers41,42 have noted that tests of executive function are frequently correlated with general cognitive ability in healthy populations, and this should be considered when individual performances in tests of executive skills are evaluated.
Standard Neuropsychological Tests Commonly Used in Assessing Executive Function
Test Batteries or Individual Tests for Executive Function?
There are now several test batteries of executive function, including the Behavioural Assessment of the Dysexecutive Syndrome (BADS),43 the Behavioral Dyscontrol Scale,44 and the Delis-Kaplan Executive Function Scale (D-KEFS).45 The D-KEFS is especially useful because of its reasonably large normative sample and the provision of multiple standardized tests in which the same outcome scale is used for comparison purposes. These batteries should be considered for use, because they allow comprehensive assessment of executive function; however, in many clinical examinations, time is limited and administration of a full test battery cannot be easily accommodated. Consequently, the neuropsychologist is faced with the decision of individual test selection.
Consideration of the cognitive and neural models of executive function, and of reports of the patient’s particular difficulties, is essential to guide the examiner in determining which aspects of the fractionated roles of executive function are critical in the assessment. The following sections summarize some of the neuropsychological tests commonly used to assess executive function. The listing is by no means exhaustive, and for more information on these and other related tests, the reader is referred to Lezak and colleagues (2004). The listing of the tests within separate domains of executive function is also debatable16,17 and is provided simply as a guide to the reader. As stated earlier, neuropsychological tests of executive function tend to be multifactorial, which precludes a simple description of the underlying properties of the tests.
Focusing Attention and Inhibiting Distraction
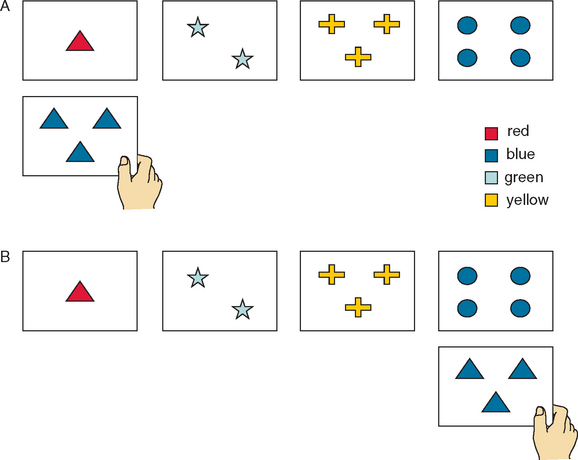
An important feature of executive function is the ability to maintain attention on task and to resist interference from distracting events or thoughts. The Stroop Test46–49 provides a classic paradigm for assessing capacity to resist interference from an automatic process (in this instance, reading words: the names of colors) on a more effortful process (identifying the colors of the ink in which the words are printed). There are multiple versions of this test (including the Color-Word interference subtest from the D-KEFS), but in the basic task, participants are timed in (1) reading aloud words that are the names of colors; (2) naming ink patches of color; and (3) naming the color of the ink in which incongruent-color words are printed: for example, stating “blue” when the word “red” is printed in blue letters.46 The increased time taken to complete this final interference trial provides an index of the capacities to focus attention on the appropriate stimulus and to inhibit distraction from the more automatic response of reading the color word (cognitive inhibition). Excessive slowing or an increase in errors on the interference trial provides indication of diminished ability to inhibit inappropriate responses (Fig. 7-4). The test is relatively quick to administer (most versions take approximately 8 minutes), and scoring is by time or error on each trial. A substantial amount of cognitive neuropsychological research50 and a number of neuroimaging studies have confirmed the role of executive function and of the prefrontal regions of the brain—specifically, the anterior cingulate/mesiofrontal circuit—in task performance.51,52
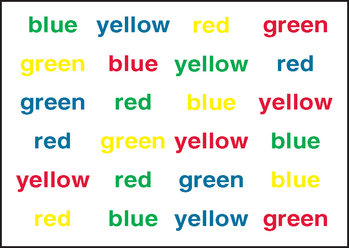
Figure 7-4 Interference trial of the Stroop test (Victoria version).
(From Spreen O, Strauss E: A Compendium of Neuropsychological Tests: Administration, Norms and Commentary, 2nd ed. New York: Oxford University Press, 1998.)
Satisfactory test-retest reliability has been reported for different test versions;6,53 for example, Houx and associates reported high test-retest reliability (r = .80) within a multicenter study of 5804 older adults.35 However, practice effects, as observed in most executive measures, are apparent. Houx and associates reported that speed of performance on the interference trial improved by approximately 5 seconds between baseline and reassessment 2 weeks later, and they argued that procedural learning in the Stroop test has to be taken into account when the course of executive function in patients is monitored over time. Impaired visual acuity or severe color blindness precludes use of the test, but it has been found to be sensitive to executive dysfunction in a range of clinical populations, including patients with traumatic brain injury54 and mildly and moderately demented patients.55 In both of these populations and in patients with Huntington’s disease,56 however, increasing severity of disorder results in increased generalized slowing of response, so that the specific Stroop effect diminishes.55,57 More recent versions of the test have increased its complexity by adding an additional switching trial to the basic color naming, word reading, and color-word interference trials.54,58,45,54 In the switching trial, participants are required to name the color of the ink as per the traditional color-word interference trial but to switch to reading the color word of any items enclosed by a rectangle, randomly positioned throughout the trial. This addition provides increased sensitivity for the identification of mild impairment in executive function.
