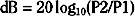CHAPTER 25 EXAMINATION OF HEARING AND BALANCE
Hearing loss and balance disorders are two of the most common reasons that patients visit their physicians. Varying degrees of hearing loss can affect patients at any age. One of every 1000 newborns is affected by some degree of hearing loss, and the prevalence of hearing loss rises with advancing age.1 By age 60, one of every three individuals is affected by hearing loss, and by age 85, one of every two is affected.1
Balance disorder, or “dizziness,” is the ninth most common complaint for which patients visit primary care physicians and the third most common complaint for 65- to 75-year-old patients.2–4 Hearing and balance disorders have a myriad of manifestations and etiologies, some of which are difficult to piece together. Treatment is often multidisciplinary, involving the neurologist, otolaryngologist, audiologist, neurosurgeon, and physical therapist, among others. It is important to recognize the signs and symptoms associated with specific types of hearing loss and balance disorders for the patient to receive proper referrals and proper treatment. The purpose of this chapter is to provide a better understanding of the otolaryngologist’s approach to the hearing and balance examination.
HEARING EXAMINATION
History
When taking a history of present illness, specific points should be emphasized. These include the patient’s perception of the degree of hearing loss, whether the hearing loss is unilateral or bilateral, and the onset of the hearing loss (sudden within 3 days, rapidly progressive within 1 week, slowly progressive over weeks to years, fluctuating, or stable). The patient may have associated symptoms, such as aural fullness, tinnitus, vertigo, disequilibrium, otalgia, otorrhea, headache, visual problems, and other neurological complaints (facial numbness or weakness, ataxia, oscillopsia, etc.), that may help point to specific causes of hearing loss. The past medical history is also very helpful: cardiovascular, renal, rheumatological, hematological, endocrine, and neurological conditions can predispose a patient to certain types of hearing loss.5 Past surgical history should also be obtained, with special emphasis on head trauma and previous otological or neurological surgery. A history of noise exposure is also important, as excessive noise exposure, either suddenly or over a period of time, can lead to hearing loss. A full account of the patient’s recent medications, including potentially ototoxic medications, should be taken. It is very important to know whether there is a family history of hearing loss, as there is a genetic predisposition for many types of hearing loss, and many genes associated with deafness and predisposition to hearing loss have been identified.1,5
Physical Examination
A complete head and neck examination can give many clues to the cause of a patient’s hearing loss. The auricle and the postauricular area should be examined for deformities, surgical incisions, the presence of a hearing aid, and patency of the external auditory canal. Something as simple as cerumen impaction can be the cause of hearing loss in some patients, but other conditions, such as foreign bodies, exostoses, canal stenosis/atresia, and carcinoma of the external canal, can be more troublesome. Pneumatic otoscopy can then be used to examine the tympanic membrane and middle ear. Here, the presence of a tympanostomy tube, tympanosclerosis (scarring of the tympanic membrane), tympanic membrane perforation, retraction pocket, fluid in the middle ear, middle ear masses, or otorrhea can be assessed. It is important to obtain a good seal with the speculum in order to assess the mobility of the tympanic membrane. External and middle ear abnormalities usually point to a conductive component of hearing loss.
Tuning fork testing is an essential part of the physical examination and can help determine if the cause of hearing loss is conductive, sensorineural, or mixed. The three types of tuning forks that can be used are 256Hz (middle C), 512Hz (octave above middle C), and 1024Hz (two octaves above middle C). The Rinne test is useful in determining if there is a conductive hearing loss and is performed by striking the tuning fork and placing it on the mastoid bone (testing bone conduction). Once the patient stops hearing the sound, the tines of the tuning fork are then placed in front of the external canal (testing air conduction) with the tines oriented in the head-frontal plane, and the patient indicates whether he or she can hear the sound. If the patient can hear the sound, air conduction is greater than bone conduction, and the result is normal, or “positive.” If the patient cannot hear the sound, bone conduction is greater than air conduction, and the result is abnormal, or “negative.” The degree of conductive hearing loss can be estimated based on the results of the Rinne test. A test that is negative at 256Hz and positive at 512 and 1024Hz indicates a mild 20- to 30-decibel (dB) conductive loss. A test that is negative at 256 and 512Hz and positive at 1024Hz indicates a moderate 30- to 45-dB conductive loss. A negative test at all three frequencies indicates a severe 45- to 60-dB conductive loss.6,7 The Weber test is a test used to lateralize the hearing loss. The tuning fork is struck and placed on the patient’s vertex, nasal bones, or maxillary teeth in the midline. The single most clinically useful fork used here is the 512-Hz variety, as the 256-Hz fork can be overly sensitive, leading to many false-positive results, and the 1024-Hz fork may not be sensitive enough.7–9 Lateralization of sound to one ear during the Weber test indicates either a conductive hearing loss in that ear or a greater sensorineural loss in the opposite ear.7 Simple tuning fork tests using only a few frequencies are far from comprehensive. If both ears are symmetrically affected by a sensorineural hearing loss, both the Rinne and Weber tests will be normal, provided the patient is able to hear the tuning fork at all.
Pure-Tone Audiometry
Pure-tone audiometry is the most commonly used test to measure auditory sensitivity. Pure-tone signals are delivered to the ear via air conduction and bone conduction at a variety of frequencies, and the patient responds to the sound by signaling the examiner with a button or by raising a hand. The response can be modified for pediatric patients or patients who lack the capacity to respond in the conventional manner. Although the entire range of human hearing is from 20 to 20,000Hz, the typical range of frequencies tested runs from 250 to 8000Hz, which is the range necessary to understand speech.10
The intensity of a sound presented is represented by a ratio of its sound pressure to a reference sound pressure, defined as the amount of pressure that can just be sensed by a normal human ear at its most sensitive frequency (0.0002dyne/cm2).11 As the pressure level of a presented sound is often many times the reference sound pressure, the simplest way to present this ratio is to use the decibel, a logarithmic unit:
where P2 is the presented sound pressure and P1 is the reference sound pressure.