Set-Shifting and Cognitive Flexibility
The ability to shift attention readily between different cognitive tasks (cognitive flexibility) is an important feature of adaptive behavior.16,58 One of the most popular tests of set-shifting is the Trail Making Test (TMT)59,60 which consists of two parts: the Trail Making Test Part A (TMT-A) and the Trail Making Test Part B (TMT-B). The TMT-A is a timed trial that requires participants to draw lines to interconnect 25 consecutively numbered circles. The TMT-B is also timed and requires participants to interconnect consecutive numbers and letters, alternating between the two sequences (i.e., 1-A-2-B-3-C-4-D …. L-13). Scoring is based on time to complete each trial (errors being reflected in the time score) and derived scores of (1) the difference in time to complete the two sections (TMT-B score minus TMT-A score) and (2) the ratio of TMT-B score to TMT-A score. The derived scores provide the advantage of removing the individual variance in speed of response before set-shifting capacity is calculated. These derived scores, as well as time taken to complete TMT-B, have frequently been used as indices of cognitive flexibility or set-shifting.61–66 The derived scores have been shown in some reports of TMT performance to provide better correlations with other measures of cognitive flexibility,61 and neuroimaging research has provided support for the critical role of the dorsolateral and medial frontal cortices in the regulation of cognitive flexibility and set-shifting, as required in the TMT.67
The TMT is quick to administer (approximately 4 to 6 minutes), but additional difficulties in visual scanning and motor control can compromise performance. To address this, the TMT subtest in the D-KEFS includes three extra conditions that allow the contributions of visual sequencing and motor speed to be evaluated more thoroughly.45 Because the TMT is predicated on familiarity with the English alphabet, the Color Trails Test68 was developed to provide a nonalphabetical parallel form for use in cross-cultural studies or clinical settings, although only limited normative data are available.69,70
Satisfactory test-retest reliability is reported for the TMT, with many reported coefficients higher than .80 and all exceeding .60.6,53 However, practice effects are again noticeable, especially on the TMT-A, and need to be considered when performance over time is evaluated. A large normative study of 911 participants aged 18 to 89 years has provided useful information on the TMT-A and TMT-B and on the effects of age and education on test performance,71 and this can be supplemented by normative information on the derived scores from a smaller study.72
The TMT has been demonstrated to be sensitive to cognitive impairment in a range of clinical conditions, including Parkinson’s disease,73,74 Alzheimer’s disease,75,76 and other dementias.77–81
The WCST82,83 is the most extensively characterized test of executive function. Although multifactorial, it is frequently described as a task of attentional set-shifting.17,84 The test requires the client to sort a set of cards on the basis of several different characteristics: color, form, and number. Clients are not given instructions on how to sort the cards but must infer the correct method of sorting from the examiner’s mention of “Correct” or “Incorrect” in response to their previous sorting attempts (Fig. 7-5). After a series of consecutive successful sorts, the sorting principle changes without notice, and the participant must both discern that the rule has changed and discover the new criterion for sorting. Scoring includes the number of correct categories achieved and the number of perseverative errors (an error of sorting within a category that was formerly correct but is no longer appropriate: that is, a failure in ability to switch response according to task demands).
The WCST has a number of drawbacks for consideration in the clinic.85 First, administration time is lengthy (approximately 30 to 45 minutes). In response to this limitation, a short form of the WCST has been developed (Modified Wisconsin Card Sorting Test86). A further limitation is that although the WCST was initially reported to be sensitive to dysfunction of the dorsolateral prefrontal cortex, especially on the left,87,88 researchers have more recently criticized the test for failing to discriminate between frontal and nonfrontal brain injury patients.89–91 This lack of specificity may result from the complexity of the task; failure on the WCST can arise as a result of a range of different deficits.29 A further problem for clinical use of the WCST is that performance is particularly prone to practice effects once the subject gains an appreciation of its principles; on the basis of results from a 3-year study of cognitive disorder in Huntington’s disease, Snowden and colleagues suggested that the WCST has limited use in longitudinal studies, although its use in cross-sectional studies remains important.56 Nevertheless, the WCST has been found to be a modest predictor of everyday functional ability after discharge from acute rehabilitation.92
Coordinating the Performance of Multiple Tasks (Dual Tasking)
Performing two tasks simultaneously requires dividing attention between the two tasks, coordination of attention, and ongoing monitoring of the effectiveness of performance.93 This skill, termed dual-tasking, provides another avenue to assessment of executive attention.14,17 A commonly used paradigm in dualtask research requires verbal performance of digit span at the same time as a paper and pencil tracking task; dualtask capacity is indexed by comparing performance level in each single task with performance of both tasks under dualtask conditions.93 Within such a paradigm, dualtask impairment has distinguished normal older adults from those with early Alzheimers’s disease94 and from older adults with nonspecific cognitive impairment.95 In a study of patients with severe head injury, Alderman found that a large dualtask decrement was associated with a poor response to behavioral intervention.96 However, as yet, dualtask paradigms have been restricted to clinical research procedures, and their adoption into routine clinical assessment will be dependent on the development of standardized tests and normative databases. In this regard, a new battery of dualtask measures may prove useful.97
Strategically Activating Information from Long-Term Memory and Manipulating Information Online
Extensive neuropsychological research has linked verbal fluency performance to executive functioning.98 Verbal fluency tasks require generation of words, usually for 60 to 90 seconds, based on either phonemic (letter fluency) or semantic (category fluency) criteria. A nonverbal analog, design fluency, has been developed and is included in the D-KEFS battery.44 Probably the best known verbal fluency task is the Controlled Oral Word Association test,99 consisting of three 1-minute trials of generating words beginning with the letters F, A, and S (or C, F, and L or with P, R and W). Scoring is based on total words generated within the time limit and is adjusted for age, gender, and education.
Phonemic fluency has been argued to be an effortful task, requiring recruitment of executive function, because retrieving words on the basis of orthographic criteria (spelling) is unusual: People normally retrieve words on the basis of their meaning.100 In contrast, semantic fluency is considered less effortful, although patients with early Alzheimer’s disease have been reported to demonstrate more difficulty with semantic fluency than with phonemic fluency, presumably as a function of impaired semantic memory caused by early involvement of the temporal neocortex.101 However, contrary to early conceptualizations concerning differential performance on phonemic and semantic fluency,102 Henry and Crawford demonstrated through meta-analysis that both forms of fluency are equivalent in sensitivity to frontal lesions, which suggests that both draw on resources of executive processes, including initiation, efficient organization of verbal retrieval and recall, and self-monitoring.103 However, semantic fluency is also sensitive to temporal lobe lesions, which suggests that impaired semantic fluency may be a result of either executive or temporal dysfunction.
Research has also confirmed that set-shifting ability contributes to verbal fluency by allowing active strategic search of relevant retrieval cues for generating words (e.g., “ship, sailor, sea …”; “soap, shower, shampoo …”),104–106 and this has promoted additional scoring measures: the number of subcategory switches and the cluster size of individual groups of words. Qualitative aspects, such as production of socially inappropriate words or rule breaking by producing proper nouns despite being able to state that these are not allowed, are important additional observations. (The latter is an example of what Walsh termed “the curious dichotomy between knowing and doing” that typifies dysexecutive behavior.107)
Neuroimaging studies identify significant activation of the left dorsolateral prefrontal cortex (or its associated network) and the left thalamic nucleus during verbal fluency tasks.108,109 A variety of populations with frontal damage, including patients with many varieties of cortical and subcortical dementia, demonstrate reduced fluency,110 although the underlying neuropsychological impairment causing the reduced fluency varies.111
An advantage of verbal fluency tests is that they are quick to administer (approximately 3 to 8 minutes). However, several variables need to be considered when performance on verbal fluency tasks is interpreted, including (1) the presence of aphasia and (2) premorbid verbal ability112 and educational and vocational achievements,46 which are correlated with fluency performance.
Planning and Hypothesis Generation
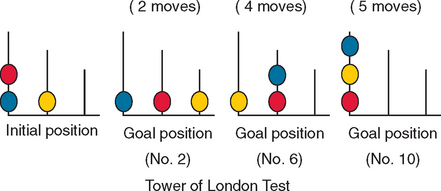
A further group of tests, such as the Zoo Map test from the BADS,43 require planning and capacity for maintaining goal-directed behavior through dependence on rule adherence. Another of a number of such instruments is the Tower of London task, which requires participants to solve increasingly more difficult spatial problems by planning several moves ahead to resolve the problems in the minimum number of moves.20 The participant is provided with a starting array of different colored beads placed on three pegs (initial position). The task is to move the beads, according to certain rules, across the pegs to achieve a target configuration; the target configurations become increasingly complex, requiring an increasing number of bead moves (Fig. 7-6). Performance is measured in accuracy, latency to initial move, and total time to completion. A computerized version of the task also exists.113 Patients with frontostriatal dysfunction tend to make more rule-breaking errors and perform poorly on the task, often being unable to find solutions to the more complex problems.9,114,115 Although the Tower of London task has been used frequently in research on executive dysfunction, concerns about its reliability116 may limit its use in clinical settings. However, there is some evidence that difficulty in thinking ahead (forming plans of action/goal-directed behavior) during the test may reflect everyday behavioral problems.9
Self-Reports and Informant Reports of Everyday and Emotional Behavior
Collateral information in the assessment of executive functioning is vital, because significant executive dysfunction invariably impairs the capacity of affected patients to gauge the effects of their actions on other people, and awareness of social performance in everyday life is frequently observed to be diminished.13 Systematic reports gathered from friends and relatives assist in clarifying the impact of executive dysfunction on everyday functioning and behavior. To this end, the Dysexecutive Questionnaire43 has been developed as a 20-item self-report and informant report on the difficulties related to executive dysfunction in everyday life. Preliminary analysis identified three factors in the questionnaire (behavior, cognition, and emotion), and a moderately high correlation was established between tests of executive functions (BADS43) and the factor scores derived from informant (family) ratings. Although some other groups were unable to replicate these relationships,117,118 Chan subsequently identified a five-factor structure within a healthy community sample and reported correlations between these Dysexecutive Questionnaire factors and tests of executive function.119 These results were supported and extended in a later study of patients with traumatic brain injury.120 The Dysexecutive Questionnaire, when completed by a health professional, has also been reported to be sensitive to executive dysfunction that follows traumatic brain injury (although it is less accurate when completed by family or patient rating), and it has been suggested that it can be used effectively as a screening instrument to identify executive disorder, through this method of administration.121
Another assessment based on a structured interview of an informant is the Neuropsychiatric Inventory.122 This 10-domain scale was initially developed to fractionate and quantify psychopathology in dementia, but it includes areas of behavior that are relevant to executive dysfunction. The Neuropsychiatric Inventory domains concern delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, and aberrant motor behavior. Its originators reported that the inventory was both reliable and valid.122 A self-administered informant-report version (Neuropsychiatric Inventory-Q123) is available.