A sound referenced to the reference sound pressure is known as the absolute sound level, presented as decibels sound pressure level (dB SPL). The normal human ear is variably sensitive to different frequencies throughout its range, so clinically, the easiest reference level to use is the sound pressure level for each tested frequency that can be heard by a normal ear. The sound level is presented as decibels hearing loss, or dB HL.11
Auditory threshold is defined as the lowest signal intensity at which the signal can be identified 50% of the time.12 Air conduction thresholds are determined by presenting sound to the ears via headphones or insert earphones, and bone conduction thresholds are determined by vibrating the mastoid directly. Air conduction thresholds measure the sensitivity of the entire auditory system from the external ear to the auditory cortex. When analyzed alone, they do not provide much information regarding the etiology of hearing loss. However, when they are analyzed together with bone conduction thresholds, which measure the degree of sensorineural hearing loss, they can provide valuable information regarding both the type and severity of the hearing loss.12 When air conduction thresholds are elevated relative to bone conduction thresholds, an “air-bone gap” exists, indicating a conductive hearing loss. Air conduction and bone conduction thresholds showing the same amount of hearing loss indicate a sensorineural hearing loss. A mixed hearing loss is present when both air and bone conduction thresholds are elevated, but air conduction thresholds are more elevated than bone conduction thresholds.
The normal region on the audiogram is from 0 to 20dB HL for adults and from 0 to 15dB HL for children. Mild hearing loss is 20 to 40dB HL, moderate loss is 40 to 55dB HL, moderately severe loss is 55 to 70dB HL, severe loss is 70 to 90dB HL, and profound loss is above 90dB HL. Hearing sensitivity within the speech frequencies is known as the pure-tone average (PTA) and can be calculated by adding the thresholds obtained at 500, 1000, and 2000Hz and dividing the result by 3.11
For audiometric results to be valid, the patient must respond to stimulation of the ear being tested. When noninsert earphones are used, sounds greater than 40dB HL presented to one ear can cross over to the opposite ear, most likely with the vibration of the earphone against the skull acting as a bone conductor. The amount of sound needed for crossover to occur is known as the interaural attenuation, which for air conduction is about 50dB HL for lower frequencies and 60dB HL for higher frequencies. The interaural attenuation is considerably higher when insert earphones are used. For bone conduction, interaural attenuation is less than 10dB HL.11 To correct for the presence of interaural attenuation when a true hearing loss is present, masking techniques are used. A narrow band “white” noise is presented to the nontest ear when the true stimulus is being given to the test ear, and with adequate masking, any sound crossing over to the nontest ear is masked by the noise. To work, the masking noise presented to the nontest must be greater than the threshold of hearing for the nontest ear.11 This can be a problem when bilateral hearing loss (especially conductive) exists, as masking presented to the nontest ear can cross back over to the test ear. This is known as a “masking dilemma.”10 In air conduction testing, masking should be used when there is a difference between the air conduction presentation level to the test ear and the bone conduction threshold of the nontest ear of greater than 40dB for lower frequencies and greater than 60dB for higher frequencies. In bone conduction testing, masking should be used whenever there is any difference in the air and bone conduction thresholds.10
Speech Audiometry
Commonly measured speech tests include the speech detection threshold (SDT), the speech reception threshold (SRT), and speech discrimination or word recognition. The SDT is the softest level at which the patient can barely detect the presence of a speech signal 50% of the time.12 The SRT is the softest level at which the patient can repeat 50% of balanced disyllabic words, or spondees (e.g. “hot dog,” “baseball”), correctly.10,12 The SDT should correspond to the PTA, whereas the SRT is usually about 8 to 9dB higher than the PTA.12 Both SDT and SRT can be measured with bone conduction testing and can be masked if necessary. Discrepancies between the PTA and the SDT or SRT can indicate malingering.
The speech discrimination score is a test of the patient’s ability to identify monosyllabic words, or phonemes, at a suprathreshold level, usually about 40dB above the SRT.10 The speech discrimination score is important in that it helps assess the patient’s ability to understand speech, to communicate effectively, and to benefit from amplification. It also provides some information regarding the patient’s central auditory function.12
In general, patients with conductive hearing loss tend to have excellent speech discrimination scores when presented with sounds loud enough for them to hear. Patients with cochlear sensorineural loss tend to have lower speech discrimination scores, and patients with retrocochlear sensorineural loss (from lesions of the eighth cranial nerve to the auditory cortex) have even lower speech discrimination scores. They may even have lower speech discrimination in the presence of normal pure-tone thresholds.12
Tympanometry
Acoustic immittance refers to either acoustic admittance (the ease with which energy flows through a system) or acoustic impedance (the blockage of energy flow through a system).12 In tympanometry, acoustic immittance measures are used to determine the status of the tympanic membrane and middle ear. A probe is placed in the ear canal and an airtight seal is obtained. A tone is introduced into the ear canal and the pressure in the canal is varied. When the pressure in the ear canal is equal to the middle ear pressure, the tympanic membrane will be at its most compliant (highest admittance) and will absorb the sound. This results in a tympanometric peak.10