Two recent caregiver-rated questionnaires, designed specifically to assess frontal behavioral change, are the 24-item Frontal Behavioral Inventory124 and the 46-item Frontal Systems Behavioral Scale.125 The items in the Frontal Behavioral Inventory were selected to reflect the core symptoms of frontal lobe–related dementias, whereas the Frontal Systems Behavioral Scale covers the three principal frontal behavioral syndromes: apathy (mesial), disinhibition (orbitofrontal), and (cognitive) executive dysfunction (dorsolateral). Good reliability has been reported for both scales, although an advantage of the Frontal Systems Behavioral Scale is availability of large-scale norms (436 persons; age range, 18 to 95 years), and the availability of self- and family informant–rating versions.125
QUALITATIVE OBSERVATIONS AND BEDSIDE TESTS
Bedside Testing
Individual Bedside Tests
A range of behaviors characteristic of frontal system disorders can provide insight into the features of executive dysfunction. Motor perseveration may be evident in copying drawings of repeating patterns (e.g., “+ 0 + + 0 + + + 0 …”), and motor impersistence—often initially evident as a failure to keep eyes closed during sensory testing—can be quantitated.126 Impairment of sequencing can be sought through motor control tests such as Luria’s fist/edge/palm test.127 Sometimes patients may even say the correct sequence aloud while performing it incorrectly: another example of the “curious dichotomy between knowing and doing.”107
Cognitive inhibition may be demonstrated on the antisaccade test,128 as well as on the conflicting tapping test (“tap once when I tap twice, and tap twice when I tap once”) and the go–no-go test (“tap once when I tap once, but don’t tap at all when I tap twice”).127 In each case, the patient should be able to repeat the instructions correctly after having performed the maneuvers incorrectly, to ensure that failure was not attributable merely to impairments of comprehension or memory.
It is traditional to test “abstraction” using proverb interpretation. Although this has been formalized as the Gorham proverb test,129 it is problematic because known proverbs may well elicit known interpretations (i.e., they may actually test semantic memory), whereas unknown proverbs may have a number of possible interpretations, which inhibits response. The California Proverbs test45 was intended to overcome this through the use of a multiple-choice format with proverbs of graded unfamiliarity, but tests of word similarities (e.g., “clock”/“thermometer,” “bicycle”/“train,” “poem”/“statue,” and “bridge”/“tunnel”) and differences (“dwarf”/“child,” “river”/“canal,” “laziness”/“idleness,” “character”/“reputation”)6 are easier to apply in the clinic or at the bedside.
Judgment is sometimes assessed by asking patients what they would do in a hypothetical situation, such as if they found water flooding into their kitchen. The difficulty with this approach is that patients may know and give the “correct” answer but do something quite different in practice. An informal adaptation of Shallice’s and Evans’s Cognitive Estimation test,130 which also has several American versions,6 is an examination of practical judgment. For example, patients might be asked how fast a racehorse can gallop (any answer of more than 40 miles/65 km per hour is incorrect), how tall the tallest building in the city is, how many slices are in a loaf of bread, or what the length of the average man’s spine is.
The Frontal Assessment Battery
A number of such bedside tests, comprising similarities, phonemic verbal fluency (generation of words beginning with “S”), Luria’s fist/edge/palm test, conflicting tapping, and the grasp response have been assembled into the Frontal Assessment Battery.131 This takes less than 10 minutes to perform, and instructions and scoring details are given in the appendix to the original article. The Frontal Assessment Battery demonstrated high interrater reliability of .87, good internal consistency, good concurrent validity against other measures thought to be sensitive to (but not specific for) executive dysfunction (including the WCST), and 89% accuracy in distinguishing patients with “frontal” disorders from controls. Its specificity for patients with executive dysfunction as distinct from other types of cognitive deficits has not, however, yet been established.
Release Signs (Primitive Reflexes)
Release signs, or primitive reflexes (e.g., palmomental, glabellar tap, snout and grasp reflexes), are sometimes sought as evidence of “frontal” involvement. They are thought to represent reappearance of infantile reflexes as a result of loss of inhibition from higher centers, but they have relatively poor localizing value,132 although asymmetrical release signs (palmomental/grasp) are more likely in asymmetrical disease (e.g., strokes) than in diffuse degenerations. Also, about 16% of normal elderly persons have at least one primitive reflex; the palmomental is the least specific for dementia and a grasp response the most specific.133 Even normal elderly individuals do not have three or more, however, and a grasp reflex is probably always abnormal.134,135 Release signs are more likely to be significant (if still nonlocalizing) in younger patients (younger than 50 years). This subject has been reviewed in depth.135
CONCLUSION
Crawford JR, Henry JD. Assessment of executive dysfunction. In: Halligan PW, Wade N, editors. The Effectiveness of Rehabilitation for Cognitive Deficits. London: Oxford University Press; 2005:233-245.
Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th ed. Oxford, UK: Oxford University Press, 2004.
Miller BL, Cummings JL, editors. The Human Frontal Lobes—Functions and Disorders. New York: Guilford Press, 1999.
Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press, 2002.
1 Macmillan M. An Odd Kind of Fame: Stories of Phineas Gage. Cambridge, MA: MIT Press, 2000.
2 Eslinger PJ, Damasio AR. Severe disturbance of higher cognition following bilateral frontal lobe ablation. Neurology. 1985;35:1731-1741.
3 Andrewes DG. Neuropsychology: From Theory to Practice. East Sussex, UK: Psychology Press, 2001.
4 Banich M. Cognitive Neuroscience and Neuropsychology, 2nd ed., Boston: Houghton Mifflin; 2004:365-392.
5 Bechara A, Damasio H, Damasio AR. Emotion, Decision Making and the Orbitofrontal Cortex. Cereb Cortex. 2000;10:295-307.
6 Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th ed. Oxford, UK: Oxford University Press, 2004.
7 Luria AR. The Working Brain. Harmondsworth, UK: Harper & Row, 1973.
8 Robbins TW. Dissociating executive functions of the prefrontal cortex. Phil Trans R Soc Lond B. 1996;351:1463-1471.
9 Shallice T, Burgess P. Deficits in strategy application following frontal lobe damage in man. Brain. 1991;114:727-741.
10 Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289-298.
11 Cahn-Weiner DA, Ready RE, Malloy PF. Neuropsychological predictors of everyday memory and everyday functioning in patients with mild Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2003;16:84-89.
12 Griffith HR, Belue K, Sicola A, et al. Impaired financial abilities in mild cognitive impairment. Neurology. 2003;60:449-457.
13 Manchester D, Priestley N, Jackson H. The assessment of executive functions: coming out of the office (a review). Brain Injury. 2004;18:1067-1081.
14 Baddeley AD. Is working memory still working? Am Psychol. 2001;56:849-864.
15 Baddeley AD, Logie R. Working memory: the multiple-component model. In: Miyake A, Shah P, editors. Models of Working Memory. New York: Cambridge University Press; 1999:28-61.
16 Baddeley AD. Exploring the central executive. Q J Exp Psychol A. 1996;49:5-28.
17 Miyake A, Friedman NP, Emerson MJ, et al. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49-100.
18 Rabbitt PMA, editor. Methodology of Frontal and Executive Functions. Hove, UK: Psychology Press, 1997.
19 D’Esposito M, Postle BR. The Neural Baiss of Working Memory Storage, Rehearsal, and Contral Processes: Evidence from Patient and Functional Magnetic Resonance Imaging Studies. Squire L, Schacter D, editors. Neuropsychology of Memory. 3rd edn. New York: Guilford Press; 2002:215-224.
20 Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B. 1982;298:199-209.
21 Cooper R, Shallice T. Contention scheduling and the control of routine activities. Cogn Neuropsychol. 2000;17:297-338.
22 Stuss DT, Shallice T, Alexander MP, et al. A multidisciplinary approach to anterior attentional functions. Ann N Y Acad Sci. 1995;769:191-212.
23 Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357-381.
24 Cummings JL. Fronto-subcortical circuits and human behaviour. Arch Neurol. 1993;50:873-880.
25 Miller BL, Cummings JL. The Human Frontal Lobes: Functions and Disorders. New York: Guilford Press, 1998.
26 Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry. An update. J Psychosom Res. 2002;53:647-654.
27 Mendez MF, Adams NL, Lewandowski KS. Neurobehavioral changes associated with caudate lesions. Neurology. 1989;39:349-354.
28 Zakzanis KK, Leach L, Kaplan E. Neuropsychological Differential Diagnosis. Lisse, The Netherlands: Swets & Zeitlinger, 1999;73-82. and 173–193.
29 Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function: a review of its promise and challenges for clinical research. J Neuropsychiatry Clin Neurosci. 2002;14:377-405.
30 Lhermitte F, Pillon B, Serdaru M. Human autonomy and the frontal lobes. Part I: Imitation and utilization behavior: a neuropsychological study of 75 patients. Ann Neurol. 1986;19:326-334.
31 Nathaniel-James DA, Fletcher P, et al. The functional anatomy of verbal initiation and suppression using the Hayling test. Neuropsychologia. 1997;35:559-566.