If eustachian tube function is normal, the middle ear pressure is equal to the atmospheric pressure and the peak occurs at 0 mm H2O—this corresponds to a type A tympanogram. If there is negative middle ear pressure, the peak occurs at a negative pressure, corresponding to a type C tympanogram. If there is no peak (flat or type B tympanogram), there is no compliance of the tympanic membrane (no admittance), indicating a middle ear effusion, tympanic membrane perforation, or patent tympanostomy tube. These can be distinguished using ear canal volume measurements, with higher volumes corresponding to a hole in the tympanic membrane. Other types of tympanograms include As (shallow peak and low compliance at 0 mm H2O), indicating ossicular chain fixation or middle ear effusion, and Ad (very high peak and high compliance at 0 mm H2O), indicating ossicular chain discontinuity or a monomeric tympanic membrane.10
Acoustic Reflex
In acoustic reflex testing, acoustic immittance measures are used to assess the neural pathway surrounding the stapedial reflex, which occurs in response to a loud sound (70 to 90dB above threshold).10 The afferent limb of the stapedial reflex is the ipsilateral eighth nerve, which leads to the brainstem. Complex pathways in the brainstem involving the ipsilateral ventral cochlear nucleus, trapezoid body, and bilateral medial superior olives lead from the eighth nerve on the ipsilateral (stimulated) side to the motor nucleus of the facial nerve on both sides of the brainstem.7,10–12 The efferent limb is the ipsilateral and contralateral facial nerves, which innervate the stapedius muscles. When the stapedius muscle contracts, the ossicular chain stiffens, causing a small change in compliance in the middle ear system that is detected by the probe.11
Patients with mild to moderate cochlear sensorineural hearing loss have reflexes bilaterally at about the same intensity level as those with normal hearing, but patients with severe or profound hearing loss have absent reflexes when the affected ear is stimulated.10
A conductive hearing loss results in absent reflexes when the affected ear is stimulated, as sound will not be loud enough to stimulate the reflex. Even when the normal ear is stimulated, the ear with the conductive loss does not have a reflex, as the middle ear condition prevents the stapedius from contracting.10
A lesion of the eighth nerve should result in absent reflexes bilaterally when the affected ear is stimulated, but reflexes should be present bilaterally when the nonaffected ear is stimulated. This can be confused with the reflex result associated with profound unilateral hearing loss (>70dB) of cochlear origin. Lesions of the brainstem affecting the central crossed pathways may result in present ipsilateral reflexes when each ear is stimulated but absent contralateral reflexes. A facial nerve lesion results in an absent reflex on the affected side, no matter which side is stimulated, provided the lesion is proximal to the branching of the nerve to the stapedius muscle.10
Auditory Brainstem Response
The auditory brainstem response (ABR) is an electrophysiological recording of responses of the distal auditory pathway (eighth nerve and brainstem) to sounds.11 The ABR involves placement of electrodes on the patient’s head and presentation of sound to the ear. When sound is presented to a normal ear, either in click form or frequency-specific tones, five to seven peaks occurring within 10 milliseconds make up the ABR.12 Usually only the first five peaks are considered. Wave I represents the action potentials from the eighth nerve near the cochlea. Wave II comes from the eighth nerve near the cochlear nucleus in the brainstem. Waves I and II are the only waves generated by ipsilateral structures. All subsequent waves represent bilateral crossed pathways. Wave III comes from the caudal pons with contributions from the cochlear nucleus, trapezoid body, and superior olive. Wave IV probably comes from the lateral lemniscus. Wave V, the most prominent wave, comes from the lateral lemniscus as it approaches the inferior colliculus.11
For audiological purposes, the latencies and amplitudes of waves I, III, and V are analyzed, and comparisons between sides are made. In normal hearing, the latencies of waves I, III, and V are within normal ranges and the latencies between ears are within 0.2 to 0.4 milliseconds of each other. In conductive hearing loss, the absolute latency of wave I is prolonged, but the latencies between waves and the amplitudes are not affected. In cochlear sensorineural loss, the wave I latency is slightly delayed and is small in amplitude, but the latencies between waves are not affected. In retrocochlear (neural) hearing loss, wave I tends to be normal, but latencies between waves I-III and I-IV are abnormally prolonged.10,11
In practice, the ABR is a good tool to definitively test hearing in uncooperative patients (newborns) and in suspected malingerers, and it can be used to evaluate the eighth nerve and brainstem structures in patients with suspected retrocochlear hearing loss. It is also used in neurotological surgical procedures, such as vestibular nerve section and acoustic neuroma removal.11
Electrocochleography
Electrocochleography is a test of the electrical activity generated by the cochlea and eighth nerve. It is most often used to aid in the diagnosis of Ménière disease, but it can also be used for intraoperative monitoring of the cochlear and eighth nerve. An electrode is placed in the ear canal, on the tympanic membrane, or on the promontory of the cochlea in the middle ear. The three main signals detected by electrocochleography are the cochlear microphonic, the summating potential, and the action potential. The cochlear microphonic and summating potential reflect cochlear electrical activity, and the action potential reflects eighth nerve activity and is the same as wave I of the ABR. The calculation of interest is the summating potential/action potential ratio. An abnormally high ratio is suggestive of endolymphatic hydrops characteristic of Meniere’s disease.10,11
Otoacoustic Emissions
Otoacoustic emissions (OAEs) represent auditory signals produced by the cochlear outer hair cells that can be picked up by a very sensitive microphone in the ear canal.12 Although they are a measure of cochlear function, abnormalities anywhere between the microphone and cochlea (e.g. middle ear) block any signals going from the cochlea to the microphone—they will not be detectable in the presence of conductive hearing loss.10 The three main types of OAEs are spontaneous, transient evoked, and distortion product.