32 D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event related fMRI studies. Exp Brain Res. 2000;133:3-11.
33 Damasio AR. Descartes’ Error: Emotion, Reason, and the Human Brain. New York: GP Putnam, 1994.
34 Gilbert SJ, Frith CD, Burgess PW. Involvement of rostral prefrontal cortex in selection between stimulus-oriented and stimulus-independent thought. Eur J Neurosci. 2005;21:1423-1431.
35 Houx PJ, Shepherd J, Blauw G-J, et al. Testing cognitive function in elderly populations: the PROSPER study. J Neurol Neurosurg Psychiatry. 2005;73:385-389.
36 Lezak M. The problem of assessing executive functions. Int J Psychol. 1982;17:281-297.
37 Burgess PW. Theory and methodology in executive function and research. In: Rabbitt P, editor. Methodology of Frontal and Executive Function. Hove, UK: Psychology Press; 1997:81-116.
38 Alderman N, Burgess PW, Knight C, et al. Ecological validity of a simplified version of the multiple errands shopping test. J Int Neuropsychol Soc. 2003;9:31-44.
39 Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. J Cogn Neurosci. 1998;10:640-656.
40 Rahman S, Sahakian BJ, Hodges JR, et al. Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain. 1999;122:1469-1493.
41 Crawford JR, Obonsawin MC, Bremner M. Frontal lobe impairment in schizophrenia: Relationship to intellectual functioning. Psychol Med. 1993;23:787-790.
42 Obonsawin MC, Crawford JR, Page J, et al. Performance on tests of frontal lobe function reflect general intellectual ability. Neuropsychologia. 2002;40:970-977.
43 Wilson BA, Alderman N, Burgess P, et al. Behavioural Assessment of the Dysexecutive Syndrome. Bury St. Edmunds, UK: Thames Valley Test Company, 1996.
44 Grigsby J, Kaye K, Shetterly SM, et al. Prevalence of disorders of executive cognitive functioning among the elderly: findings from the San Luis Valley Health and Aging Study. Neuroepidemiology. 2002;21:213-220.
45 Delis D, Kaplan E, Kramer J. Delis-Kaplan Executive Function Scale. San Antonio, TX: Psychological Corporation, 2001.
46 Stroop JR. Studies of interference in serial verbal reaction. J Exp Psychol. 1935;18:643-662.
47 Dodrill CB. A neuropsychological battery for epilepsy. Epilepsia. 1978;19:611-623.
48 Golden C. Stroop Color and Word Test Manual (Cat. 30150M). Chicago: Stoelting, 1978.
49 Trennery MR, Crosson B, DeBoe J, et al. Stroop Neuropsychological Screening Test. Tampa, FL: Psychological Assessment Resources, 1989.
50 Stuss DT, Floden D, Alexander MP, et al. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771-786.
51 Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1-47.
52 Peterson BS, Skudlarski P, Getenby JC, et al. An fMRI study of Stroop Word-Color Interference: evidence for anterior cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237-1258.
53 Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary, 2nd ed. New York: Oxford University Press, 1998.
54 Bohnen N, Jolles J, Twijnstra A. Modification of the Stroop Color Word Test improves differentiation between patients with mild head injury and matched controls. Clin Neuropsychol. 1992;6:178-184.
55 Bondi MW, Serody AB, Chan AS, et al. Cognitive and neuropathologic correlates of Stroop Color-Word Test Performance in Alzheimer’s disease. Neuropsychology. 2002;16:335-343.
56 Snowden J, Craufurd D, Griffiths H, et al. Longitudinal evaluation of cognitive disorder in Huntington’s disease. J Int Neuropsychol Soc. 2001;7:33-44.
57 Ponsford J, Kinsella G. Attentional deficits following closed head injury. J Clin Exp Neuropsychol. 1992;14:822-838.
58 Burgess PW, Veitch EJ, Costello Ade L, et al. The cognitive and neuroanatomical correlates of multitasking. Neuropsychologia. 2000;38:848-863.
59 Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation, 2nd ed. Tucson, AZ: Neuropsychology Press, 1993.
60 Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: research findings and clinical application. In: Kaufman AS, editor. Specific Learning Disabilities and Difficulties in Children and Adolescents: Psychological Assessment and Evaluation. New York: Cambridge University Press; 2001:309-346.
61 Arbuthnott K, Frank J. Trail Making Test Part B as a measure of executive control: validation using a set-switching paradigm. J Clin Exp Neuropsychol. 2000;22:518-528.
62 Korrte KB, Horner MD, Windham WK. The Trail Making Test Part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9:106-109.
63 Crowe SF. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on Parts A and B of the Trail Making Test. J Clin Psychol. 1998;54:585-591.
64 Lamberty GJ, Putnam SH, Chatel DM, et al. Derived Trail Making Test indices: a preliminary report. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:230-234.
65 Ratti MT, Bo P, Giardini A, et al. Chronic alcoholism and the frontal lobe: which executive functions are impaired? Acta Neurol Scand. 2002;105:276-281.
66 Stuss DT, Bisschop SM, Alexander MP, et al. The Trail Making Test: a study in focal lesion patients. Psychol Assess. 2001;13:230-239.
67 Moll J, De Oliveiro-Souza R, Moll FT, et al. The cerebral correlates of set-shifting. An fMRI study of the Trail Making Test. Arq Neuropsiquiatr. 2002;60:900-905.
68 Maj M, D’Elia L, Satz P, et al. Evaluation of two new neuropsychological tests designed to minimise cultural bias in the assessment of HIV-1 seropositive persons: a WHO study. Arch Clin Neuropsychol. 1993;8:123-135.
69 Ponton MO, Gonzalez JJ, Hernandez I, et al. Factor analysis of the Neuropsychological Screening Battery for Hispanics (NeSBHIS). Appl Neuropsychol. 2000;7:32-39.
70 Lee TM, Chan CC. Are trail making and color trails tests of equivalent constructs? J Clin Exp Neuropsychol. 2000;22:529-534.
71 Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203-214.
72 Hester RL, Kinsella GJ, Ong B, et al. Demographic influences on baseline and derived scores from the Trail Making Test in healthy older Australian adults. Clin Neuropsychol. 2005;19:45-54.
73 Goldman WP, Baty JD, Buckles VD, et al. Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol. 1998;55:674-680.
74 Pillon B, Gouider-Khouja N, Deweer B, et al. Neuropsychological pattern of striatonigral degeneration: comparison with Parkinson’s disease and progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 1995;58:174-179.
75 Chen P, Ratcliffe G, Belle SH, et al. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847-1853.
76 Chen P, Ratcliffe G, Belle SH, et al. Patterns of cognitive decline in presymptomatic Alzheimer disease: a prospective community study. Arch Gen Psychiatry. 2001;58:853-858.
77 Hestad K, Aukrust P, Ellertsen B, et al. Neuropsychological deficits in HIV-1 seropositive and seronegative intravenous drug users (IVDUs): a follow-up study. J Int Neuropsychol Soc. 1996;2:126-133.
78 Heun R, Papassotiropoulos A, Jennssen F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int J Geriatr Psychiatry. 1998;13:368-380.
79 Lunn S, Skydsbjerg M, Schulsinger H, et al. A preliminary report on the neuropsychologic sequelae of human immunodeficiency virus. Arch Gen Psychiatry. 1991;48:139-142.
80 Paul R, Moser D, Cohen R, et al. Dementia severity and pattern of cognitive performance in vascular dementia. Appl Neuropsychol. 2001;8:211-217.
81 Selnes OA, Jacobson L, Machado AM, et al. Normative data for a brief neuropsychological screening battery. Multicenter AIDS Cohort Study. Percept Mot Skills. 1991;73:539-550.
82 Grant DA, Berg EA. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J Exp Psychol. 1948;38:404-411.
83 Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test. Manual Revised and Expanded. Odessa, FL: Psychological Assessment Resources, 1993.
84 Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277-2284.
85 Parker DM, Crawford JR. Assessment of frontal lobe function. In: Crawford JR, Parker DM, McKinlay WW, editors. A Handbook of Neuropsychological Assessment. London: Erlbaum; 1992:267-291.
86 Nelson HE. A modified card sorting test sensitive to frontal lobe damage. Cortex. 1976;12:313-324.
87 Drewe EA. The effect of type and area of brain lesions on Wisconsin Card Sorting Test performance. Cortex. 1974;10:159-170.
88 Milner B. Effects of different brain lesions on card sorting. Arch Neurol. 1963;9:90-100.
89 Anderson CV, Bigler ED, Blatter DD. Frontal lobe lesions, diffuse damage, and neuropsychological functioning in traumatically brain-injured patients. J Clin Exp Neuropsychol. 1995;17:900-908.
90 Axelrod BN, Goldman RS, Heaton RK, et al. Discriminability of the Wisconsin Card Sorting Test using the standardization sample. J Clin Exp Neuropsychol. 1996;18:338-342.
91 Mountain MA, Snow WG. Wisconsin Card Sorting Test as a measure of frontal pathology: a review. Clin Neuropsychol. 1993;7:108-118.
92 Hanks RA, Rapport LJ, Millis SR, et al. Measures of executive functioning as predictors of functional ability and social integration in a rehabilitation sample. Arch Phys Med Rehabil. 1999;80:1030-1037.
93 Logie RH, Cocchini G, Della Sala S, et al. Is there a specific executive capacity for dual task coordination? Evidence from Alzheimer’s disease. Neuropsychology. 2004;18:504-513.
94 Baddeley AD, Baddeley HA, Bucks RS, et al. Attentional control in Alzheimer’s disease. Brain. 2001;124:1492-1508.
95 Holtzer R, Burright RG, Donovick PJ. The sensitivity of dualtask performance to cognitive status in aging. J Int Neuropsychol Soc. 2004;10:230-238.
96 Alderman N. Central executive deficit and response to operant conditioning methods. Neuropsychol Rehabil. 1996;6:161-186.
97 Wilson BA, Evans JJ, Greenfield E, et al. Test of Divided Attention. London: Thames Valley Test Company, 2005.
98 Henry JD, Crawford JR. A meta-analytic review of verbal fluency performance following focal cortical lesions. Neuropsychology. 2004;18:284-295.
99 Benton AL, Hamsher KdeS. Multilingual Aphasia Examination. Iowa City: AJA Associates, 1989.
100 Crowe SF. Dissociation of two frontal lobe syndromes by a test of verbal fluency. J Clin Exp Neuropsychol. 1992;14:327-339.