Spontaneous OAEs occur in the absence of a stimulus, but they only occur in less than one half to 60% of normal hearing individuals.10,11 Transient evoked OAEs (OAEs) are elicited by a brief click or tone burst. Distortion product OAEs (OAEs) are generated when two pure-tone stimuli of different frequencies are presented to the ear simultaneously. In response to these tones, the outer hair cells generate signals called distortion products that are related to the frequencies of the presented tones. Transient evoked OAEs are used to determine mainly if there is good cochlear function, whereas distortion product OAEs can be used to generate a curve resembling an audiogram based on frequency-specific responses of the cochlea.10–12
OAEs are useful in that they are specific to cochlear function. They are not present in conductive hearing loss or cochlear hearing loss greater than 25 to 30dB HL. However, they can be present in retrocochlear (neural) hearing loss, which can help differentiate cochlear from retrocochlear lesions.10 OAEs are noninvasive and easy to perform—they can be used to screen hearing in infants, to confirm audiometric testing in young children, to monitor the effects of ototoxic medications, to detect cochlear abnormalities in patients with tinnitus and normal audiograms, and to help detect malingerers.10,11
Radiographic Testing
Magnetic resonance imaging (MRI) of the internal auditory canals is extremely useful in the diagnosis of unilateral or asymmetric sensorineural hearing loss. It is more sensitive and specific than ABR for the detection of acoustic neuromas and is the gold standard in the diagnosis of acoustic neuromas as a cause of retrocochlear (neural) hearing loss.5,13 MRI with gadolinium enhancement is able to detect small tumors less than 1 cm in diameter, which results in better facial and hearing function after tumor removal.13 MRI should also be heavily considered in the face of sudden sensorineural hearing loss, even if it resolves with steroids, because as many as 19% of patients with acoustic neuromas can present with sudden hearing loss.14 Some reports state that as many as 47.5% of cases of sudden hearing loss may be caused by an acoustic neuroma.14,15
BALANCE EXAMINATION
The diagnosis and treatment of patients with “dizziness” can be very challenging and frustrating for the patient, the neurologist, the otolaryngologist, and the audiologist. A huge variety of disorders can cause the patient to have a sensation of dizziness, and a huge variety of terms can be used to describe it (lightheadedness, spinning, “swimming sensation,” “things not being right in the head”).16 Often, the diagnosis is made by piecing together many different pieces of information. It is important to realize that not every case of dizziness can be completely cured or diagnosed exactly. An organized, systematic approach is necessary in order to make a reasonably accurate diagnosis and to avoid confusion. Key components in the evaluation of dizziness include the history, physical examination, electronystagmography, rotary chair testing, and computerized dynamic posturography testing.
History
Obtaining a careful history is probably the most important step in the diagnosis of dizziness, but it often requires patience. Symptoms are often vague and difficult for the patient to describe. It may seem faster to begin by asking a lot of leading questions, but the physician will actually save time by allowing the patient to describe what he or she is feeling in the patient’s own words. Especially important is the patient’s description of the first episode of dizziness, although this may be difficult to elicit in patients who are so consumed by their dizziness that they cannot focus on the initial event and in patients who have already seen multiple specialists and/or lawyers.16
When the patient describes his or her symptoms, it is important to distinguish whether the patient is experiencing a sensation of movement, such as a spinning sensation or a falling sensation. Vertigo, a false sensation of movement, should be distinguished from dizziness, which is any kind of altered sense of orientation.17 Lightheadedness refers to a sensation characteristic of presyncope, which may include temporary blurred vision and pale facial color. It should be distinguished from vertigo and is usually caused by nonvestibular problems such as the cardiac or vasovagal reflex, both of which can result in cerebral hypoxia.17 A sense of imbalance refers to the inability to maintain the center of gravity, which causes the patient to feel unsteady and as if he or she is going to fall.17 This can be caused by both vestibular and nonvestibular disorders.
When the patient describes vertigo, further information must be gathered in order to differentiate whether it is caused by a peripheral or central lesion. Vertigo can be caused by lesions anywhere from the vestibular end organs (utricle, saccule, and semicircular canals), the vestibular nuclei, the cerebellum, brainstem pathways, and the cortex (rarely).17 An important characteristic to ascertain is whether the vertigo is episodic or continuous. If episodic, how long the attacks last, how often they occur, and whether they occur with head movement or positioning are important points to know. Associated auditory symptoms, such as hearing loss, aural fullness, and tinnitus, are all important to ask about. Also important are associated neurological symptoms, such as headache with or without aura, visual changes, oscillopsia, numbness, weakness, ataxia, seizure, and loss of consciousness. Asking if the vertigo is more intense with a Valsalva maneuver is also helpful. A full otological history including history of infection, otalgia, otorrhea, and previous otological surgery is essential. In addition, it is imperative to obtain a full past medical history, past surgical history, history of head trauma, recent medications (with attention to ototoxic medications, blood pressure medications, stimulants, depressants, and illegal drugs), diet, allergies, social history, and family history of hearing loss or vestibular problems.16
Sorting out the history is important in suggesting possible diagnoses as well as recognizing more extensive and complex conditions. Episodic intense vertigo lasting up to one minute associated with head positioning or movement and not associated with other auditory symptoms is characteristic of benign paroxysmal positional vertigo (BPPV),17 but brief 5- to 10-second episodes associated with head movement may also be a sign of vascular compression of the eighth nerve complex.2 Episodic vertigo lasting minutes to hours sometimes associated with fluctuating hearing loss, tinnitus, and/or aural fullness is suggestive of Meniere’s disease, but vertigo lasting 2 to 20 minutes may be associated with transient ischemic attacks, especially when associated with visual changes, ataxia, and other neurological findings.17 An isolated attack of continuous vertigo lasting longer than 24 hours with a sudden onset is suggestive of vestibular neuronitis when not associated with hearing loss and with viral labyrinthitis when associated with hearing loss.17 However, sudden-onset vertigo associated with hearing loss and tinnitus can also represent a brainstem stroke.18 Vertigo brought about by straining or other Valsalva-like maneuvers are associated with perilymphatic fistula, Chiari malformation, and superior semicircular canal dehiscence.17 There is also vertigo induced by sound, which is known as the Tullio phenomenon. This can be associated with perilymph fistula, Meniere’s disease, congenital inner ear malformations, Lyme disease, and superior semicircular canal dehiscence.19
Physical Examination
Head and Neck Examination
The head and neck examination is similar to that described previously. Additional information can be found by performing a fistula test, which can be done by either tragal pressure or pneumatic otoscopy. The patient is instructed to look straight ahead, and continuous positive and negative pressure is applied. Normally, the eyes will not drift, but a positive fistula test (Hennebert’s sign) is manifest by the eyes drifting away from the tested ear with positive pressure and toward the tested ear with negative pressure. A positive fistula test is associated with a perilymph fistula, Meniere’s disease, or superior semicircular canal dehiscence.17,19
The cranial nerve examination should be as thorough as possible, as every cranial nerve may be potentially affected in disease processes that cause vertigo. Oculomotor examination documenting the function of cranial nerves III, IV, and VI should be performed. Internuclear ophthalmoplegia produced by lesions in the medial longitudinal fasciculus of the lower midbrain and pons is important to recognize, as vertigo may be one of the manifesting signs of multiple sclerosis.16 Subtle abnormalities in cranial nerves V, VII, and VIII may indicate a retrocochlear lesion. These can be tested by closely examining facial symmetry at rest and during movement, performing the corneal blink reflex test, and performing tuning fork testing. Usually, though, patients with retrocochlear lesions will present with hearing loss rather than tinnitus or vertigo.16 Finally, cranial nerves IX, X, XI, and XII should be thoroughly examined.
Oculomotor Function Testing
The basis for nystagmus and oculomotor testing revolves around the vestibulo-ocular reflex (VOR). The VOR is a pathway that associates the activity of paired semicircular canals to a set of extraocular muscles.20 There are two main types of VOR: the angular reflex associated with the semicircular canals and the linear reflex associated with the utricle and saccule. The purposes of the reflex are to maintain binocular vision and to stabilize images on the fovea during head movement.2 The pathway involves the vestibule, the vestibular nuclei, and the oculomotor nuclei with modulation between cerebellar centers. The easiest reflex pathway to test is the paired horizontal semicircular canals with cranial medial and lateral recti muscles. For example, in a normal individual, there is an equal tonic firing rate of both vestibular nerves in the absence of head movement, but when the head turns to the left, the endolymph in the left horizontal canal moves to the right. This displaces the cupula and consequently the cilia toward the kinocilium, leading to an increased firing rate in the left superior vestibular nerve. The opposite effect occurs on the right side. Pathways in the brainstem then cause activation of the left medial rectus and the right lateral rectus, while the left lateral rectus and right medial rectus are inhibited. The eyes then move conjugately to the right in the exact opposing fashion to head rotation until they reach a limit. At this point, a saccade to the left brings the eyes back to the midline.
When a patient has a unilateral left vestibular lesion, tonic input from the left vestibular nerve ceases, resulting in unopposed input from the right vestibular nerve. This leads to conjugate eye movements to the left (slow phase), followed by corrective saccades to the right (fast phase). The direction of nystagmus is defined by its fast phase. This is a right-beating spontaneous nystagmus. Right-beating torsional nystagmus would also occur from unopposed stimulation of the right superior and inferior canals. Upbeating or downbeating nystagmus is not characteristic of peripheral vestibular lesions and usually is caused by central lesions. Spontaneous peripheral nystagmus can be suppressed by visual fixation. The use of Frenzel lenses that do not allow visual fixation are useful to increase the examiner’s sensitivity to the patient’s nystagmus.2 Nystagmus can also be enhanced by having the patient look toward the intact side. Vestibular suppressants, alcohol, and antiepileptic medications decrease the amplitude of the nystagmus and can make evaluation difficult.16
Gaze nystagmus can be identified by having the patient look at the examiner’s index finger held at off-center positions. Gaze-evoked nystagmus is often a side effect of drugs such as anticonvulsants, benzodiazepines, or alcohol, but when it is present in the absence of these drugs, it almost always indicates a central disorder involving the brainstem, cerebellum, or midbrain depending on its direction, and also tends to be direction changing.17,20
Head-shaking nystagmus is assessed by having the patient shake his or her head very rapidly back and forth in the horizontal plane while wearing Frenzel lenses. Shaking is abruptly stopped, and nystagmus is assessed. Normal individuals usually have just a beat or two of nystagmus, but individuals with a unilateral vestibular lesion show nystagmus with the fast phase toward the intact side.2 Patients with central lesions such as cerebellar dysfunction may also have post head-shaking nystagmus, often in the vertical direction.2
Nonlinearity testing, or head thrust testing, is performed by applying quick head thrusts about 15 degrees in the plane of each semicircular canal from the neutral position while the patient attempts to fix his gaze on the examiner’s nose. A normal patient is able to keep his or her gaze on the examiner’s nose, but a patient with a lesion affecting a semicircular canal demonstrates a corrective saccade after the head thrust toward the lesioned side.17
Positional Testing
The first positional test that should be performed is the Dix-Hallpike maneuver to detect the presence of benign paroxysmal positional vertigo of the posterior semicircular canal. In this test, the patient is sitting upright on an examination table and the head is turned 45 degrees to the side in question. The head is brought quickly down to a position where the head hangs off the edge of the table and the patient is instructed to look straight ahead with the eyes open (the patient may also wear Frenzel lenses if desired).2,17 This position is held for 30 seconds, and in the presence of BPPV, classically the patient has horizontorotary nystagmus with the fast phase beating toward the down ear (geotropic), which is delayed in onset and fatigable. Almost all persons with BPPV have a sensation of spinning.16 Nystagmus of central origin may also manifest itself during the Dix-Hallpike maneuver, but it usually lasts indefinitely while the patient is in the supine position.16
Postural Control Testing
A simple way to think of this is that balance in gravity depends on three peripheral components: vision, proprioception, and the vestibular system. These three components are bilateral peripheral inputs to the brain, which integrates balance, whereas the cerebellum is considered a central input. Taking away one of the inputs places the burden of maintaining balance on the other two inputs, and taking away two of the inputs places all of the burden on the one remaining input. This is analogous to a person standing in darkness having to rely on vestibular inputs and proprioception to maintain balance.16 The Romberg test was originally described for tabes dorsalis and primarily tests proprioception.16,21 In this test, the patient stands with both feet together with the arms either folded in front or down at the sides. Then the patient closes his or her eyes and attempts to keep balance. Patients with a unilateral vestibular lesion tend to fall toward the lesioned side. The tandem Romberg test is a variant that requires patients to stand with one foot directly in front of the other. This increases its sensitivity.16
The Fukuda stepping test is performed with the patient’s arms straight out in front and the eyes closed. The patient then marches in place. A vestibular lesion is indicated if the patient is turned more than 30 degrees from the original position after approximately 50 steps. Usually, patients turn toward the diseased side. Patients with a vestibular lesion with a positive Fukuda stepping test are usually surprised by the result, as they do not sense that they are rotating during the test.16