101 Salmon DP, Heindel WC, Lange KL. Differential decline in word generation from phonemic and semantic categories during the course of Alzheimer’s disease: implications for the integrity of semantic memory. J Int Neuropsychol Soc. 1999;5:692-703.
102 Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12:323-330.
103 Henry JD, Crawford JR. Verbal fluency deficits in Parkinson’s disease: a meta-analysis. J Int Neuropsychol Soc. 2004;10:608-622.
104 Baldo J, Shimamura AP, Delis DC, et al. Verbal and design fluency in patients with frontal lobe lesions. J Int Neuropsychol Soc. 2001;7:586-596.
105 Rende B, Ramsberger G, Miyake A. Commonalities and differences in the working memory components underlying letter and category fluency tasks: a dualtask investigation. Neuropsychology. 2002;16:309-321.
106 Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138-146.
107 Walsh K. Understanding Brain Damage, 2nd ed. Edinburgh: Churchill Livingstone, 1991.
108 Brannen JH, Badie B, Moritz CH, et al. Reliability of functional MR imaging with word-generation tasks for mapping Broca’s area. AJNR Am J Neuroradiol. 2001;22:1711-1718.
109 Paulescu E, Goldacre B, Scifo P, et al. Functional heterogeneity of left inferior frontal cortex as revealed by fMRI. Neuroreport. 1997;8:2011-2016.
110 Stuss DT, Alexander MP, Hamer LP, et al. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4:265-278.
111 Henry JD, Crawford JR, Phillips LH. Verbal fluency performance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia. 2004;42:1212-1222.
112 Crawford JR, Moore JW, Cameron IM. Verbal fluency: a NART-based equation for the estimation of premorbid performance. Br J Clin Psychol. 1992;31:327-329.
113 Robbins TW, James M, Owen AM, et al. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: implications for theories of executive functioning and cognitive aging. J Int Neuropsychol Soc. 1998;4:474-490.
114 Lewis SJ, Cools R, Robbins TW, et al. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645-654.
115 Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10:525-557.
116 Humes G, Welsh MC, Retzlaff P, et al. Towers of Hanoi and London: reliability and validity of two executive function tasks. Assessment. 1997;4:249-257.
117 Bogod NM, Mateer CA, McDonald SWS. Self-awareness after traumatic brain injury: a comparison of measures and their relationship to executive functions. J Int Neuropsychol Soc. 2003;9:450-458.
118 Norris G, Tate RL. The Behavioural Assessment of the Dysexecutive Syndrome (BADS): ecological, concurrent, and construct validity. Neuropsychol Rehabil. 2000;10:33-45.
119 Chan RC. Dysexecutive symptoms among a non-clinical sample: a study with the use of the Dysexecutive Questionnaire. Br J Psychol. 2001;92:551-565.
120 Chan RCK, Manly T. The application of “dysexecutive syndrome” measures across cultures: performance and checklist assessment in neurologically healthy and traumatically brain-injured Hong Kong Chinese volunteers. J Int Neuropsychol Soc. 2002;8:771-780.
121 Bennett PC, Ong B, Ponsford J. Measuring executive dysfunction in an acute rehabilitation setting: Using the Dysexecutive Questionnaire (DEX). J Int Neuropsychol Soc. 2005;11:376-385.
122 Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308-2314.
123 Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233-239.
124 Kertesz A, Nadkarni N, Davidson W, et al. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6:460-468.
125 Grace J, Malloy PF. Frontal Systems Behavior Scale. Professional manual. Odessa, FL: Psychological Assessment Resources, 2001.
126 Benton AL, Sivan AB, Hamsher Kde S, et al. Contributions to Neuropsychological Assessment, 2nd ed., New York: Oxford University Press; 1994:137-149.
127 Christensen A-L. Luria’s Neuropsychological Investigation, 2nd ed., Copenhagen: Munksgaard; 1979:44.
128 Currie J, Ramsden B, McArthur C, et al. Validation of a clinical antisaccade eye movement test in the assessment of dementia. Arch Neurol. 1991;48:644-648.
129 Gorham DR. A proverbs test for clinical and experimental use. Psychol Rep Monogr. 1956;2:1-12.
130 Shallice T, Evans ME. The involvement of the frontal lobes in cognitive estimation. Cortex. 1978;14:294-303.
131 Dubois B, Slachevsky A, Litvan I, et al. The FAB: a frontal assessment battery at bedside. Neurology. 2000;55:1621-1626.
132 Jenkyn L, Walsh D, Culver C, et al. Clinical signs in diffuse cerebral dysfunction. J Neurol Neurosurg Psychiatry. 1977;40:956-966.
133 Hogan DB, Ebly EM. Primitive reflexes and dementia: results from the Canadian Study of Health and Aging. Age Ageing. 1995;24:375-381.
134 Di Legge S, Di Piero V, Altieri M, et al. Usefulness of primitive reflexes in demented and nondemented cerebrovascular patients in daily clinical practice. Eur Neurol. 2000;45:104-110.
135 Walterfang M, Velakoulis D. Cortical release signs in psychiatry. Aust N Z J Psychiatry. 2005;39:317-327.