Another test of vestibulospinal function is the tandem gait test, in which the patient is asked to step heel-to-toe with his/her eyes closed. Normal individuals can do this for at least 10 steps, but patients with vestibular disorders fail this test.17 The past pointing test is done by having the patient and examiner stand facing each other with arms extended forward and their index fingers in contact with one another. The patient then raises his or her arms up and brings his or her fingers into contact again with the examiner’s, first with the eyes open and then with the eyes closed. Failure of this test can indicate an abnormality in the vestibulospinal pathway.2,17
Electronystagmography
Electronystagmography is a combination of tests based on the VOR that provides important information about the vestibular and ocular systems. Results of the electronystagmographic battery should be used in conjunction with findings from the history, the physical examination, and other studies to arrive at a diagnosis.22
Electro-oculography
Electro-oculography is used to record eye movements during electronystagmographic testing. It is based on the corneoretinal potential (difference in electrical charge between the cornea and the retina), with the long axis of the eye acting as a dipole. Movements of the eye relative to the surface electrodes placed around the eye produce an electrical signal that corresponds to eye position. Recordings of eye movement are accurate to about 0.5 degree, but it is still less sensitive than visual inspection, which can perceive movements of about 0.1 degree.2 Therefore, visual inspection with Frenzel lenses is sometimes still necessary to document nystagmus of low amplitude. Another limitation of electro-oculography is that torsional eye movements cannot be monitored. Again, visual inspection with Frenzel lenses is sometimes necessary to document torsional nystagmus.2
Fortunately, new techniques have been developed to provide greater accuracy and breadth for oculomotor testing. The most clinically useful technique that has been developed is the infrared video electronystagmographic system. Here, the patient wears goggles that illuminate the eyes with infrared light (invisible to the patient), allowing a small video camera to pick up and project an image of the eyes onto a monitor. This can also assess eye movement in horizontal, vertical, and torsional directions and is more accurate than electro-oculography.22
Oculomotor Testing
Oculomotor testing measures the accuracy, latency, and velocity of eye movements in response to a stimulus (usually an LED light). The tests performed include tests for saccades, smooth pursuit, and optokinetic nystagmus. Saccades are rapid eye movements that bring objects from the peripheral visual fields onto the fovea. They are controlled by the occipitoparietal cortex, the frontal lobe, the basal ganglia, the superior colliculus, the cerebellum, and the brainstem.17 During saccade testing, the patient follows the LED, which flashes sequentially in positions 15 to 20 degrees to the right or left of center. The test is repeated vertically. The latency, peak eye velocity, and accuracy are then calculated. The latency is the time lag between presentation of the stimulus and the beginning of a saccade. Prolonged or shortened latency, as well as differences in latency between eyes, are usually indicative of neurodegenerative disease. Abnormally slow peak velocities can be caused by sedative drugs, drowsiness, cerebellar disorders, basal ganglia disorders, and brainstem lesions. Abnormally fast velocities are found with calibration errors and eye muscle restrictions. Asymmetrical velocities are caused by internuclear ophthalmoplegia, eye muscle restriction, and cranial nerves III and VI palsies. Poor accuracy, described as overshoot or undershoot dysmetria, usually indicates cerebellar, brainstem, or basal ganglia abnormalities.17
Smooth pursuit describes eye movements that are generated when tracking moving objects. In smooth pursuit testing, the patient follows an LED moving back and forth between two points at a constant velocity. The gain and phase are then calculated. Gain is the ratio of the eye velocity to the target velocity. Abnormally low gain is suggestive of a central disorder (brainstem or cerebellum).17 Phase is the difference in time between eye movement and target movement. Abnormalities here also indicate central nervous system disorders.17 The morphology of the smooth pursuit tracing can be analyzed. A saccadic pattern of smooth pursuit is associated with a cerebellar disorder.22 Acute peripheral vestibular lesions can also impair smooth pursuit when the eyes are trying to move opposite the slow phase of spontaneous nystagmus.17
Optokinetic nystagmus is tested by having the patient look ahead while seated in a rotating drum with black and white stripes on it. When the patient tries to look straight ahead, there will be small involuntary excursions of the eye (stare nystagmus). When the patient follows a target, smooth pursuit is tested (look nystagmus). Both types of nystagmus are probably responsible for eye movement during stimulation. However, when the lights go out, the patient with an intact optokinetic system will continue to have nystagmus for about 25 seconds—optokinetic after nystagmus (OKAN).22 The optokinetic system is distributed widely throughout the brainstem and cerebellum, so abnormalities are difficult to localize. However, absence or asymmetry of OKAN can occur with peripheral vestibular lesions. Bilateral lesions tend to greatly reduce or eliminate OKAN, whereas unilateral lesions can result in asymmetrical OKAN with prolonged nystagmus directed at the site of lesion.22,23
Spontaneous and Gaze Nystagmus
The electronystagmogram can record eye movements associated with spontaneous and gaze-evoked nystagmus similar to that described earlier (see Physical Examination). An advantage of electronystagmography over physical examination is that eye movements can be monitored with the eyes closed. If during any part of the test nystagmus is identified with the eyes closed, the patient is then told to open the eyes so that changes in nystagmus can be detected. Patients with peripheral causes of nystagmus and a normal central pathway are able to suppress the nystagmus with the eyes open. This is called fixation suppression. A central lesion is suggested when there is no fixation suppression and the nystagmus continues with the eyes open.22
Positional and Positioning Tests
Positional tests measure the response to changes in the direction of gravitational force. With the eyes closed, the patient is moved slowly into a series of stationary positions, and the presence of nystagmus is assessed, which can be fixed or direction changing. Positional nystagmus from a peripheral lesion can fatigue with repeated testing, is usually fixed in direction, and usually does not change independent of head movement. Nystagmus that changes in direction independent of head movement is suggestive of a central lesion.22
Positioning tests include the Dix-Hallpike maneuver, among others. The patient is positioned as described earlier (see Physical Examination), and the presence of nystagmus is noted. If the patient has nystagmus, the test is repeated to see if the response fatigues. If the response fatigues, it is suggestive of a peripheral disorder, but if it does not, it suggests a central lesion.22
Caloric Testing
Caloric testing is a very important part of electronystagmography in that it is one of the few tests that allows one labyrinth to be examined independently of the other.2 Horizontal nystagmus is induced by stimulation of the horizontal semicircular canal using a cold and warm stimulus (air or water). The patient lies in the supine position with the head tilted 30 degrees upward to bring the horizontal canal into the vertical plane (direction of gravity), making it more sensitive to the flow of endolymph.17 The external canal is irrigated with 250 mL of water at 30°C and 44°C for about 30 seconds each. Alternatively, air at temperatures of 24°C and 50°C can be used.
The measured value of the induced nystagmus for each stimulus is the peak slow-phase velocity averaged over a 10-second period.17 The difference between the sides is calculated, and any difference greater than 20% to 25% between sides is considered significant and indicates weakness of the vestibular labyrinth or nerve on the less active side. Directional preponderance, which compares the peak slow-phase velocities of eye movements to the right with the left, can also be calculated. A difference of 25% to 30% is considered significant and indicates an imbalance but is a nonlocalizing measure.16
Rotational Chair Testing
Rotational chair testing measures the VOR response to small rotations of the body around an axis. It can be useful in monitoring changes in vestibular function over time (especially bilateral lesions or lesions from vestibulotoxic medications), monitoring compensation following acute injury, and identifying residual vestibular function in patients with no response during caloric testing.22 The easiest canal to test is the horizontal canal. The patient is fitted with electro-oculographic electrodes and rotated slowly around a vertical axis with the eyes covered. The patient then undergoes sinusoidal harmonic acceleration, during which the patient is rotated back and forth at gradually increasing frequencies to a peak angular velocity of about 50 degrees per second.16 The three values analyzed are phase, gain, and symmetry.
Phase measures the timing of eye movement relative to head movement. In individuals with an intact VOR, the direction of slow phase eye velocity is exactly opposite head velocity, but patients with a vestibular or cerebellar lesion have an abnormal phase, with either a phase lead or lag. Gain is the ratio of the slow phase eye velocity to the head velocity. Abnormally low gain may indicate bilateral peripheral vestibular weakness, whereas abnormally high gain may be seen in cerebellar lesions.17 Symmetry measures the difference between slow phase velocities associated with rightward and leftward rotation and can suggest involvement of the central pathways or peripheral vestibular dysfunction.17
Computerized Dynamic Posturography
Posturography is a quantitative test of the vestibulospinal reflex. It has the same basis as the Romberg test, where three peripheral inputs of vision, the labyrinth, and proprioception are integrated for a patient to maintain balance. If one of these inputs is taken away, the patient has to rely on the remaining inputs to maintain balance. No one input can be measured by itself. Patients with cerebellar lesions and certain cortical lesions are characteristically ataxic and will have poor results on posturography.16 There are two tests in posturography: the sensory organization test and the motor control test.
In the sensory organization test, the patient is subjected to six conditions. In condition 1, the patient stands on a fixed platform with the eyes open and looks at a fixed visual surround. In condition 2, the platform is fixed, but the eyes are covered, forcing the patient to rely on proprioceptive and vestibular cues. In condition 3, the platform is fixed and the eyes are open, but the visual surround moves in reference to body sway, forcing the patient to ignore the visual stimulus and rely on proprioceptive and vestibular cues. In condition 4, the eyes are open and the visual surround is fixed, but the platform sways, taking away proprioception, which forces the patient to rely on visual and vestibular cues. Patients with vestibular dysfunction still tend to do well in condition 4. In condition 5, the platform sways and the eyes are covered, forcing the patient to rely on vestibular cues alone—patients with vestibular dysfunction tend to fall here. In condition 6, the eyes are open, but both the platform and visual surround move, forcing the patient to rely on vestibular cues while ignoring inaccurate proprioceptive and visual cues.2 Patients with vestibular dysfunction tend to fall here as well. The parameter measured is the patient’s anterior and posterior body sway, and is measured on a 0-to-100 scale (fall = 0, no sway = 100).22
Motor control tests evaluate the automatic postural responses to forward and backward horizontal movements of the platform. The main parameter tested here is latency. A prolonged latency in both directions suggests a central lesion, whereas a prolonged latency in only one direction suggests either a peripheral or central lesion.17
Although posturography results tend not to localize lesions, they are useful for planning vestibular rehabilitation. Posturography may also aid in the detection of malingerers, who tend to have inconsistent results and may do more poorly on conditions 1 and 2 than on conditions 5 and 6.2
Other Testing
Additional tests that may be useful in the balance evaluation are audiometric tests, radiographic tests, and blood tests. Audiometric tests are extremely important in the evaluation of dizziness. Every patient should at least have an audiogram and immittance testing. A unilateral hearing loss supports a peripheral cause of vertigo, and reduced speech discrimination scores may prompt a search for a retrocochlear abnormality such as an acoustic neuroma.16 Radiographic tests such as an MRI will be able to detect acoustic neuromas, multiple sclerosis, and brainstem strokes. CT scans may detect middle and inner ear anomalies such as a cholesteatoma eroding into the semicircular canals or a superior semicircular canal dehiscence. Finally, blood tests looking for thyroid function, glucose tolerance, syphilis, rheumatoid factor, and ANA may also be useful in helping to diagnose a dizzy patient.
Chole RA, Cook GB. The Rinne test for conductive deafness. A clinical reappraisal. Arch Otolaryngol Head Neck Surg. 1998;114:399-403.
Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope. 2004;114:1686-1692.
Kileny PR, Zwolan TA. Diagnostic and rehabilitative audiology. In: Lustig LR, Cummings CW, editors. Cummings Otolaryngology Head and Neck Surgery. St Louis: Mosby Elsevier; 2004:3483-3502.
Satar B. Vestibular testing. In: Lalwani AK, editor. Current Diagnosis and Treatment in Otolaryngology—Head and Neck Surgery. New York: McGraw-Hill; 2004:643-658.
1 McGee J, Walsh EJ. Cochlear transduction and the molecular basis of peripheral auditory pathology. In: Lustig LR, Cummings CW, editors. Cummings Otolaryngology—Head and Neck Surgery. St Louis: Mosby Elsevier; 2004:3402-3465.
2 Hullar TE, Minor LB, Zee DS. Evaluation of the patient with dizziness. In: Lustig LR, Cummings CW, editors. Cummings Otolaryngology—Head and Neck Surgery. St Louis: Mosby Elsevier; 2004:3160-3192.
3 Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685-1689.
4 Kroenke K, Mangelsdorff AD. Common symptoms in ambulatory care: Incidence, evaluation, therapy, and outcome. Am J Med. 1989;86:262-266.
5 Arts HA. Sensorineural hearing loss: evaluation and management in adults. In: Lustig LR, Cummings CW, editors. Cummings Otolaryngology—Head and Neck Surgery. St Louis: Mosby Elsevier; 2004:3535-3561.
6 Diagnosis of ear disease. In: Glasscock ME, Shambaugh GE, Johnson GD, editors. Surgery of the Ear. Philadelphia: WB Saunders, 1990.
7 Backous DD, Niparko JN. Evaluation and surgical management of conductive hearing loss. In: Lustig LR, Cummings CW, editors. Cummings Otolaryngology—Head and Neck Surgery. St Louis: Mosby Elsevier; 2004:3522-3534.
8 Miltenburg DM. The validity of tuning fork tests in diagnosing hearing loss. J Otolaryngol. 1994;23:254-259.
9 Chole RA, Cook GB. The Rinne test for conductive deafness. A clinical reappraisal. Arch Otolaryngol Head Neck Surg. 1998;114:399-403.
10 Sweetow RW, Bold JM. Audiologic testing. In: Lalwani AK, editor. Current Diagnosis and Treatment in Otolaryngology—Head and Neck Surgery. New York: McGraw-Hill; 2004:631-641.
11 Hall JW, Antonelli PJ. Assessment of peripheral and central auditory function. In: Bailey BJ, editor. Head and Neck Surgery—Otolaryngology. Philadelphia: Lippincott Williams and Wilkins; 2001:1659-1672.
12 Kileny PR, Zwolan TA. Diagnostic and rehabilitative audiology. In: Lustig LR, Cummings CW, editors. Cummings Otolaryngology—Head and Neck Surgery. St Louis: Mosby Elsevier; 2004:3483-3502.
13 Cueva RA. Auditory brainstem response versus magnetic resonance imaging for the evaluation of asymmetric sensorineural hearing loss. Laryngoscope. 2004;114:1686-1692.
14 Nageris BI, Popovtzer A. Acoustic neuroma in patients with completely resolved sudden hearing loss. Ann Otol Rhinol Laryngol. 2003;112:395-397.
15 Chaimoff M, et al. Sudden hearing loss as a presenting symptom of acoustic neuroma. Am J Otolaryngol. 1999;20:157-160.
16 Linstrom CJ. Office management of the dizzy patient. Otolaryngol Clin North Am. 1992;25:745-780.
17 Satar B. Vestibular testing. In: Lalwani AK, editor. Current Diagnosis and Treatment in Otolaryngology—Head and Neck Surgery. New York: McGraw-Hill; 2004:643-658.
18 Lee H, et al. Sudden deafness and anterior inferior cerebellar artery infarction. Stroke. 2002;33:2807-2812.
19 Mong A, et al. Sound- and pressure-induced vertigo associated with dehiscence of the roof of the superior semicircular canal. AJNR Am J Neuroradiol. 1999;20:1973-1975.
20 Brandt T, Strupp M. General vestibular testing. Clin Neurophysiol. 2005;116:406-426.
21 Moffat DA, et al. Unterberger’s stepping test in acoustic neuroma. J Laryngol Otol. 1989;103:839-841.
22 Driscoll CL, Green JD. Balance function tests. In: Bailey BJ, editor. Head and Neck Surgery—Otolaryngology. Philadelphia: Lippincott Williams and Wilkins; 2001:1652-1658.
23 Hain TC, et al. Localizing value of optokinetic afternystagmus. Ann Otol Rhinol Laryngol. 1994;103:806-811.